Method for totally synthesizing natural product (+/-)-rupestine G and resolving enantiomers
A technology for enantiomers, natural products, applied in the field of organic synthesis technology and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] a. Compound 1 was 2.0 g of 2-methyl-5-bromopyridine, (12 mmol) was dissolved in 30 ml of dichloromethane solution, and 2.9 g of m-chloroperoxybenzoic acid with a purity of 85% was added in batches under an ice bath, ( 13.9mmol) after naturally warming up to room temperature, stirring overnight reaction, after the reaction, the reaction solution was poured into saturated sodium sulfite solution to quench, after stirring for 1 hour, extract 3 times with dichloromethane 150ml, combine the organic phases, and use Dry over magnesium sulfate, remove the organic solvent to obtain the nitrogen oxidation product Compound 2 is 5-bromo-2-methyl-N-oxypyridine, the yield is 93%, 2.0g;
[0054] b. Under the protection of nitrogen, dissolve 1.0g (5.4mmol) of compound 2 as 5-bromo-2-methyl-N-oxypyridine in 20ml of acetonitrile solution, and then add 2.1g of trimethylsilyl cyanide, (21.6mmol ) and triethylamine 2.2ml, (16.2mmol), after reflux 12 hours, remove organic solvent, be COMBIFL...
Embodiment 2
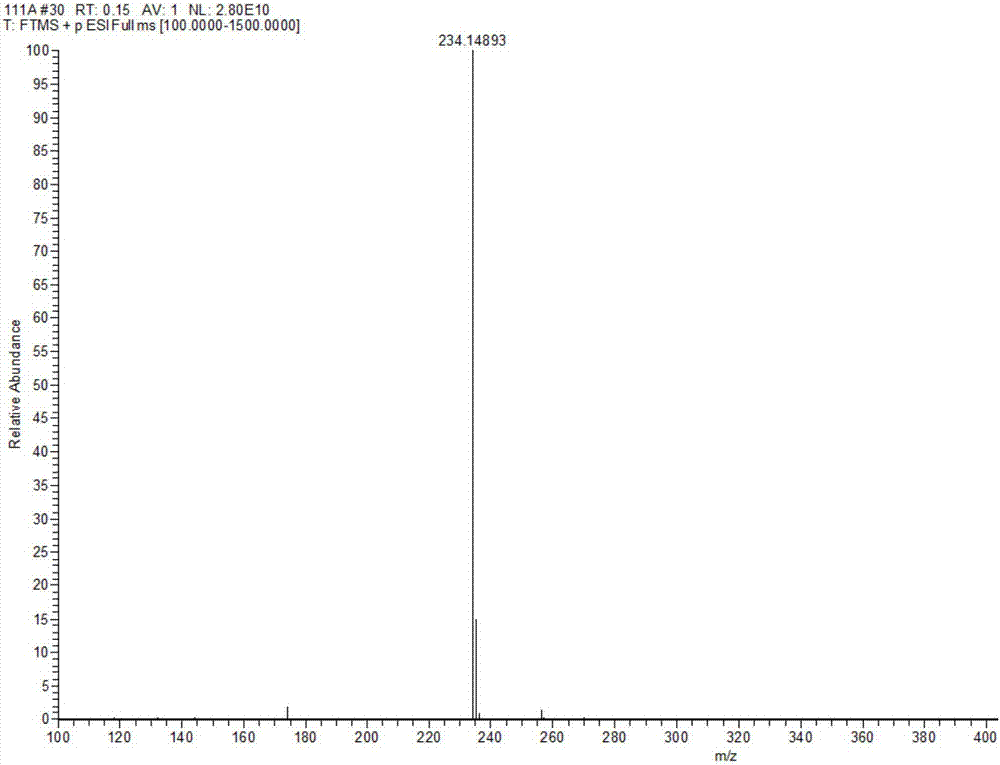
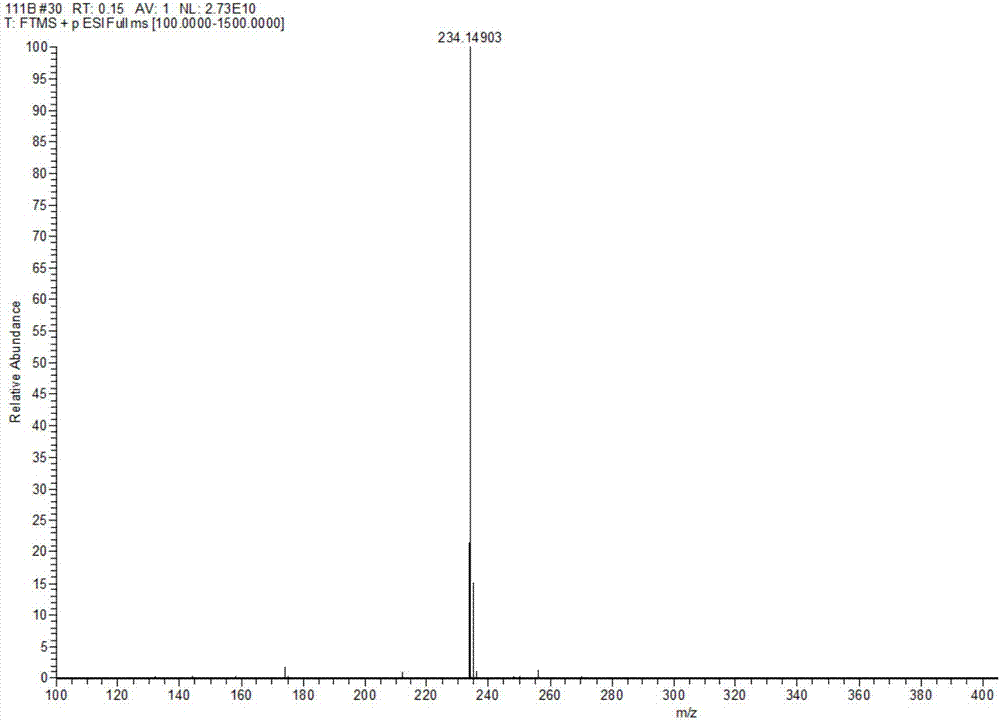
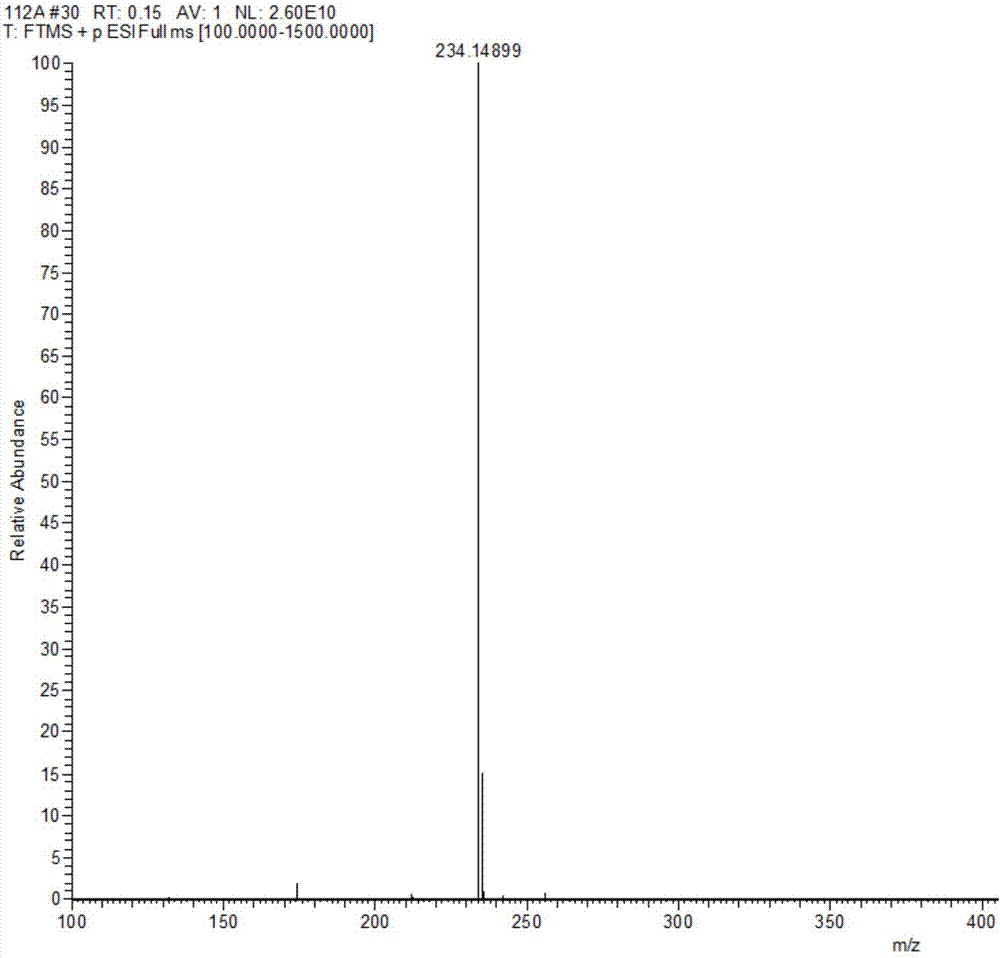
[0075] Rupestine G (Rupestine G) and its three isomers Compound 13 is (5S,8R)-Rupestine G; Compound 14 is (5R,8S)-Rupestine G; Compound 15 is (5R, 8R)-one branch of artemisinine G was measured by the QstarElite quadrupole-time-of-flight hybrid high-resolution mass spectrometer of AB SCIEX, USA, its precise molecular weight was measured, the optical rotation value was measured by Rudolph RS Autopol VIautomatic polarimeter, and the EDC was measured experimentally by Chirascan circular dichroism spectrometer German COSMOlogic GmbH&Co.KG TmoleX 3.4 software calculates the ECD spectrum and compares it to determine its absolute configuration. For the high-resolution mass spectrum of Rupestine G (Rupestine G) and its isomers, see Figure 1-Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com