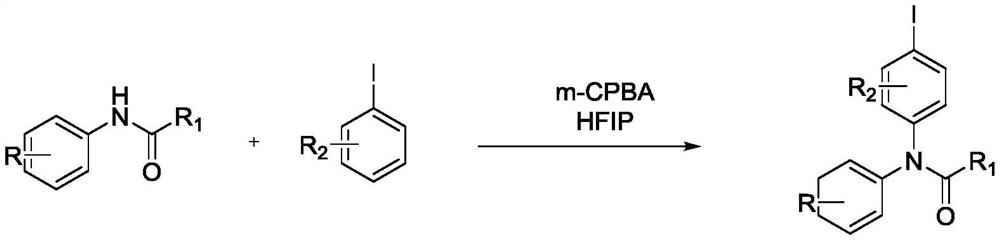
Preparation method of N-iodobenzene-N-phenyl amide compound
A technology for phenyl amides and compounds, which is applied in the field of novel preparation of aryl amide compounds, can solve the problems of poor applicability, environmental pollution, dangerous preparation conditions, etc., and achieves the effects of mild reaction conditions, high selectivity and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
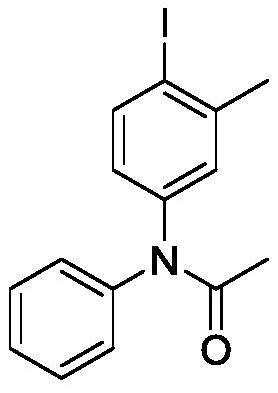
[0018] Embodiment 1, the preparation of N-(4-iodo-3-toluene)-N-phenylacetamide
[0019]
[0020] Add 0.2mmol of acetanilide, 0.3mmol of 2-iodotoluene, 0.3mmol of m-chloroperoxybenzoic acid, 1mL of hexafluoroisopropanol and one No. 5 magnet in sequence, pass the condensed water through the condenser tube from bottom to top, and then close the reactor Put it in an oil bath at 40-80°C and heat it for 2-8 hours, add 15mL of water, extract three times with 10mL of ethyl acetate each time, combine the obtained organic phases, spin dry with a rotary evaporator, and separate and purify the crude product by column chromatography , to obtain 65.3 mg of N-(4-iodobenzene)-N-acetanilide as a yellow solid with a yield of 93%.
[0021] The structure of the product is determined by H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 ): δ2.05(s,3H),2.38(s,3H),6.79(d,J=7.96Hz 2H),7.16(s,1H),7.24(d,J=8.2Hz 2H),7.38(s ,1H),7.76(s,1H); 13 C NMR (100MHz, CDCl 3 ): δ24.3, 28.2, 127.9, 129.6, 139.5, 142...
Embodiment 2
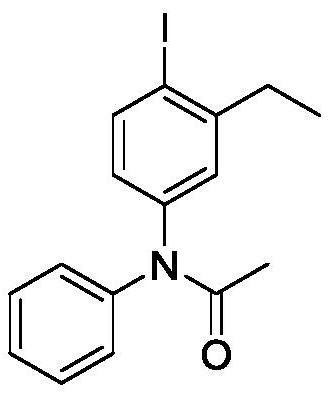
[0022] Embodiment 2, the preparation of N-(4-iodo-3-ethylbenzene)-N-phenylacetamide
[0023]
[0024] Add 0.2mmol of acetanilide, 0.3mmol of 2-ethyl iodobenzene, 0.3mmol of m-chloroperoxybenzoic acid, 1mL of hexafluoroisopropanol and one No. 5 magnet in sequence, pass condensed water through the condenser tube from bottom to top The reactor was placed in a 40-80°C oil bath and heated for 2-8 hours. Add 15mL of water and extract three times with 10mL of ethyl acetate each time. The obtained organic phases were combined and spin-dried by a rotary evaporator. The crude product was subjected to column chromatography After separation and purification, 64.2 mg of N-(4-iodo-3-ethylbenzene)-N-acetanilide was obtained as a yellow solid with a yield of 88%.
[0025] The structure of the product is determined by H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 ): δ1.17(t, J=7.32Hz, 3H) 2.06(s, 3H), 2.68(d, J=6.52Hz, 2H), 6.80(q, J=2.48Hz J=5.88Hz 1H), 7.14 (d, J=2.28Hz 1H), 7.25(d, J=7.72H...
Embodiment 3
[0026] Embodiment 3, the preparation of N-(1,3-dimethyl-2-iodobenzene)-N-phenylacetamide
[0027]
[0028] Add 0.2mmol of acetanilide, 0.3mmol of 1,3-dimethyl-2-iodobenzene, 0.3mmol of m-chloroperoxybenzoic acid, 1mL of hexafluoroisopropanol and one No. After passing the condensed water to the top, place the reactor in an oil bath at 40-80°C and heat for 2-8 hours, add 15mL of water, extract three times with 10mL of ethyl acetate each time, combine the obtained organic phases, and spin dry with a rotary evaporator , the crude product was separated and purified by column chromatography to obtain 45.99 mg of N-(4-iodobenzene)-N-acetanilide as a yellow solid with a yield of 63%.
[0029] The structure of the product is determined by H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 ): δ2.05(s,3H),2.43(s,6H),6.98(s,2H),7.25-7.27(m,3H),7.37(s,2H); 13 C NMR (100MHz, CDCl 3 ): δ23.8, 29.7, 125.1, 126.6, 128.2, 129.7, 142.9, 170.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com