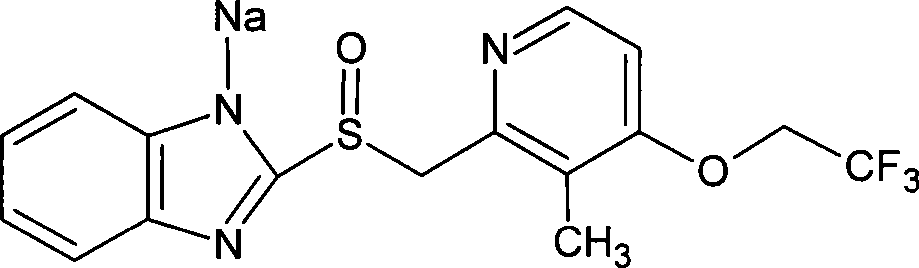
Method for synthesizing lansoprazole and salt thereof
A synthetic method, the technology of lansoprazole, applied in the field of medicinal chemistry, can solve the problems of expensive vanadium catalyst, poor product purity, unsatisfactory effect, etc., and achieve the goals of reducing side reactions, increasing product yield, and shortening reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthesis of 2-[[[3-methyl-4-(2,2,2 trifluoroethoxy)-2-pyridine]-methyl]sulfanyl]-1H-benzimidazole
[0040] Dissolve 276g (1mol) of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride in 5L of acetone while adding 5g (0.033mol) of iodine Sodium chloride, then add 180g (1.2mol) 2-mercapto-5-methoxyl group-1H-benzimidazole and 80g (2mol) sodium hydroxide, this reaction mixture is heated to reflux 1.5 hours, is cooled to room temperature, separates out solid, Filter, wash with water, and dry under vacuum at 40 °C to give 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]-methyl]sulfanyl]-1H - Benzimidazole 335g, yield: 95%, mp: 150-152°C.
[0041] Elemental analysis C: 54.3%, H: 4%, O: 4.6%, F: 16.2%, N: 11.9%, S: 9.0% (theoretical value C: 54.4%, H: 4%, O: 4.5%, F : 16.1%, N: 11.9%, S: 9.1%)
Embodiment 2
[0042] The synthesis of embodiment 2 Lansoprazole
[0043] Dissolve 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]-methyl]sulfanyl]-1H-benzimidazole 212g (0.6mol) in In 4000ml of ethyl acetate, cool with an ice-salt bath to control the internal temperature of the system at 0°C, add dropwise a mixed solution of 120g (0.7mol) of m-chloroperoxybenzoic acid and 1500ml of ethyl acetate, and control the rate of addition to ensure that the reaction temperature is at Between 0-2°C, continue to react at 2°C for 2 hours after the dropwise addition, then wash with 1500ml of saturated sodium carbonate solution, and then wash twice with 2000ml of water, dry the organic phase with anhydrous sodium sulfate, filter, and depressurize Ethyl acetate was distilled, then recrystallized with ethanol 2500ml, activated carbon decolorized, filtered, and vacuum-dried at 40-50°C to obtain 177g of white solid lansoprazole, yield 80%, purity (HPLC) 99.9%, mp: 170- 171°C.
[0044] Elemental analysis...
Embodiment 3
[0045] The synthesis of embodiment 3 lansoprazole sodium
[0046] Add 128g of lansoprazole to 500ml of isopropanol, add 140ml of 10% aqueous sodium hydroxide solution, stir at room temperature for 30 minutes, it becomes a clear liquid, filter, remove insoluble matter, cool the reaction system to 10°C, and precipitate a solid , filtered, and vacuum-dried at 40° C. to obtain 126 g of lansoprazole sodium, with a yield of 93% and a purity of 99.9%.
[0047] Elemental analysis C: 49.1%, H: 3.3%, O: 8.2%, F: 14.5%, N: 10.8%, S: 8.3%, Na: 5.8% (theoretical value C: 49%, H: 3.4%, O : 8.2%, F: 14.6%, N: 10.7%, S: 8.2%, Na: 5.9%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com