Method for preparing epsilon-caprolactone by using micro-reaction device
A technology of micro-reaction device and caprolactone, which is applied in the direction of organic chemistry, can solve the problems of low yield, difficult to control the reaction, and high cost, and achieve the effects of reducing production cost, improving quality, and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
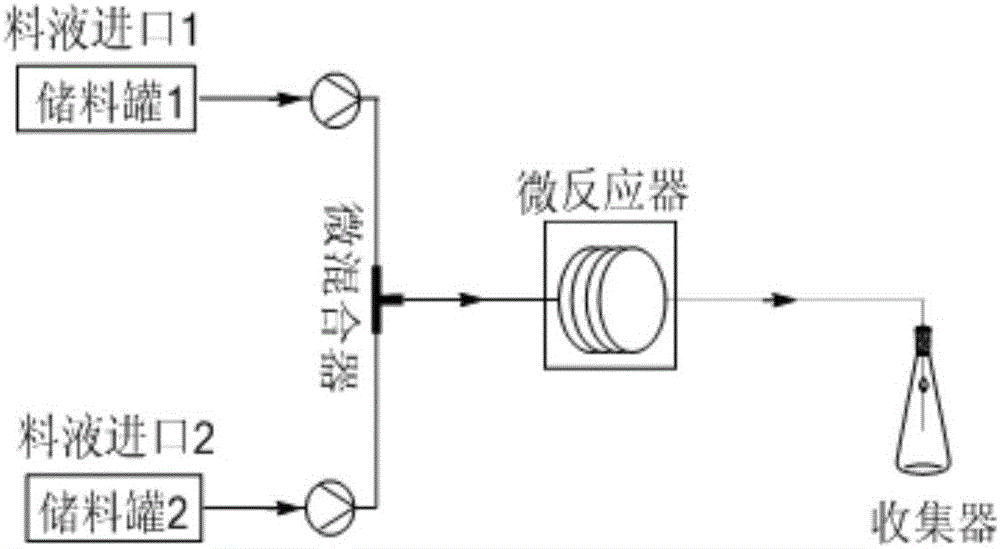
[0031] Dissolve m-chloroperoxybenzoic acid and cyclohexanone in ethyl acetate respectively, the molar ratio of m-chloroperoxybenzoic acid and cyclohexanone is 1.2:1; And cyclohexanone are pumped into the micro-mixer in the micro-reaction device at the same time, the flow rate is 0.5ml / min and 0.5ml / min respectively; after thorough mixing, the mixed system is passed into the micro-structure reactor for reaction, and the reaction temperature is 30°C , the reaction residence time is 10min; after the reaction is completed, collect the product (reaction solution), wash the organic phase to pH=7.0 with 5wt% sodium carbonate solution and distilled water respectively, collect the organic phase, and then use an organic solvent to carry out the above-mentioned aqueous phase Extraction, the organic extract is combined with the organic phase, the solvent is removed by rotary evaporation, and the target product ε-caprolactone is obtained; a certain amount of acid is added to the aqueous pha...
Embodiment 2
[0033]Dissolve m-chloroperoxybenzoic acid and cyclohexanone in ethyl acetate respectively, the molar ratio of m-chloroperoxybenzoic acid and cyclohexanone is 1.7:1; and cyclohexanone are pumped into the micro-mixer in the micro-reaction device at the same time, the flow rates are 1.172ml / min and 0.828ml / min respectively; after thorough mixing, the mixed system is passed into the micro-structure reactor for reaction, and the reaction temperature is 80°C , the reaction residence time is 5min; after the reaction is completed, collect the product (reaction solution), wash the organic phase to pH=7.0 with 5wt% sodium carbonate solution and distilled water respectively, collect the organic phase, and then use an organic solvent to carry out the above-mentioned water phase Extraction, the organic extract is combined with the organic phase, the solvent is removed by rotary evaporation, and the target product ε-caprolactone is obtained; a certain amount of acid is added to the water pha...
Embodiment 3
[0035] Dissolve m-chloroperoxybenzoic acid and cyclohexanone in dichloromethane respectively, and the molar ratio of m-chloroperoxybenzoic acid and cyclohexanone is 2:1; dissolve m-chloroperoxybenzoic acid in ethyl acetate and cyclohexanone are pumped into the micro-mixer in the micro-reaction device at the same time, the flow rates are 1.25ml / min and 0.75ml / min respectively; after thorough mixing, the mixed system is passed into the micro-structure reactor for reaction, and the reaction temperature is 100°C , the reaction residence time is 5min; after the reaction is completed, collect the product (reaction solution), wash the organic phase to pH=7.0 with 5wt% sodium carbonate solution and distilled water respectively, collect the organic phase, and then use an organic solvent to carry out the above-mentioned water phase Extraction, the organic extract is combined with the organic phase, the solvent is removed by rotary evaporation, and the target product ε-caprolactone is obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com