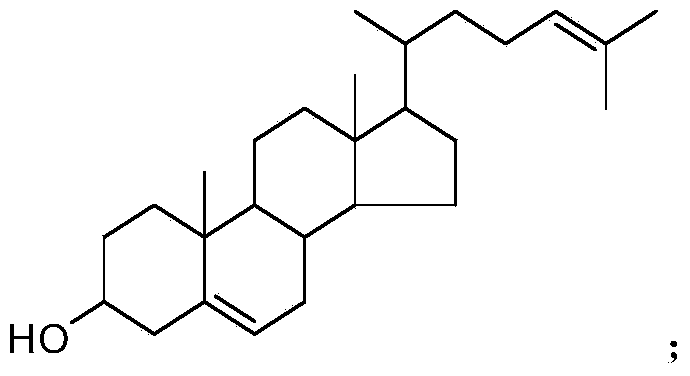
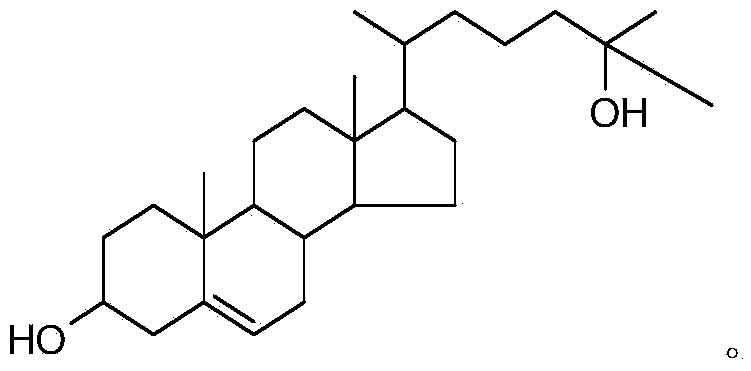
Synthetic method of 25-hydroxycholesterol
A technology of hydroxycholesterol and synthesis method, applied in the direction of steroids, organic chemistry, etc., can solve the problems of poor selectivity and low yield, and achieve the effects of high selectivity, lower reaction temperature, and higher yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, a kind of synthetic method of 25-hydroxycholesterol, carry out the following steps successively:
[0047] 1) Using 120mL pyridine as solvent and 30mL pyridine (about 0.372mol) as acid binding agent, add 20g of 24-dehydrocholesterol (about 0.052mol) and 0.2g of DMAP, and dropwise add 10g (about 0.097mmol) at room temperature ) acetic anhydride (about 30 minutes to complete). The reaction process was monitored by TLC. After the dropwise addition, the reaction was continued at room temperature for 10 h, and the reaction was completed.
[0048] The reaction solution was washed with water (the amount of water was 50ml×2), and acid washed (with a volume concentration of 5% dilute hydrochloric acid solution, the amount was 50ml×2), and then extracted with dichloromethane (the amount was 30ml×3), and the extract was Washed with saturated sodium bicarbonate solution until neutral, dried with anhydrous sodium sulfate (about 5 g), and then dehydrated the solvent (ie...
Embodiment 2
[0056] Embodiment 2, a kind of synthetic method of 25-hydroxycholesterol,
[0057] In this embodiment 2, steps 1) and 2) are different from those in embodiment 1, and the difference lies in:
[0058] 1) Using 100 mL of dichloromethane as a solvent, add 20 g of 24-dehydrocholesterol (about 0.052 mol), 0.2 g of DMAP, 10.1 g (about 0.1 mmol) of triethylamine, and dropwise add 10 g (about 0.097 mmol) of vinegar at room temperature anhydride. The reaction process was monitored by TLC, and the reaction was continued at room temperature for 10 h after the dropwise addition, and the reaction was completed.
[0059] The reaction solution was washed successively with dilute hydrochloric acid (volume concentration 5%, dosage 50ml×2), saturated sodium bicarbonate solution (30ml×2), saturated sodium chloride solution (30ml), and then dried with anhydrous sodium sulfate (about 5g) , and the solvent (ie, dichloromethane) was removed by rotary evaporation under reduced pressure (0.1 MPa) to...
Embodiment 3
[0062] Embodiment 3, a kind of synthetic method of 25-hydroxycholesterol,
[0063] In this embodiment 3, steps 1) and 2) are different from those in embodiment 1, and the difference lies in:
[0064] 1) Using 100mL of dichloromethane as a solvent, add 20g of 24-dehydrocholesterol (about 0.052mol), 0.2g of DMAP, 10.1g (about 0.1mmol) of triethylamine, and dropwise add 14.1g (about 0.1mol) at room temperature ) benzoyl chloride, the temperature was raised to reflux after the dropwise addition, and the reaction was terminated after 24 hours of reaction.
[0065] The rest is basically equivalent to step 1) of Example 2, to obtain 23.2 g of benzoylated 24-dehydrocholesterol with a yield of 95.0%.
[0066] 2) Use 40% peracetic acid 0.4g (2mmol) as the epoxidation reagent to replace "38% (mass concentration) peracetic acid 0.4g (2mmol)" in Example 1, and the reaction temperature is changed from -10 ℃ to -20°C.
[0067] The rest are basically equivalent to step 2) of Example 1; 0.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com