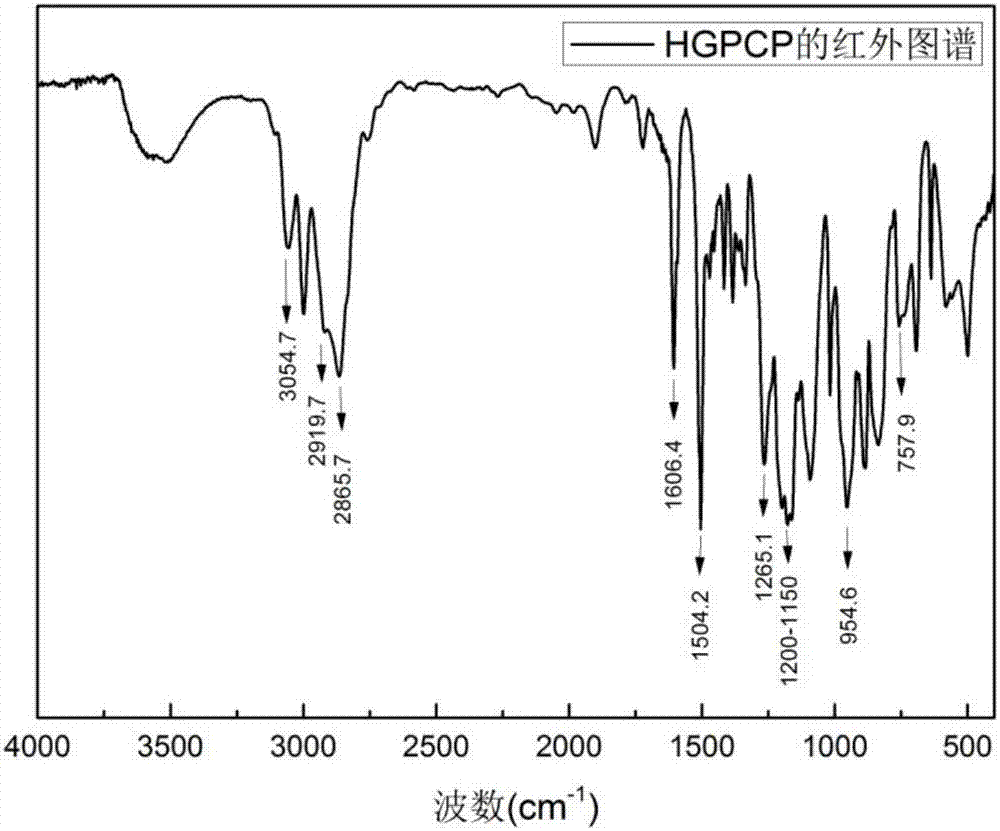
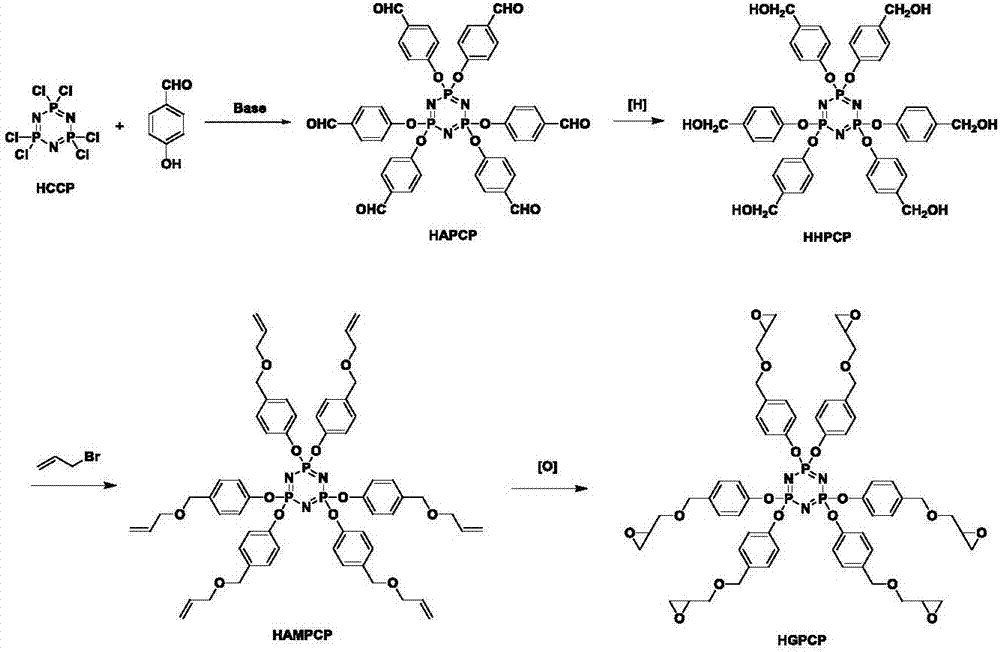
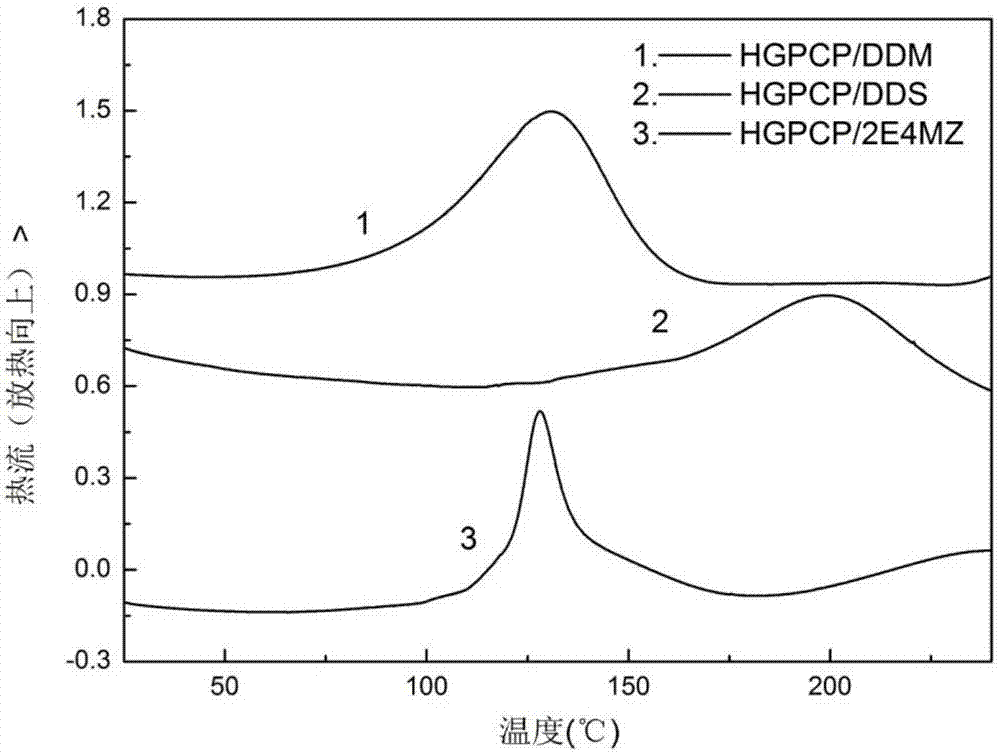
Preparation method of six-functionality epoxy resin based on cyclotriphosphazene
A hexafunctional epoxy resin technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of poor comprehensive performance of the flame retardant system and achieve flame retardant performance Excellent, the effect of improving mechanical properties and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1. Dissolve 51.7g of potassium carbonate and 10g of hexachlorocyclotriphosphazene (HCCP) in 150mL of tetrahydrofuran, weigh 24.6g of p-hydroxybenzaldehyde (PHB) and dissolve in 100mL of tetrahydrofuran, and slowly add it dropwise to the above system in an ice bath After the dropwise addition was completed, the ice bath was removed, and the reaction was performed at room temperature for 0.5h, and then moved to an oil bath to reflux for 48h. The filtrate was concentrated, extracted with chloroform, washed with saturated sodium chloride, and dried over anhydrous sodium sulfate. Filter and remove the solvent under reduced pressure to obtain a crude product. Recrystallize with dichloromethane and dry in vacuo to obtain 22.7g of white crystalline solid, i.e. hexa(4-formylphenoxy)cyclotriphosphazene (HAPCP), structural formula:
[0029]
[0030] The yield was 89%.
[0031] NMR 1 H NMR (400MHz, CDCl 3 )δ9.94 (s, 1H), 7.74 (d, 2H), 7.15 (d, 2H). 31 P NMR (162MHz, CD...
Embodiment 2
[0061] Step 1. Under the protection of ice bath and inert gas, 24.6g of PHB and 20.4g of triethylamine (TEA) were added to 150mL of tetrahydrofuran, and HCCP (10g) dissolved in 50mL of tetrahydrofuran was added dropwise. After the dropwise addition, the reaction system was moved into an oil bath, heated to reflux, and reacted for 24 hours. After the reaction was finished, the hydrochloride of triethylamine was removed by suction filtration, and after the solvent was removed by rotary evaporation, 200mL of water was added to precipitate the crude product. Finally, it was recrystallized with ethyl acetate and vacuum-dried to obtain 22.8g of pure HAPCP. The yield was 92%, the structural formula is:
[0062]
[0063] NMR 1 H NMR (400MHz, CDCl 3 )δ9.94 (s, 1H), 7.74 (d, 2H), 7.15 (d, 2H). 31 P NMR (162MHz, CDCl 3 ) δ7.3.
[0064] Step 2. Under ice-bath conditions, add 8.1g of potassium borohydride in batches to HAPCP (20g) dissolved in 100mL of methanol and tetrahydrofuran ...
Embodiment 3
[0076] Step 1. Add 121.8g of cesium carbonate and 10g of hexachlorocyclotriphosphazene into 150mL of tetrahydrofuran, weigh 24.6g of p-hydroxybenzaldehyde and dissolve it in 100mL of tetrahydrofuran, and slowly drop it into the above reaction system under ice bath conditions, and the dropwise addition is completed Finally, the ice bath was removed, and the reaction was carried out at room temperature for 0.5 h, and then stirred at reflux for 48 h. Filtrate with suction, concentrate the filtrate, extract with chloroform, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. Filter and remove the solvent under reduced pressure to obtain a crude product. Recrystallized from dichloromethane and dried under vacuum to obtain 23.3 g of HAPCP with a yield of 94%. The structural formula is:
[0077]
[0078] Step 2. 20 g of HAPCP was dissolved in 100 mL of tetrahydrofuran and methanol mixed solvent, and 5.7 g of sodium borohydride was added in batches under i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com