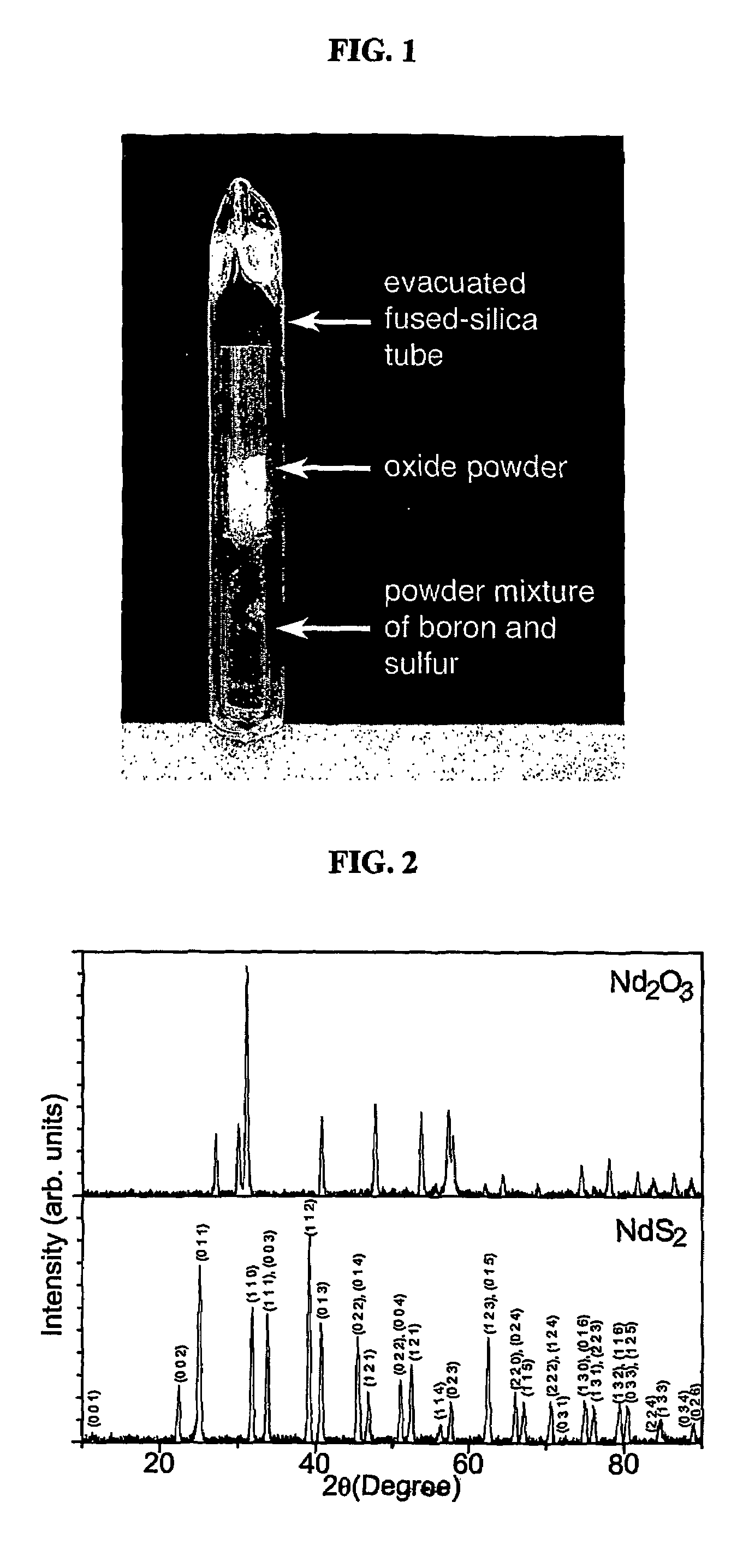
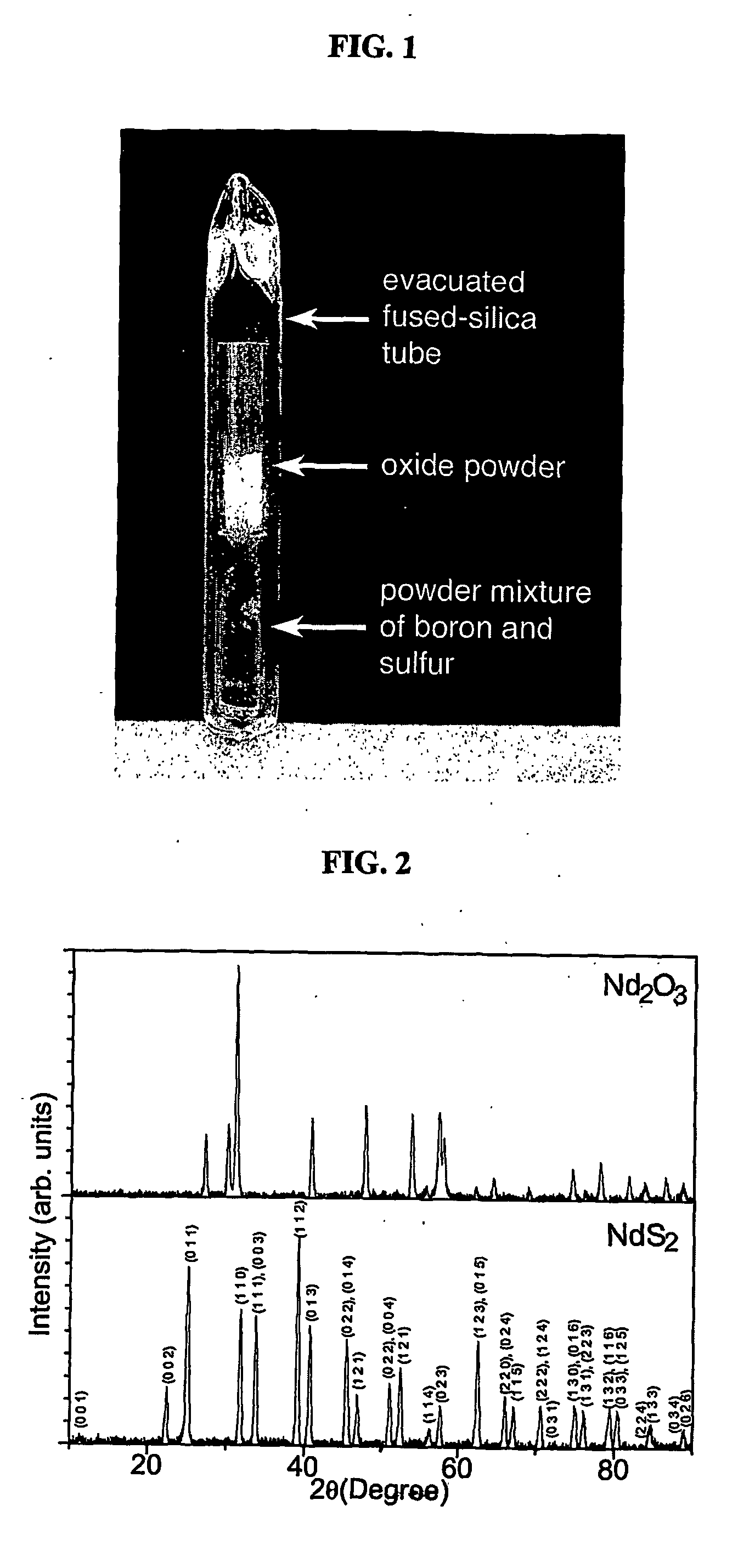
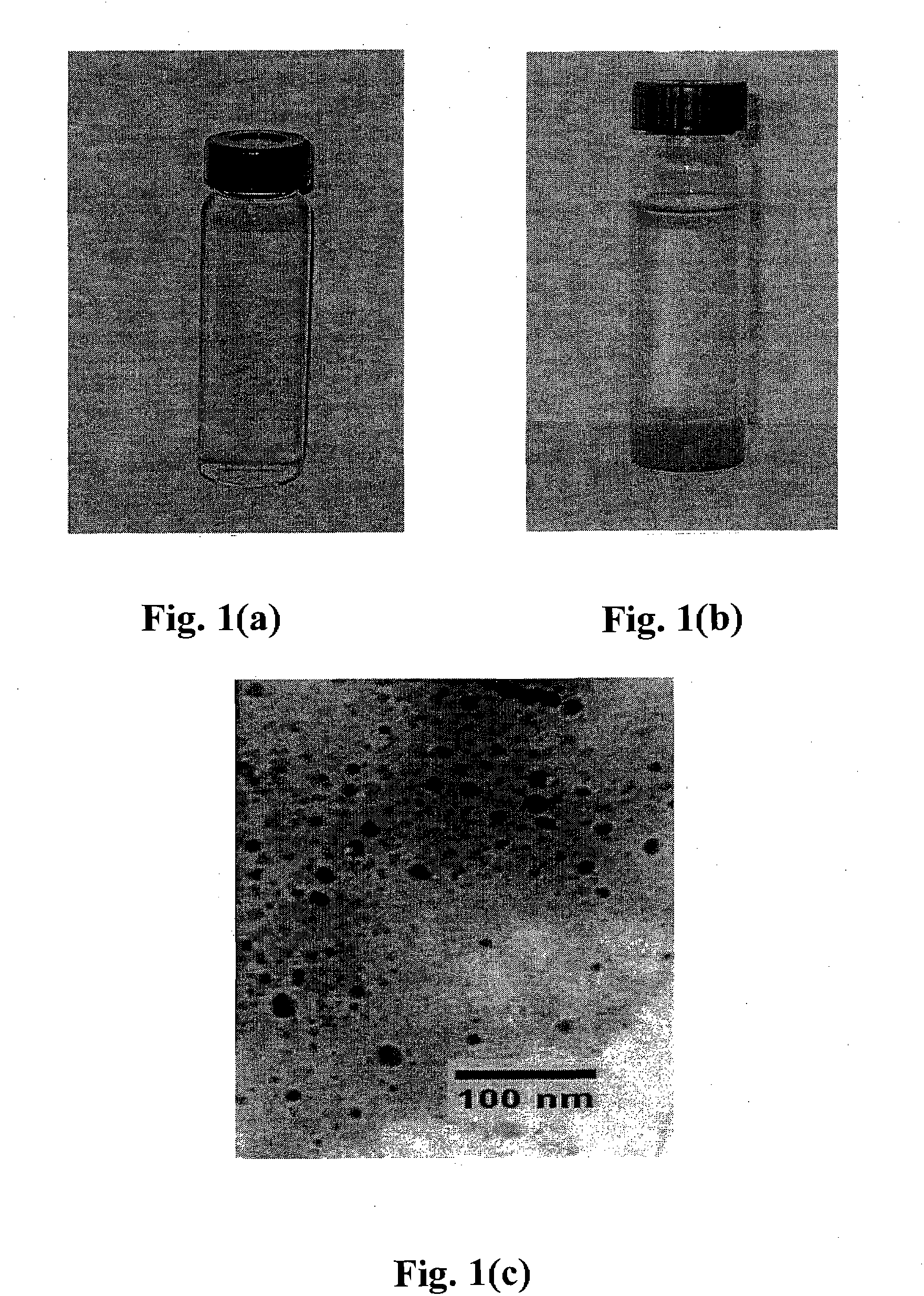
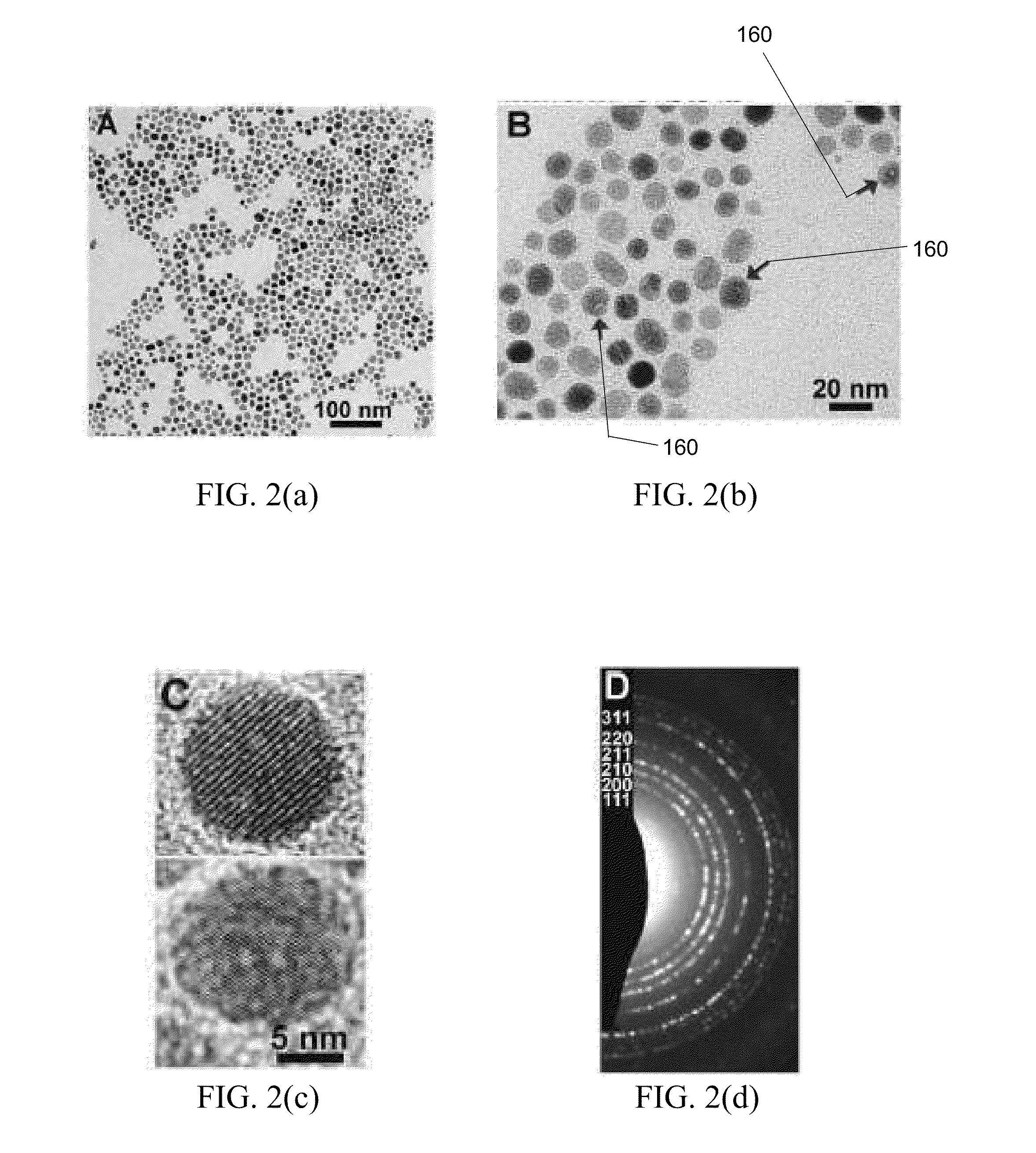
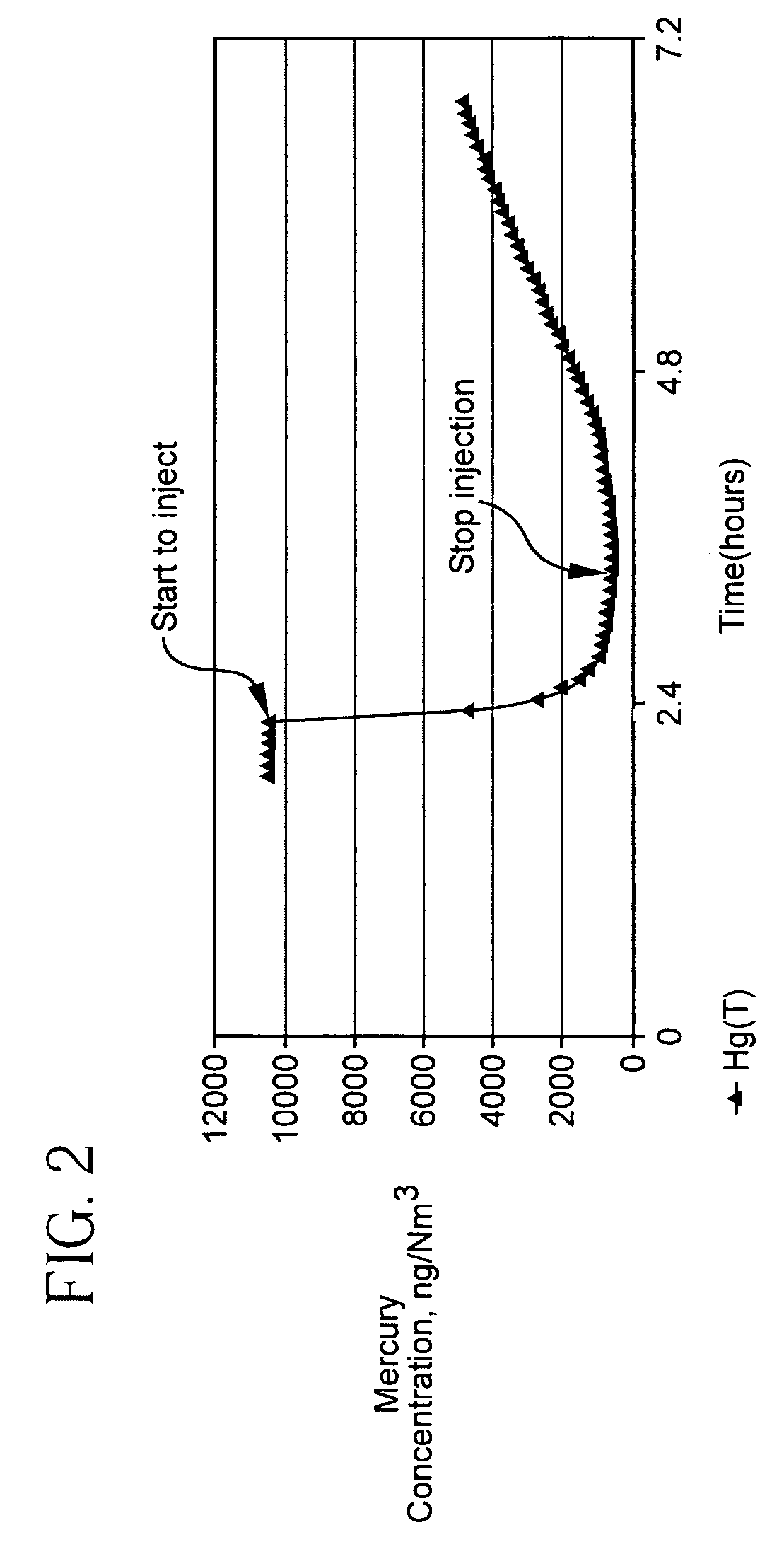
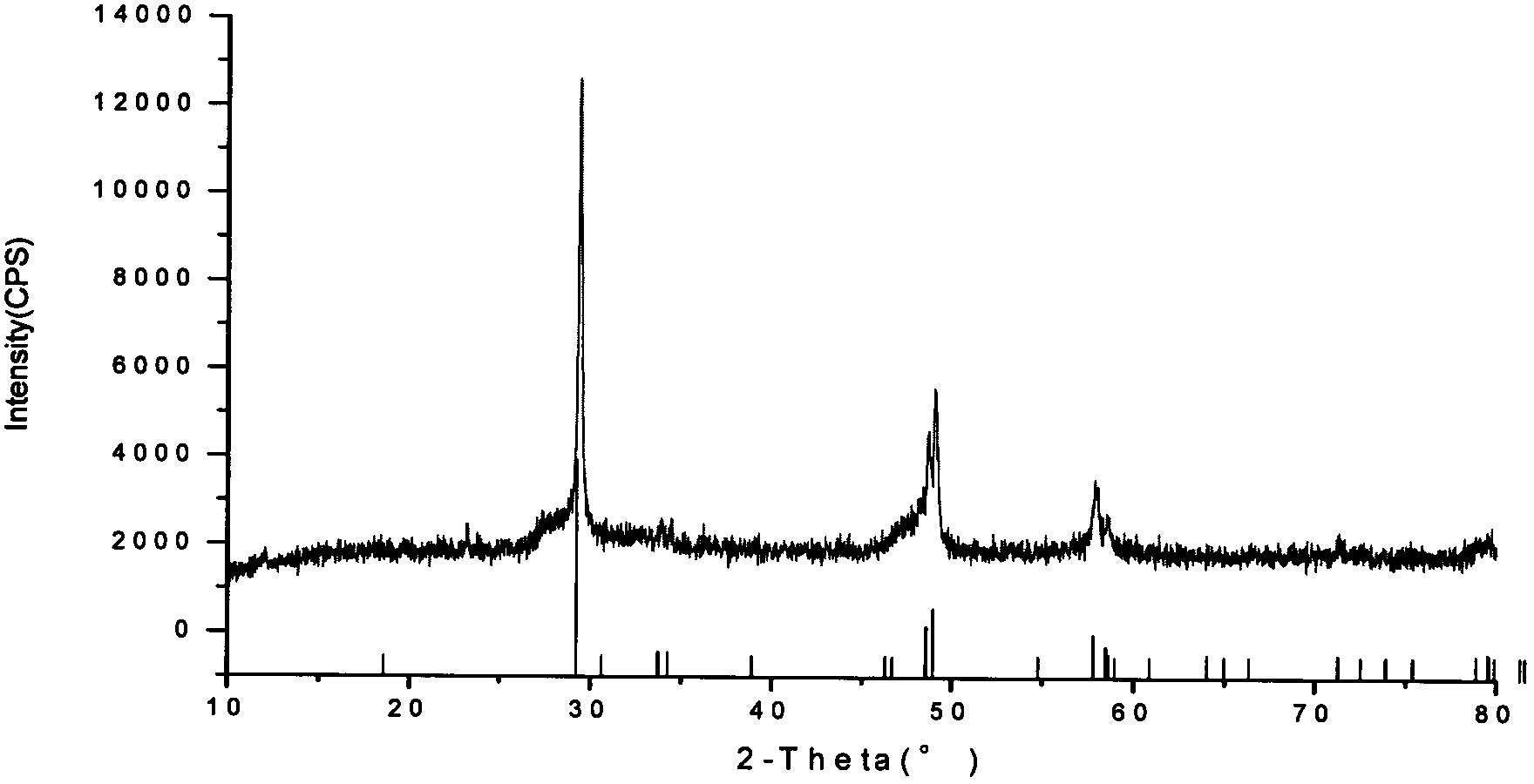
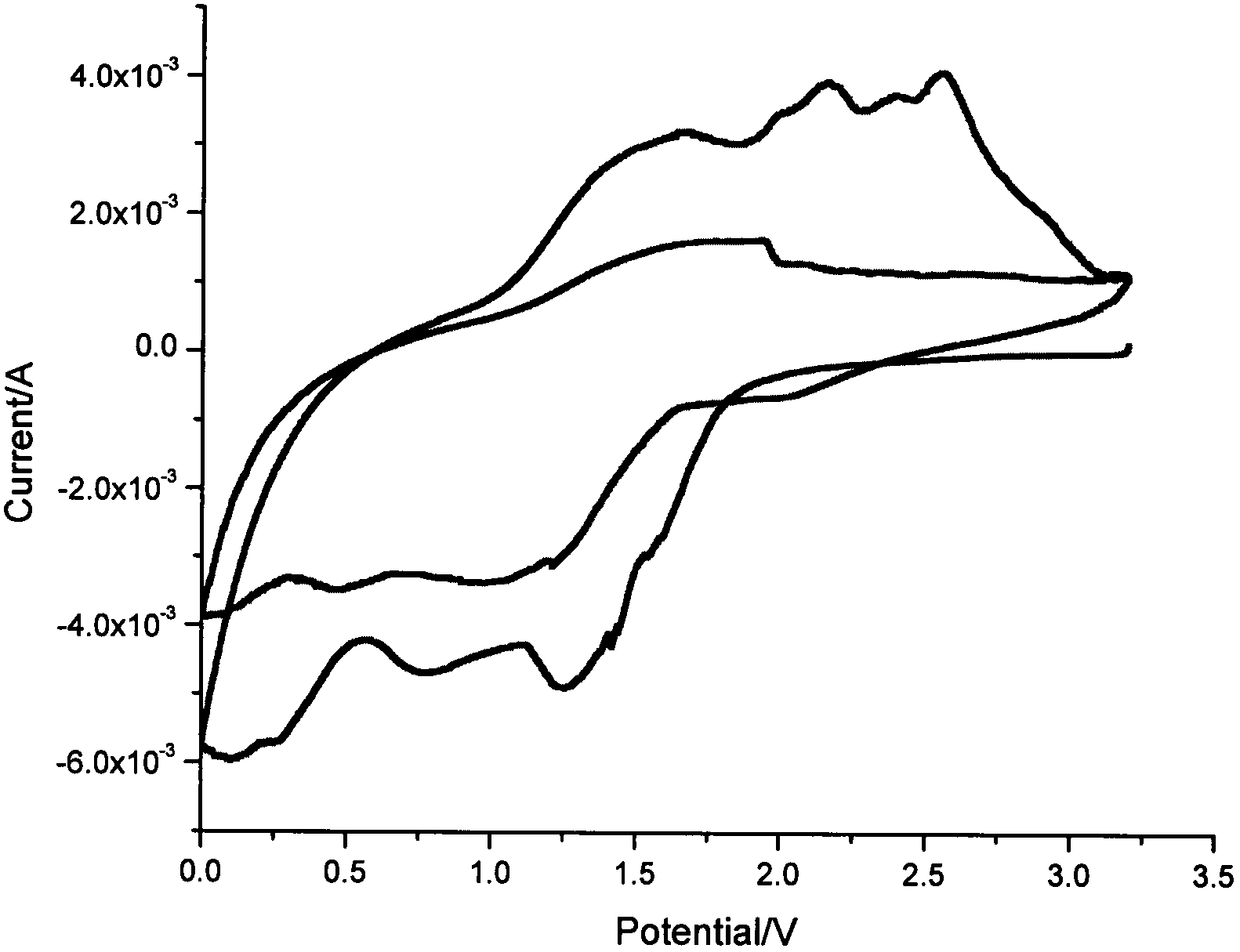
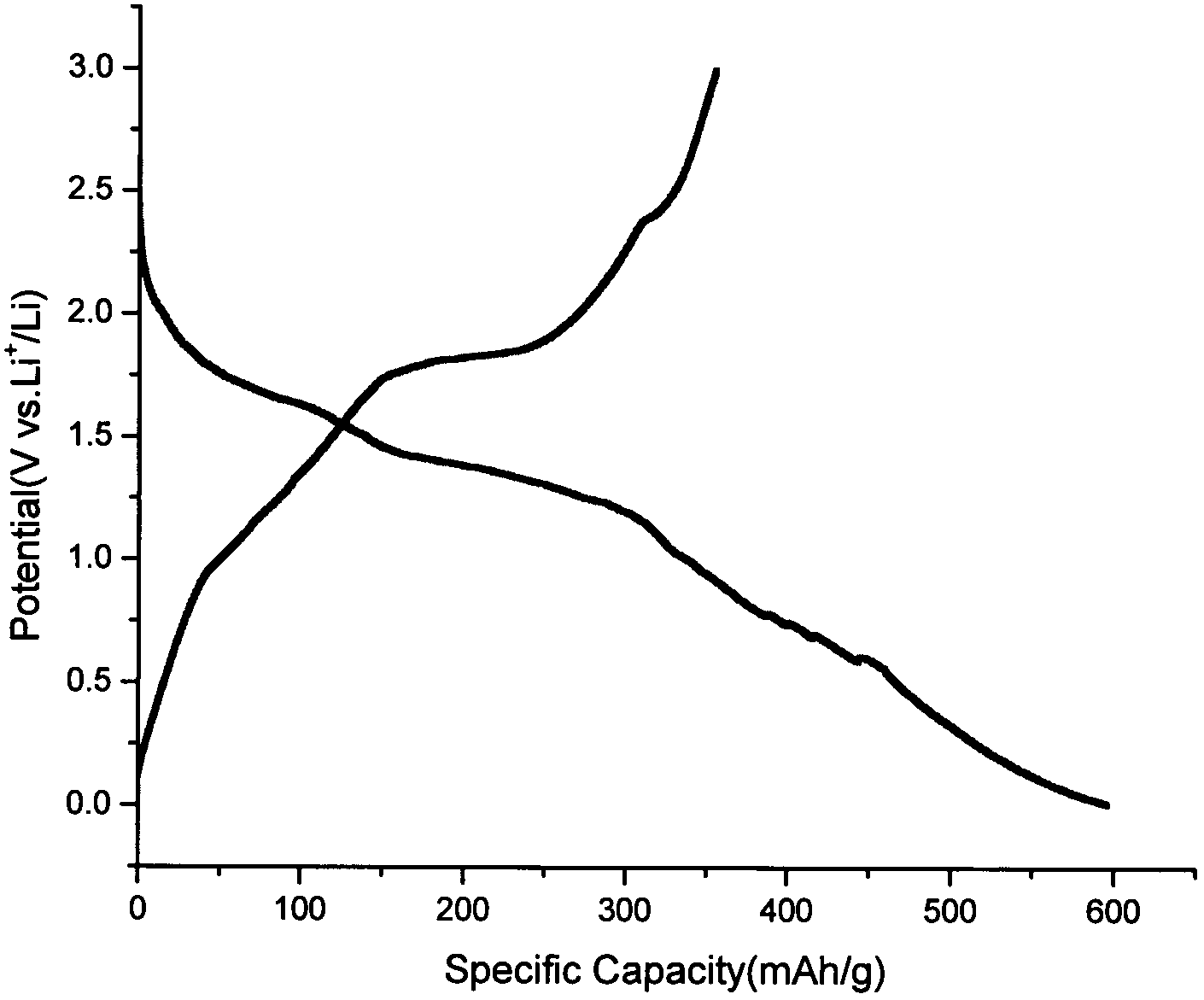
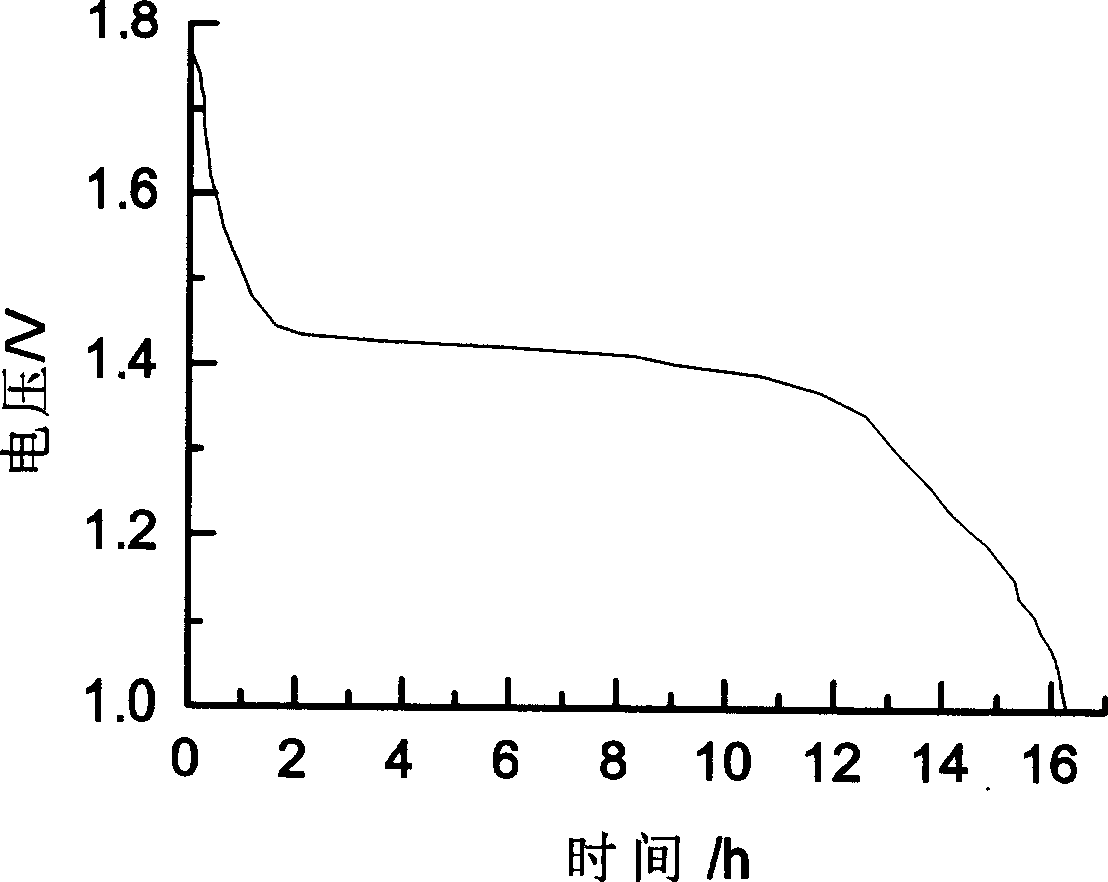
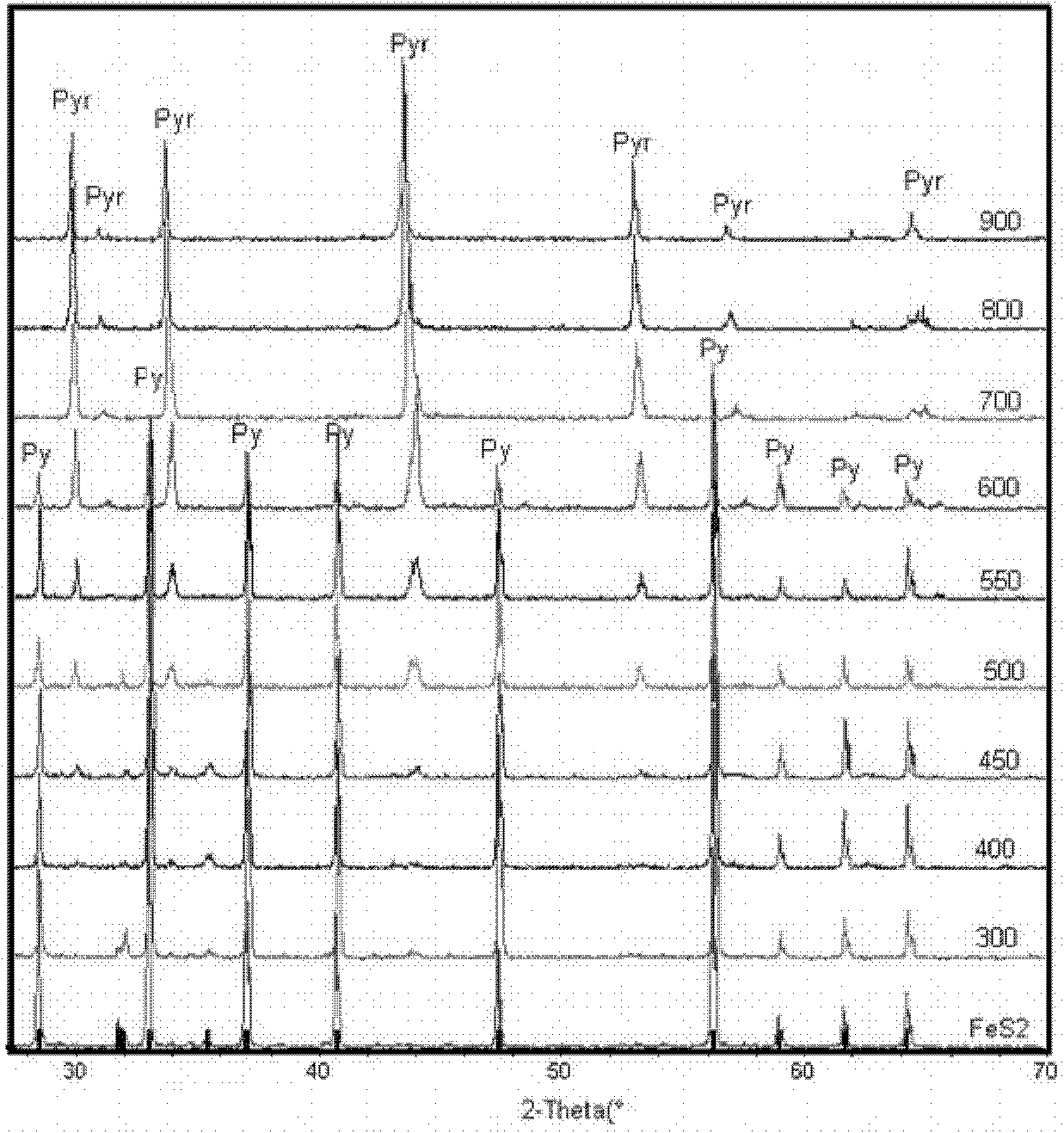
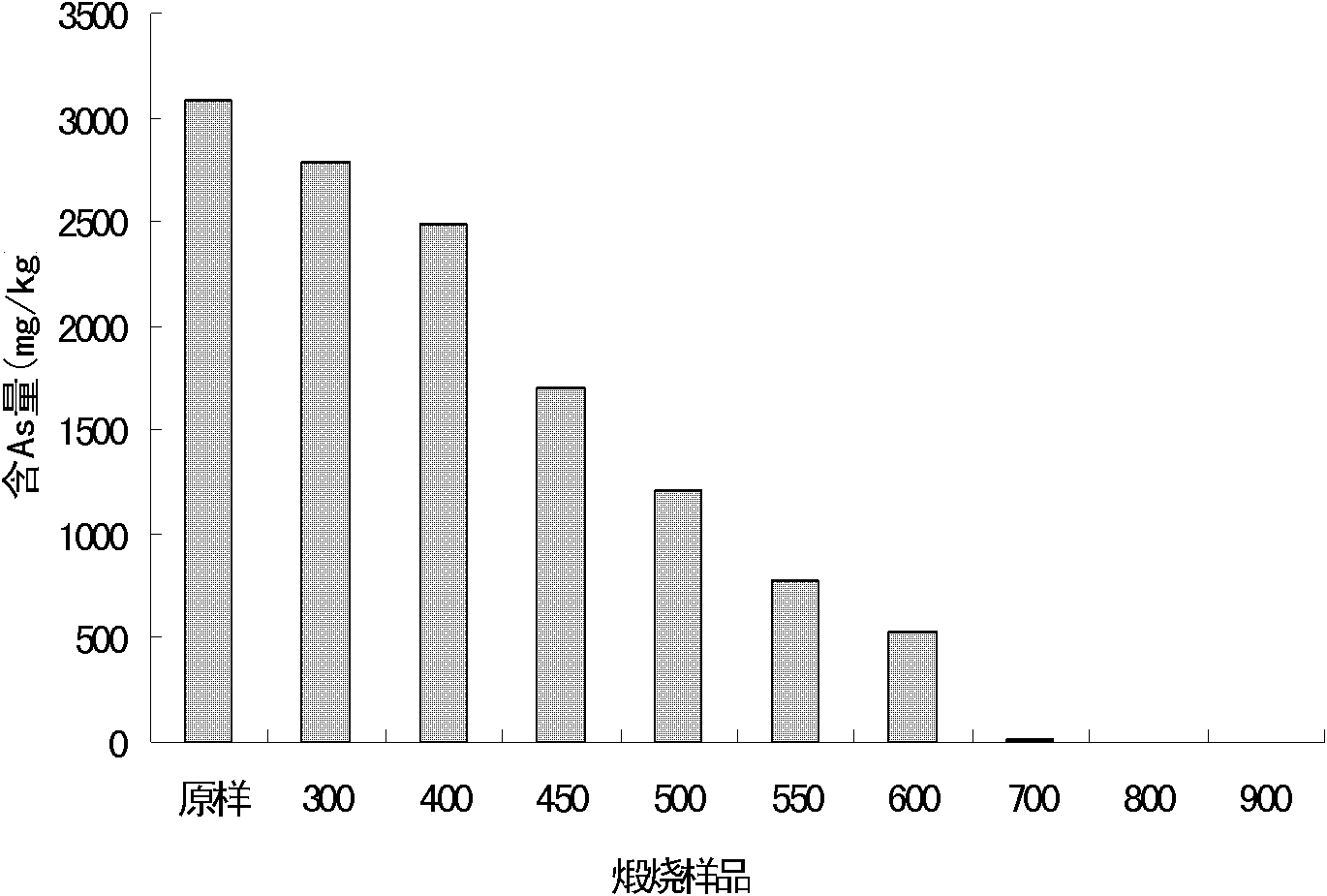
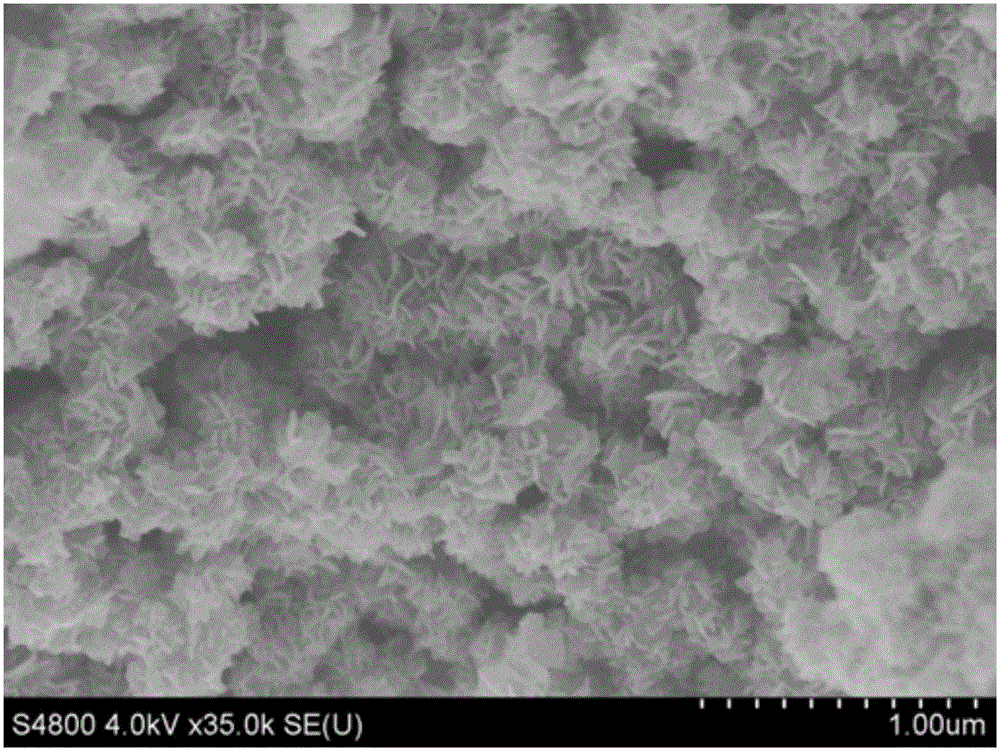
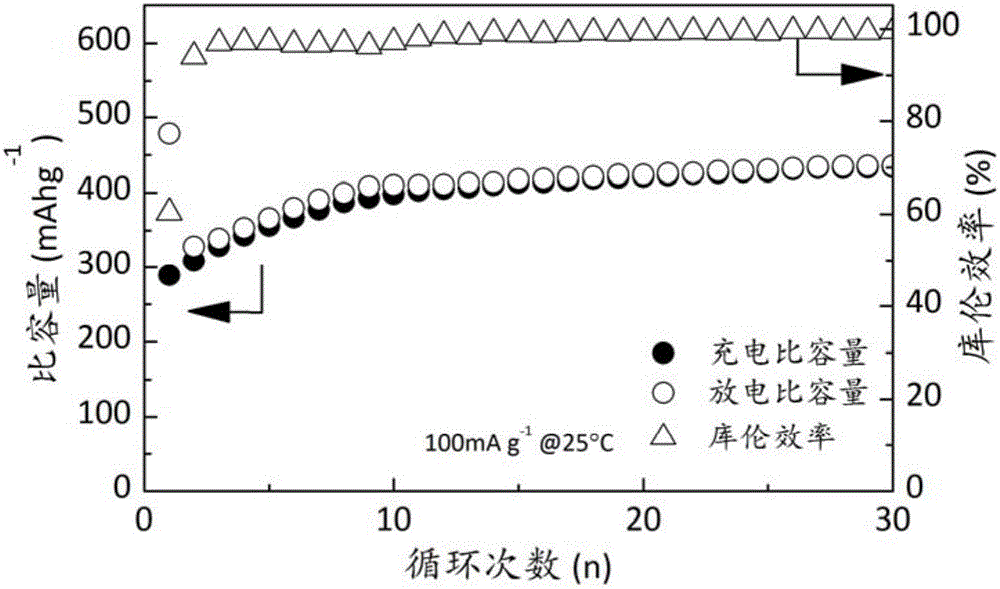
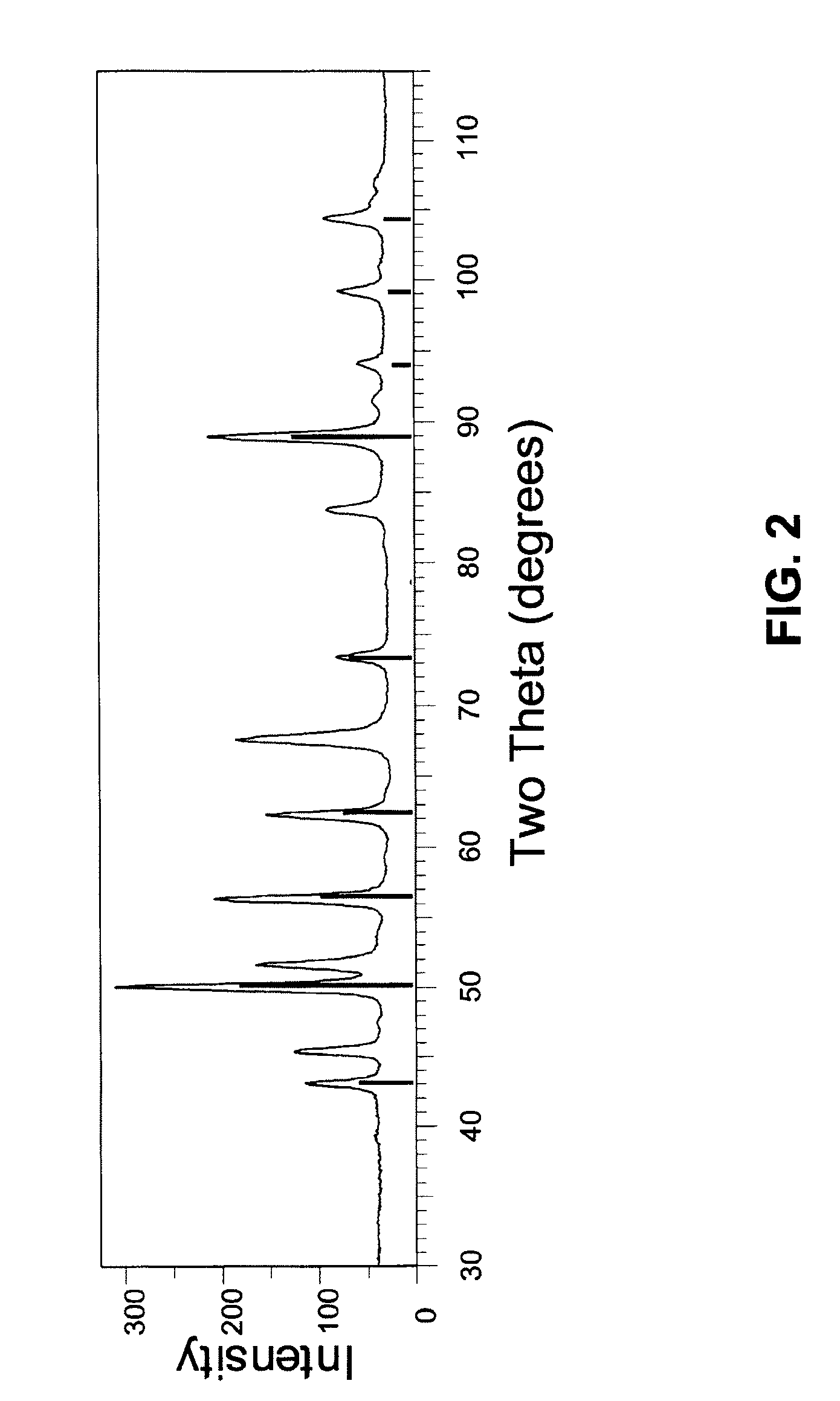
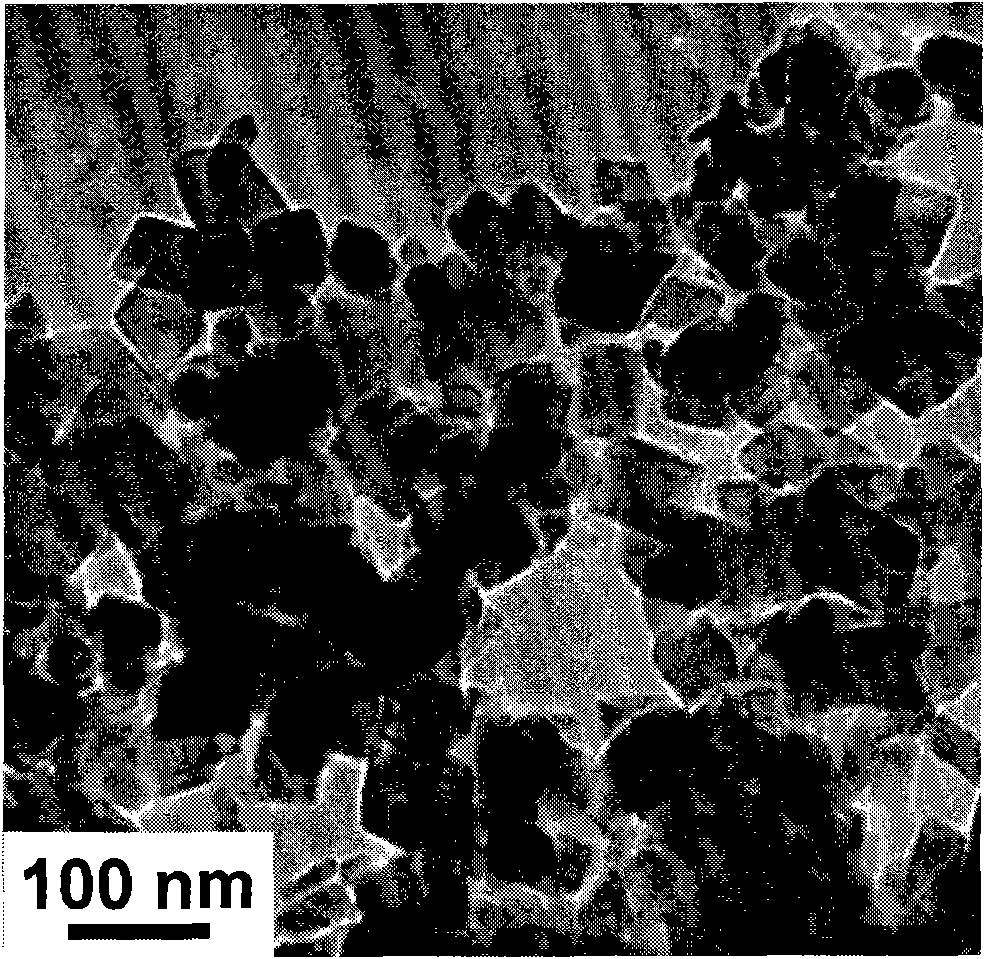
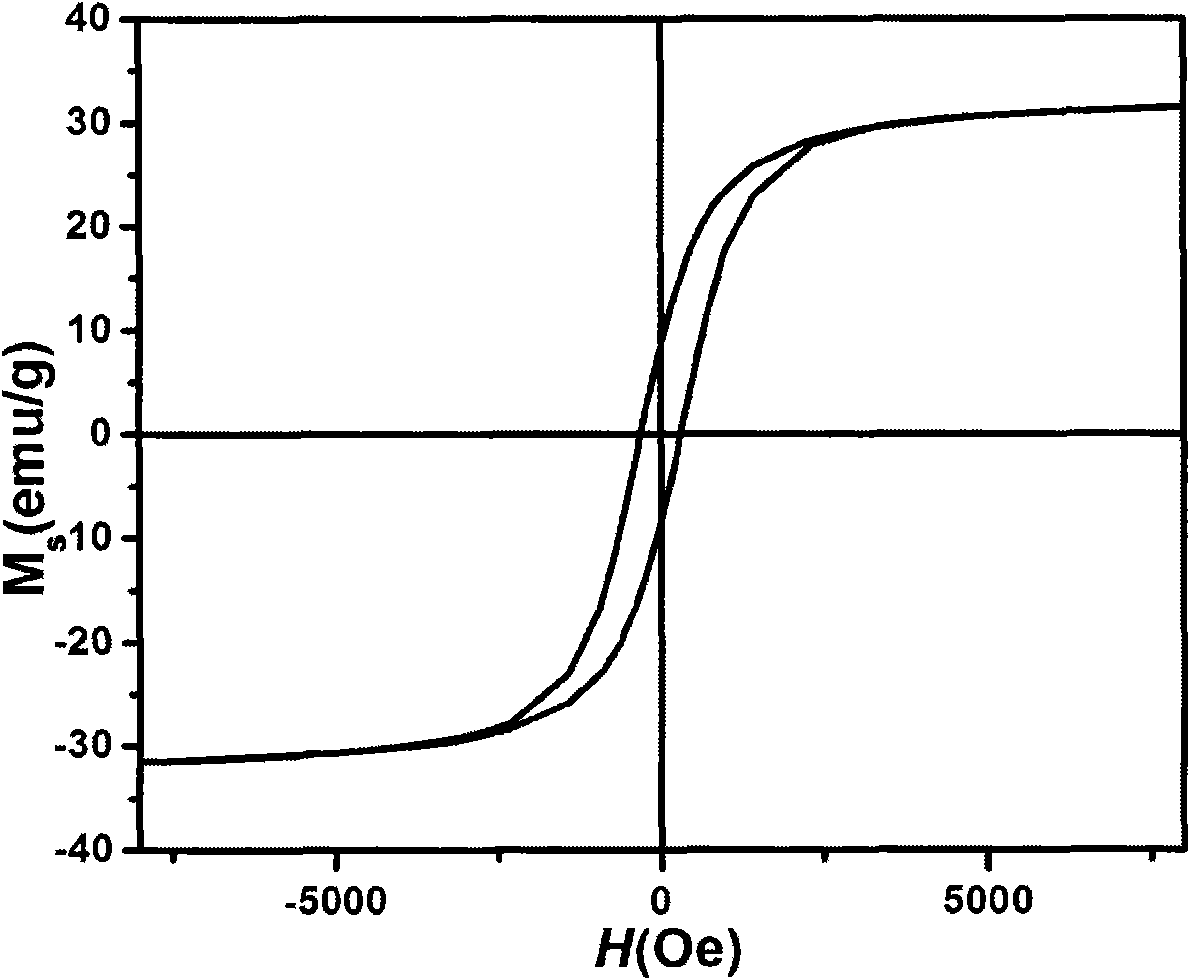
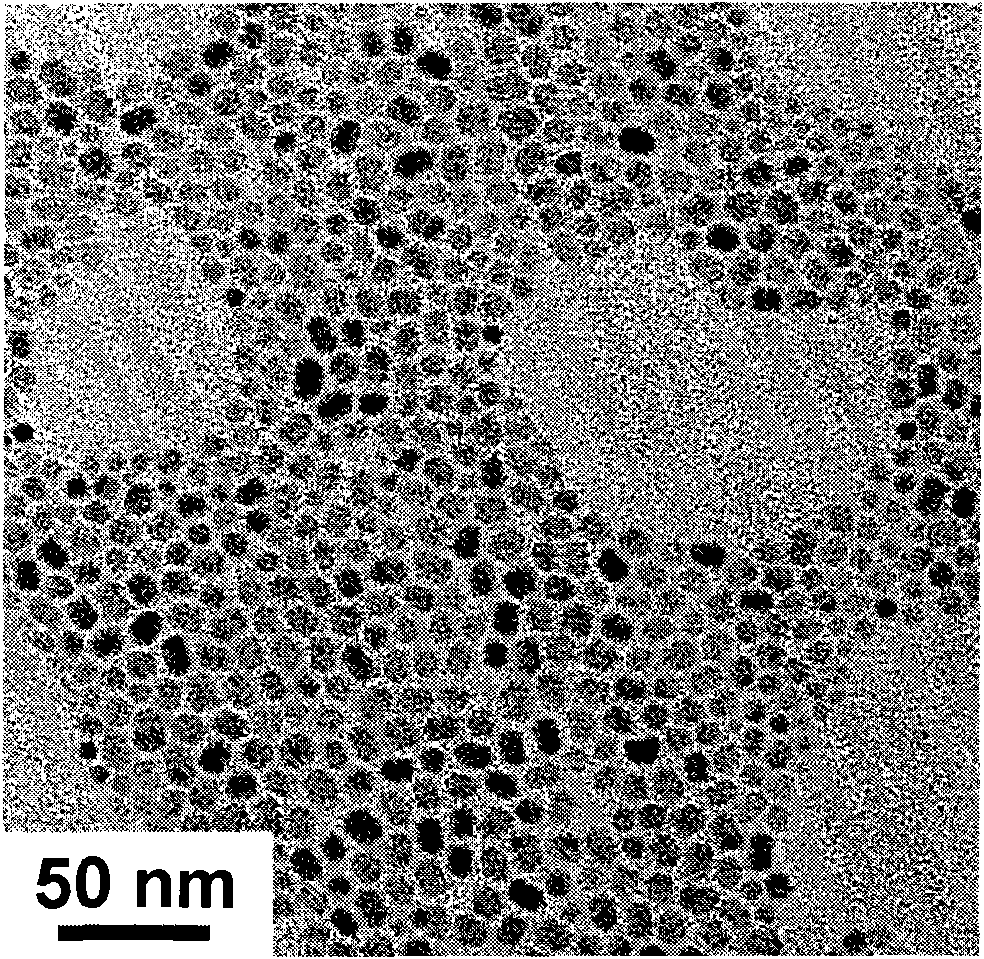
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
333results about "Iron sulfides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
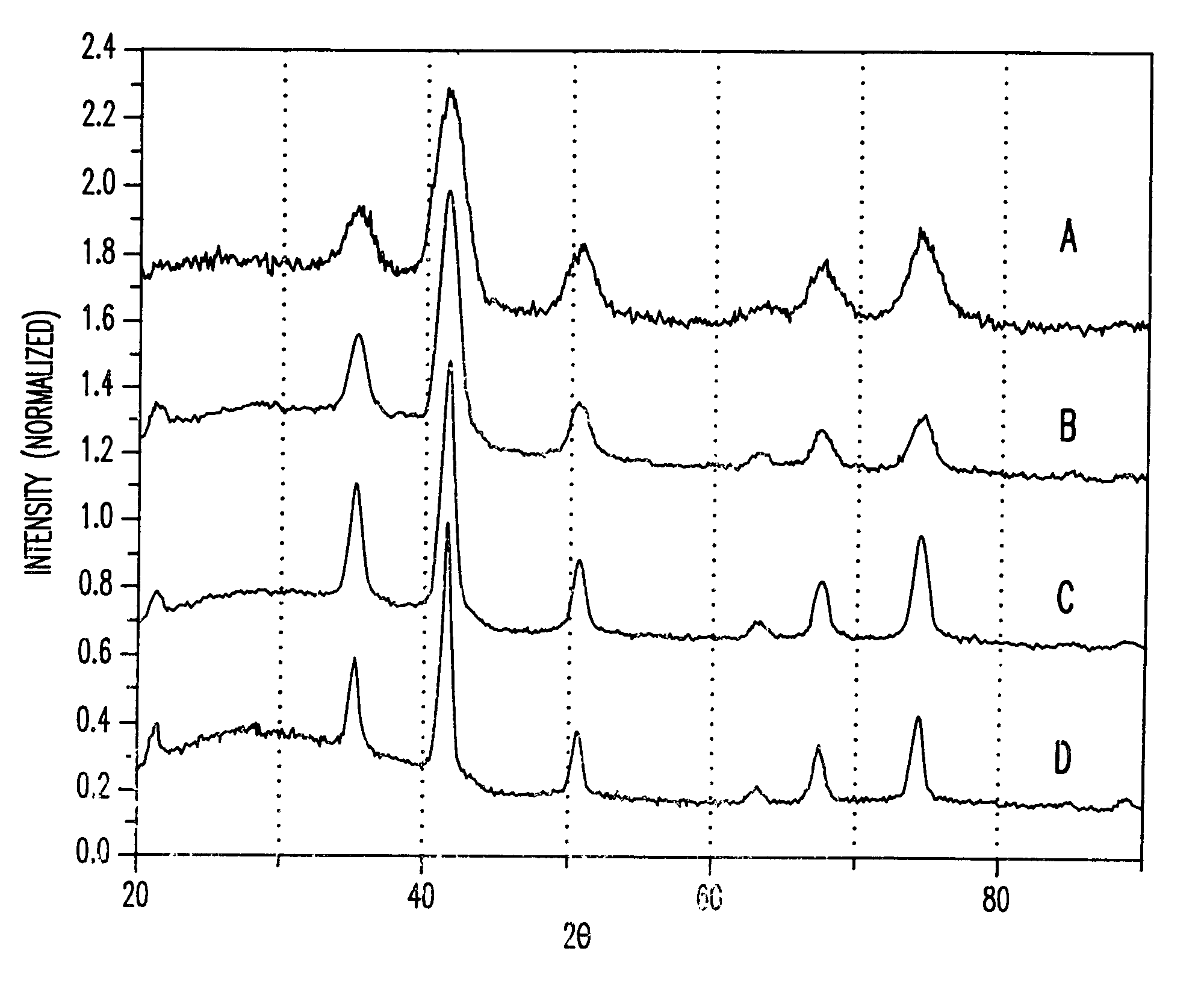
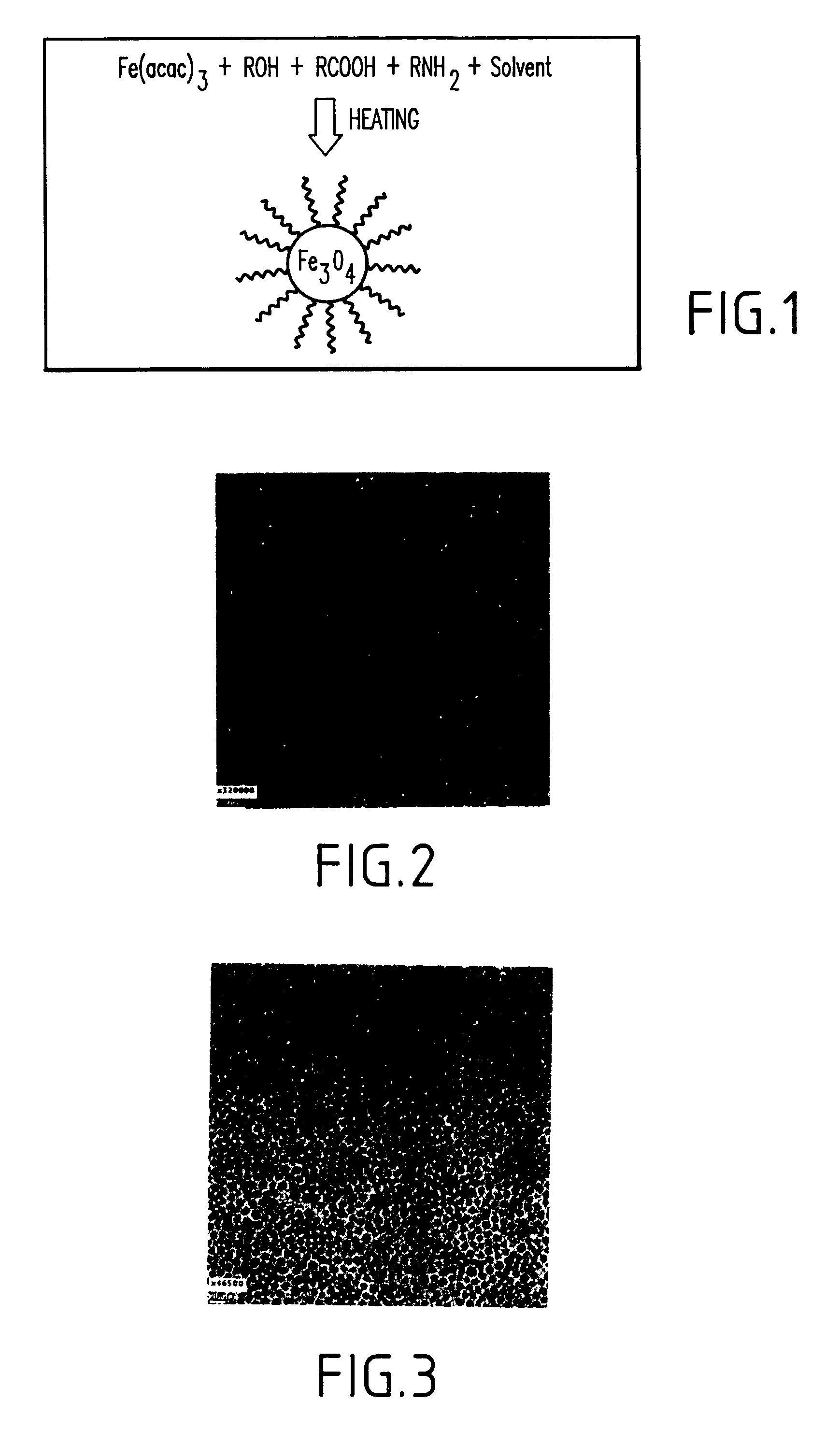
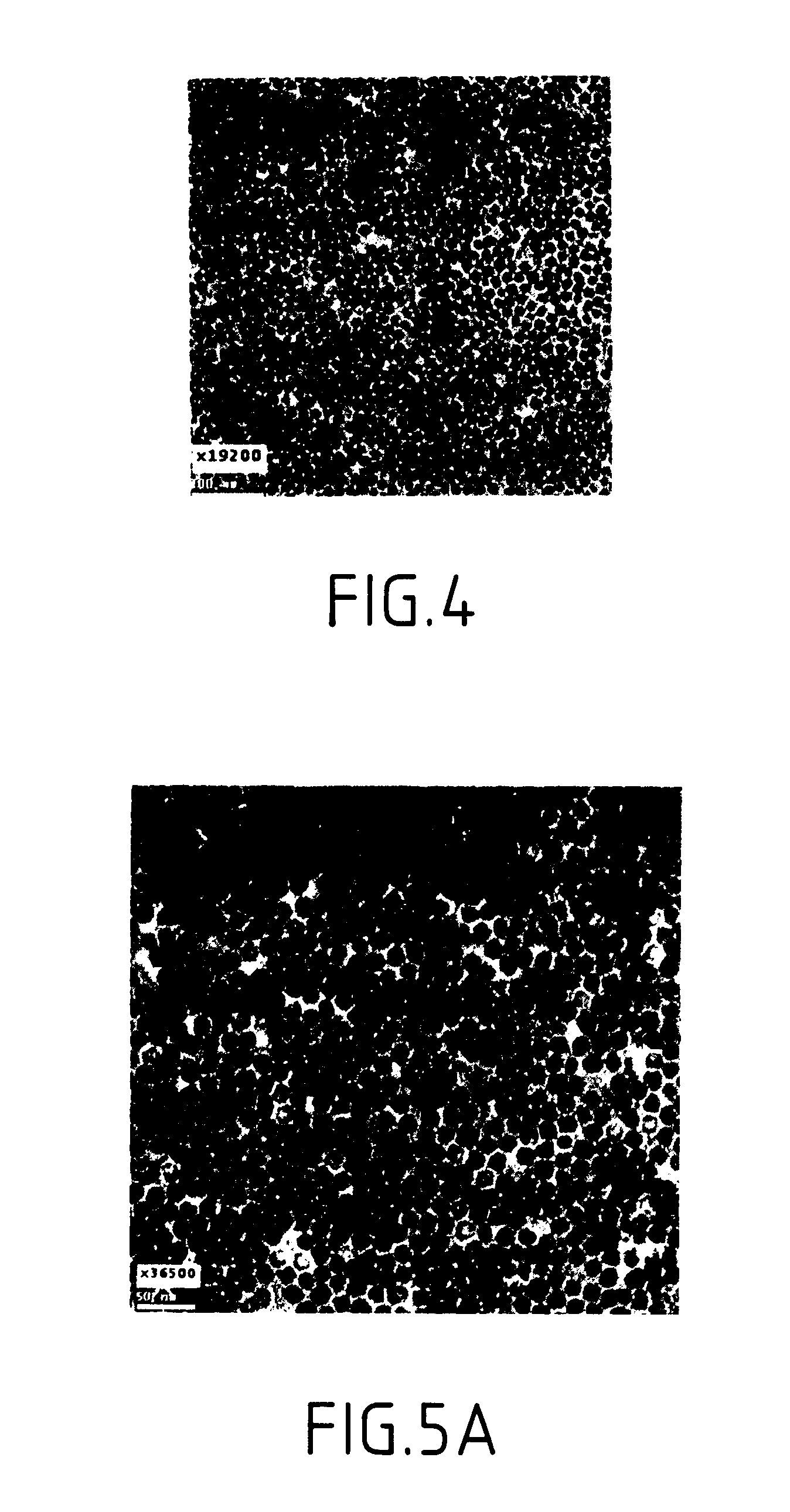
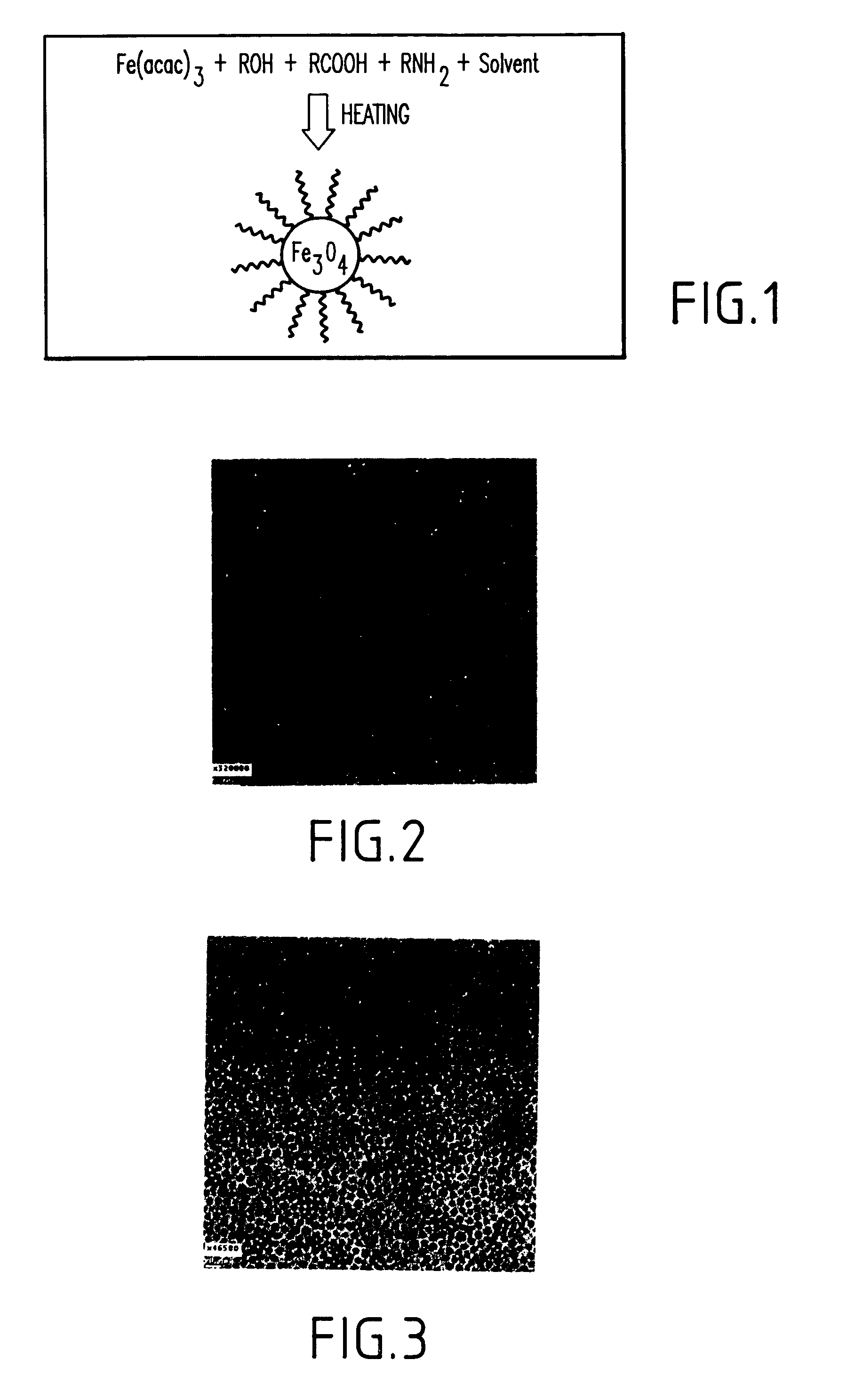
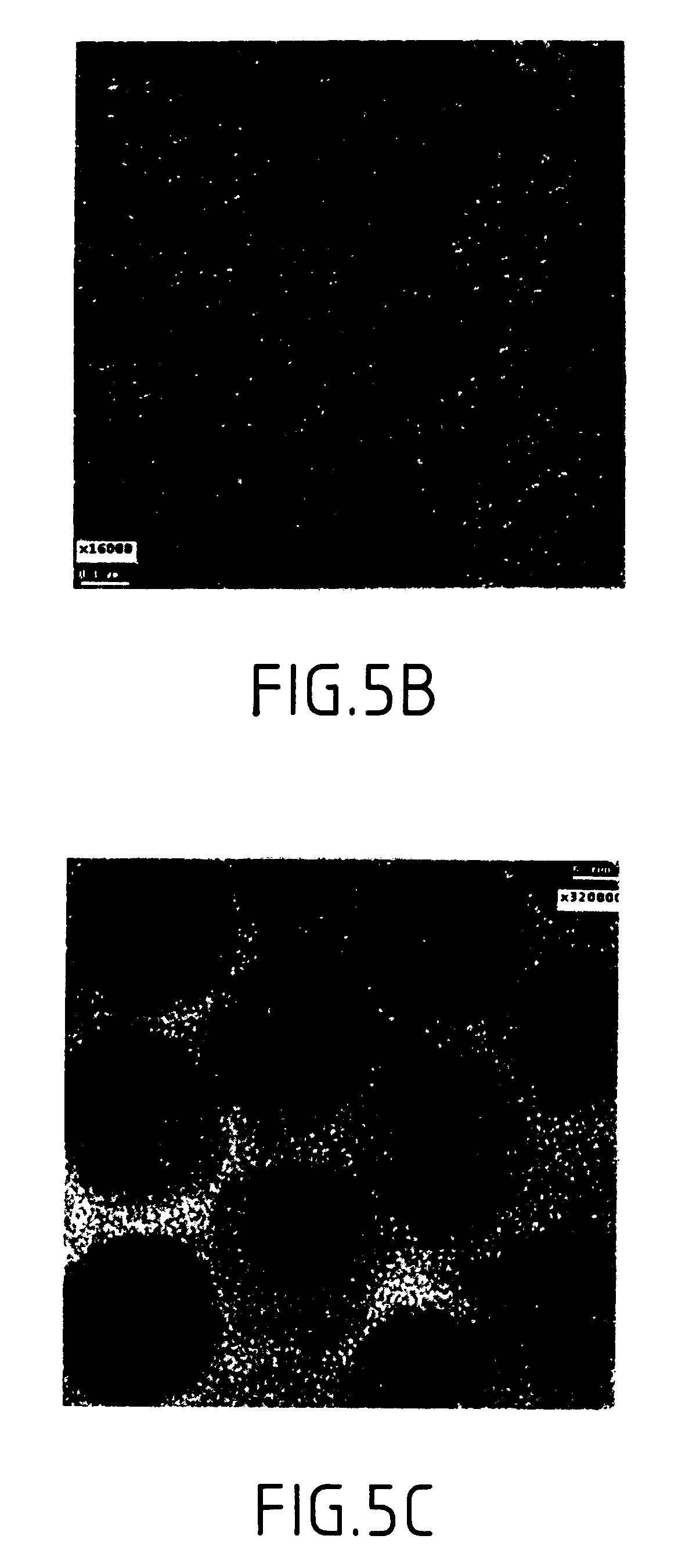
Synthesis of magnetite nanoparticles and the process of forming Fe-based nanomaterials
InactiveUS6962685B2Small sizeNarrow size distributionMaterial nanotechnologyNanomagnetismIron saltsMagnetite Nanoparticles
A method and structure for making magnetite nanoparticle materials by mixing iron salt with alcohol, carboxylic acid and amine in an organic solvent and heating the mixture to 200–360 C is described. The size of the particles can be controlled either by changing the iron salt to acid / amine ratio or by coating small nanoparticles with more iron oxide. Magnetite nanoparticles in the size ranging from 2 nm to 20 nm with a narrow size distribution are obtained with the invention. The invention can be readily extended to other iron oxide based nanoparticle materials, including M Fe2O4 (M=Co, Ni, Cu, Zn, Cr, Ti, Ba, Mg) nanomaterials, and iron oxide coated nanoparticle materials. The invention also leads to the synthesis of iron sulfide based nanoparticle materials by replacing alcohol with thiol in the reaction mixture. The magnetite nanoparticles can be oxidized to γ-Fe2O3, or α-Fe2O3, or can be reduced to bcc-Fe nanoparticles, while iron oxide based materials can be used to make binary iron based metallic nanoparticles, such as CoFe, NiFe, and FeCoSmx nanoparticles.
Owner:INT BUSINESS MASCH CORP
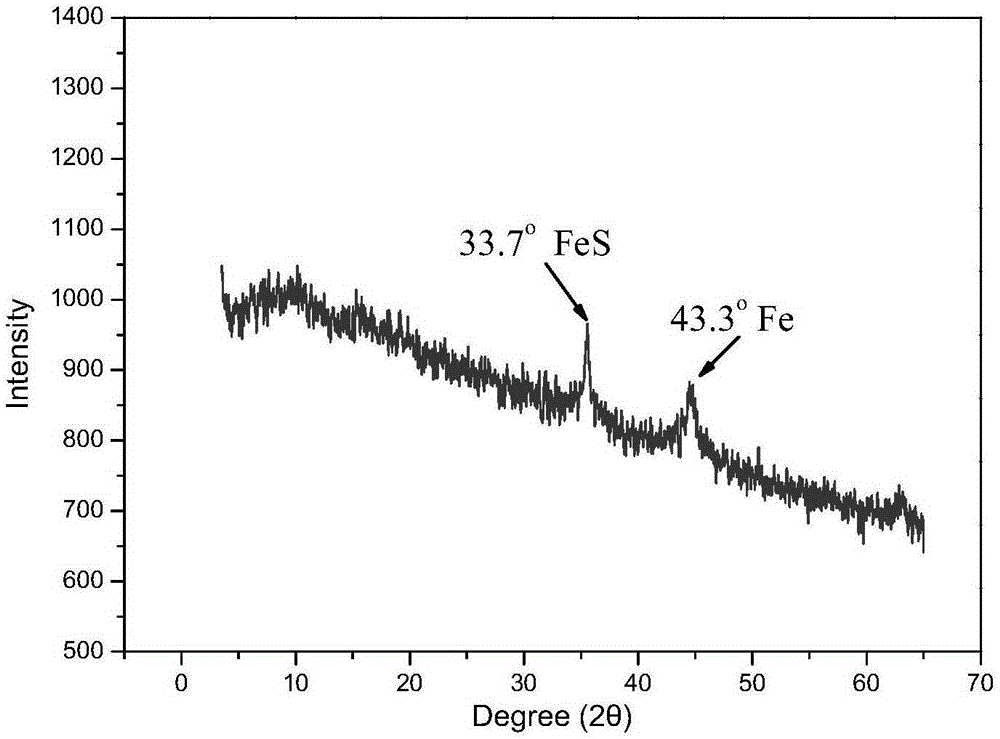
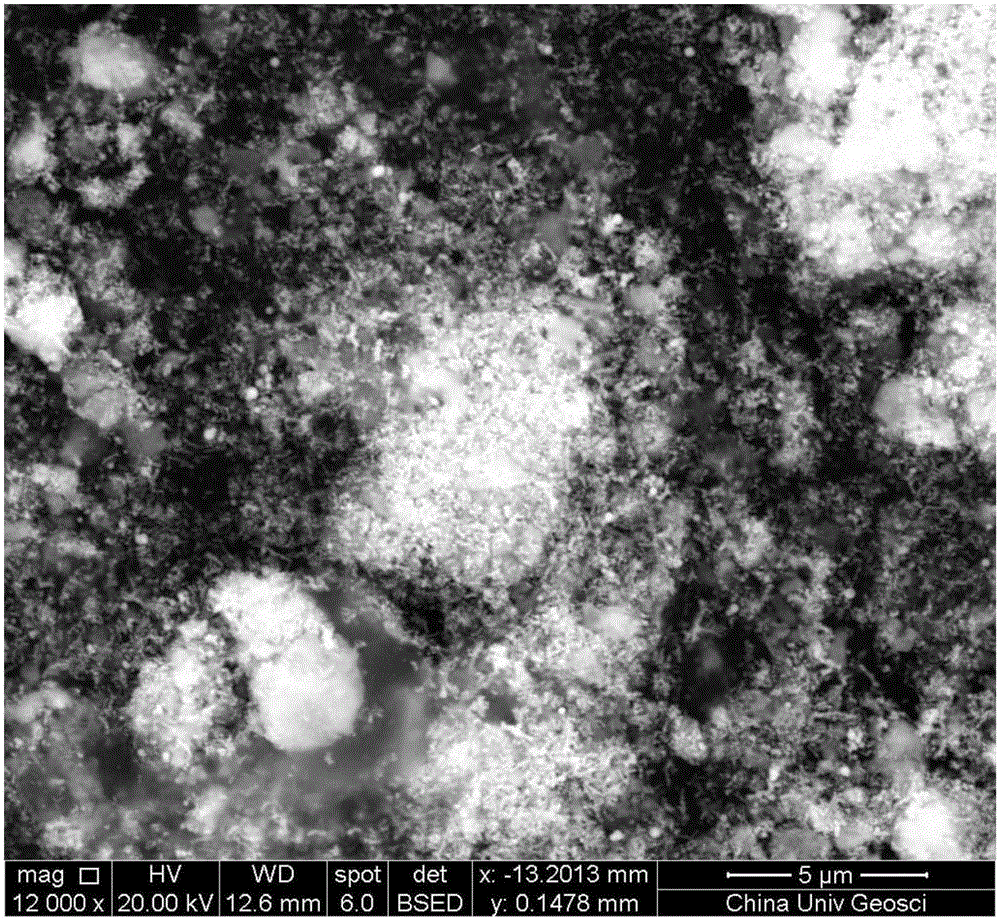
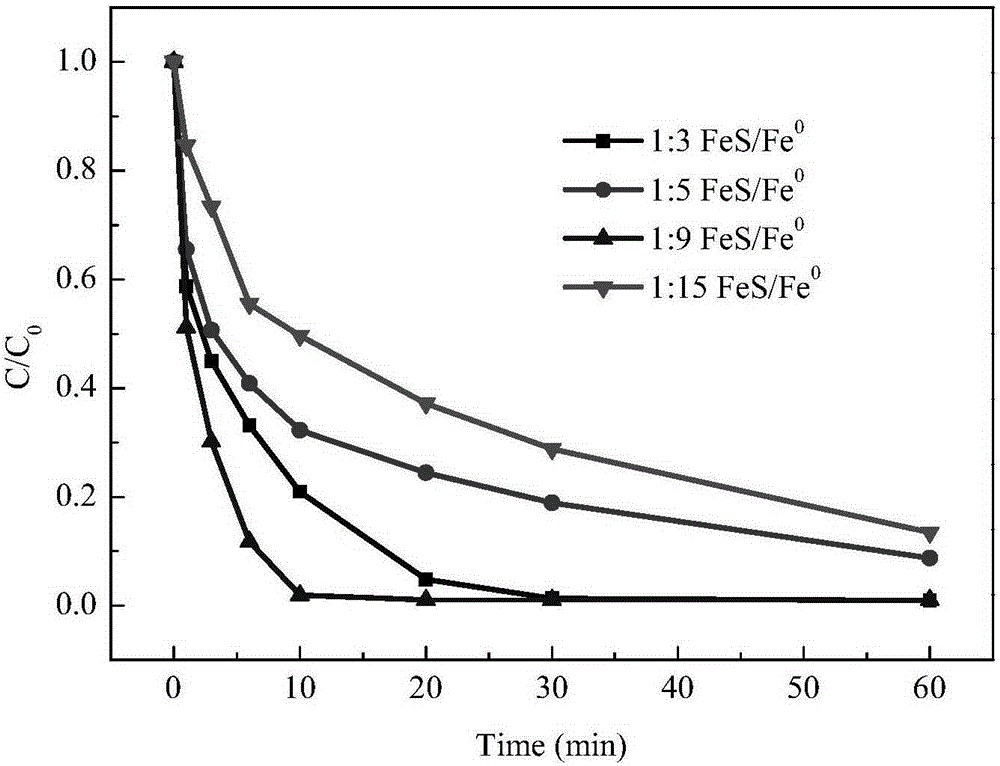
FeS and Fe0 composite and preparation method and application thereof
InactiveCN105174414AEfficient degradationHigh reactivityIron sulfidesWater/sewage treatment by reductionWastewaterSurface water
The invention belongs to the field of chemical materials, and particularly relates to a FeS and Fe0 composite and a preparation method and application thereof. The FeS and Fe0 composite is formed by compositing nano FeS and nano Fe0, the surface of the nano Fe0 is coated with the nano FeS, and the molar ratio of the nano FeS to the nano Fe0 is 2:1 to 15:1. According to the FeS and Fe0 composite and the preparation method and application thereof, the preparation process is simple, convenient, environmentally friendly and feasible, the prepared FeS and Fe0 composite is high in reaction activity, capable of efficiently and rapidly reducing and adsorbing heavy metal ions in waste water within a wider temperature range, a pH value range and a dissolved oxygen content range and further capable of rapidly and efficiently activating hydrogen peroxide and persulfate to make a system generate hydroxyl free radicals and sulfate free radicals to degrade and mineralize organic pollutants, and therefore the FeS and Fe0 composite can be widely applied to degradation of the organic pollutants in surface water and underground water.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Synthesis of magnetite nanoparticles and the process of forming fe-based nanomaterials
InactiveUS20050191231A1Small sizeNarrow size distributionMaterial nanotechnologyNanostructure manufactureIron saltsMagnetite Nanoparticles
A method and structure for making magnetite nanoparticle materials by mixing iron salt with alcohol, carboxylic acid and amine in an organic solvent and heating the mixture to 200-360 C. is described. The size of the particles can be controlled either by changing the iron salt to acid / amine ratio or by coating small nanoparticles with more iron oxide. Magnetite nanoparticles in the size ranging from 2 nm to 20 nm with a narrow size distribution are obtained with the invention. The invention can be readily extended to other iron oxide based nanoparticle materials, including MFe2O4 (M=Co, Ni, Cu, Zn, Cr, Ti, Ba, Mg) nanomaterials, and iron oxide coated nanoparticle materials. The invention also leads to the synthesis of iron sulfide based nanoparticle materials by replacing alcohol with thiol in the reaction mixture. The magnetite nanoparticles can be oxidized to γ-Fe2O3, or α-Fe2O3, or can be reduced to bcc-Fe nanoparticles, while iron oxide based materials can be used to make binary iron based metallic nanoparticles, such as CoFe, NiFe, and FeCoSmx nanoparticles.
Owner:IBM CORP
Preparation of metal chalcogenides from reactions of metal compounds and chalcogen
A method of preparing metal chalcogenides from elemental metal or metal compounds has the following steps: providing at least one elemental metal or metal compound; providing at least one element from periodic table groups 13-15; providing at least one chalcogen; and combining and heating the chalcogen, the group 13-15 element and the metal at sufficient time and temperature to form a metal chalcogenide. A method of functionalizing the surface of semiconducting nanoparticles has the following steps: providing at least one metad compound; providing one chalcogenide having a cation selected from the group 13-15 (B, Al, Ga, In, Si, Ge, Sn, Pb, P, As, Sb and Bi); dissolving the chalcogenide in a first solution; dissolving the metal compound in a second solution; providing and dissolving a functional capping agent in at least one of the solutions of the metal compounds and chalcogenide; combining all solutions; and maintaining the combined solution at a proper temperature for an appropriate time.
Owner:ARIZONA STATE UNIVERSITY
Preparation of metal chalcogenides from reactions of metal compounds and chalcogen
InactiveUS20060239882A1Rare earth metal sulfidesSelenium/tellurium compundsSufficient timeNanoparticle
A method of preparing metal chalcogenides from elemental metal or metal compounds has the following steps: providing at least one elemental metal or metal compound; providing at least one element from periodic table groups 13-15; providing at least one chalcogen; and combining and heating the chalcogen, the group 13-15 element and the metal at sufficient time and temperature to form a metal chalcogenide. A method of functionalizing the surface of semiconducting nanoparticles has the following steps: providing at least one metad compound; providing one chalcogenide having a cation selected from the group 13-15 (B, Al, Ga, In, Si, Ge, Sn, Pb, P, As, Sb and Bi); dissolving the chalcogenide in a first solution; dissolving the metal compound in a second solution; providing and dissolving a functional capping agent in at least one of the solutions of the metal compounds and chalcogenide; combining all solutions; and maintaining the combined solution at a proper temperature for an appropriate time.
Owner:ARIZONA STATE UNIVERSITY
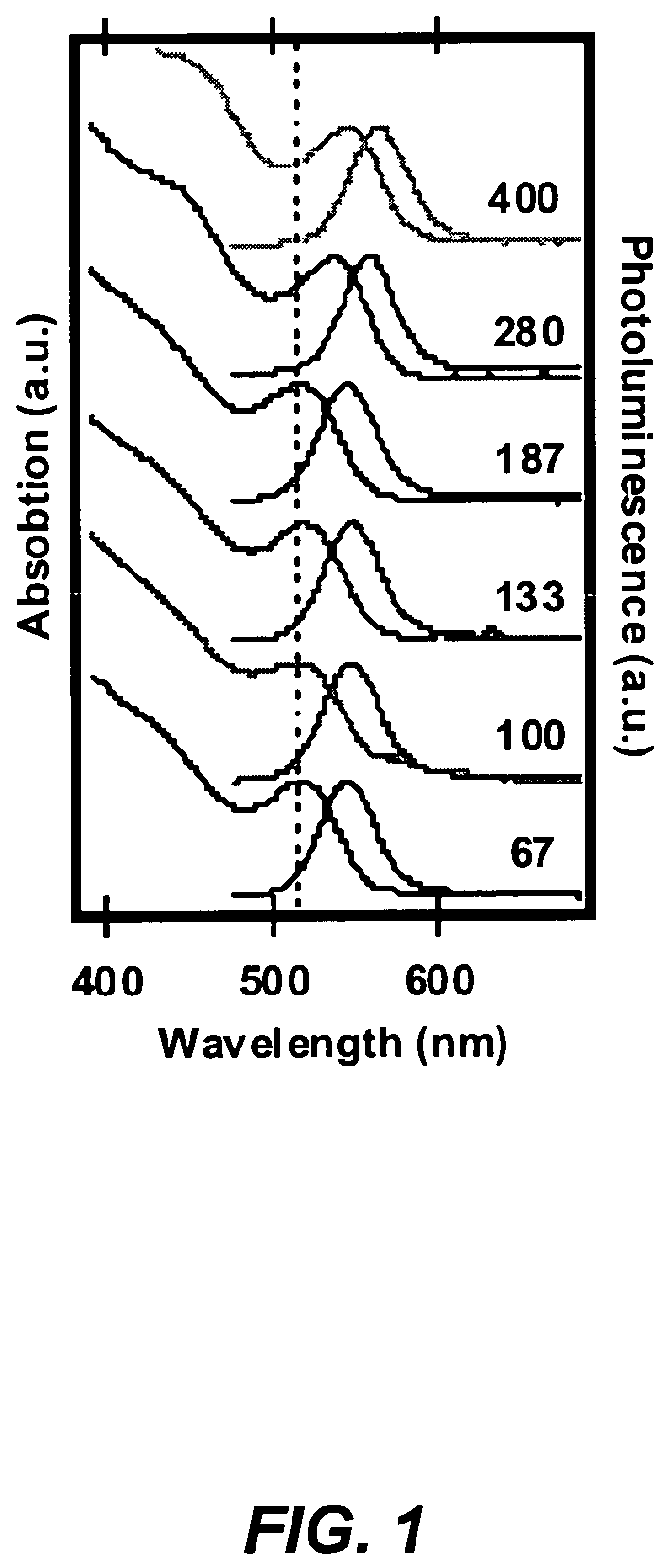
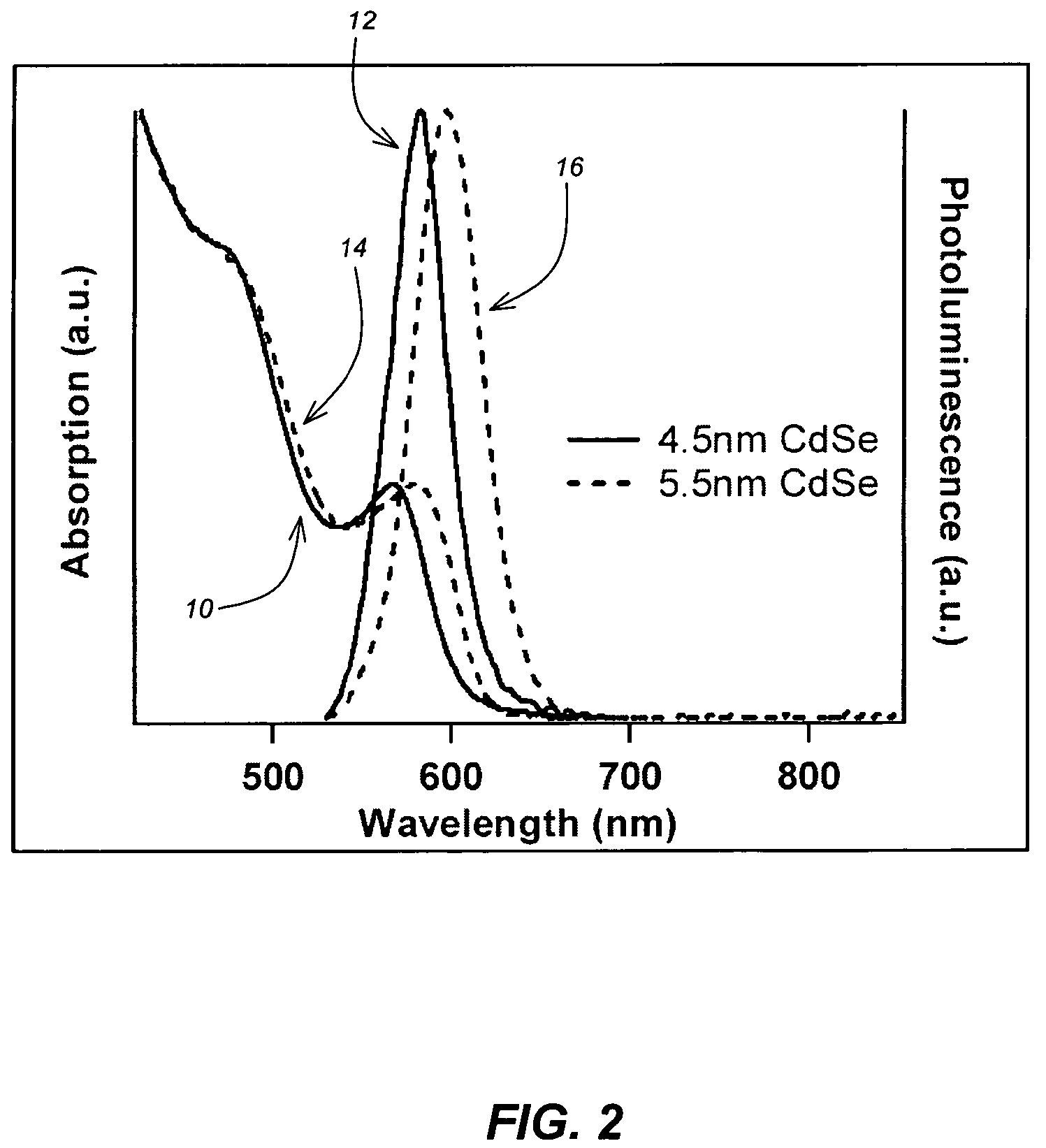
Method for synthesis of colloidal nanoparticles
InactiveUS20060060998A1Large-scale, safe, convenient, reproducible, and energy-efficient productionHigh crystallinityDielectric heatingNanoinformaticsColloidal nanoparticlesContinuous flow
A method for synthesis of high quality colloidal nanoparticles using comprises a high heating rate process. Irradiation of single mode, high power, microwave is a particularly well suited technique to realize high quality semiconductor nanoparticles. The use of microwave radiation effectively automates the synthesis, and more importantly, permits the use of a continuous flow microwave reactor for commercial preparation of the high quality colloidal nanoparticles.
Owner:RGT UNIV OF CALIFORNIA
Sequestration of a gas emitted by an industrial plant
InactiveUS20110182799A1Eliminate needCalcium/strontium/barium carbonatesAmmonium nitratesNitrogenNitrogen gas
A method of sequestering a multi-element gas emitted by an industrial plant is described herein, the method comprising: contacting a solution, including a first reactant comprising a multi-element gas emitted by an industrial plant and at least one gas absorber comprising nitrogen, for example ammonia or an amine, with a solid, including a second reactant, under conditions that promote a reaction between the first reactant and the second reactant to provide a first product, which incorporates one or more elements of the multi-element gas, thereby sequestering the multi-element gas.
Owner:RUTGERS THE STATE UNIV
In Situ Immobilization of Metals in Contaminated Sites Using Stabilized Nanoparticles
InactiveUS20070203388A1Low costProcess environmental protectionMaterial nanotechnologyWater treatment compoundsCelluloseNanoparticle
A method for preparing a class of highly stabilized and soil-dispersible nanoparticles and using the nanoparticles as a remediation technology for immobilizing toxic metals at toxic metal contaminated sites. The method employs a composition containing select polysaccharides (starch or cellulose) as a stabilizer for the nanoparticles in a liquid carrier, and results in suspensions of nanoparticles of desired size and mobility in water, soils or sediments. The stabilizer can facilitate controlling the dispersibility of the nanoparticles in the liquid carrier. An effective amount of the composition is delivered to a contaminated site so that the nanoparticles can immobilize one or more toxic metals of the contaminated site.
Owner:AUBURN UNIV
Method To Synthesize Colloidal Iron Pyrite (FeS2) Nanocrystals And Fabricate Iron Pyrite Thin Film Solar Cells
InactiveUS20110240108A1Excellent manufacturing scalabilityLow costMaterial nanotechnologySulfur compoundsHeterojunctionMetal foil
Systems and methods are provided for the fabrication and manufacture of efficient, low-cost p-n heterojunction pyrite solar cells. The p-n heterojunction pyrite solar cells can include a pyrite thin cell component, a window layer component, and a top surface contact component. The pyrite thin cell component can be fabricated from nanocrystal paint deposited onto metal foils or microcrystalline pyrite deposited onto foil by chemical vapor deposition. A method of synthesizing colloidal pyrite nanocrystals is provided. Methods of manufacturing the efficient, low-cost p-n heterojunction pyrite solar cells are also provided.
Owner:RGT UNIV OF CALIFORNIA
Pollutant emission control sorbents and methods of manufacture
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing fly ash particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the fly ash particles.
Owner:BASF CATALYSTS LLC
Method for synthesis of colloidal nanoparticles
InactiveUS7575699B2Large-scale, safe, convenient, reproducible, and energy-efficient productionHigh crystallinityDielectric heatingNanoinformaticsColloidal nanoparticlesContinuous flow
A method for synthesis of high quality colloidal nanoparticles using comprises a high heating rate process. Irradiation of single mode, high power, microwave is a particularly well suited technique to realize high quality semiconductor nanoparticles. The use of microwave radiation effectively automates the synthesis, and more importantly, permits the use of a continuous flow microwave reactor for commercial preparation of the high quality colloidal nanoparticles.
Owner:RGT UNIV OF CALIFORNIA
FeS nano-material and preparation method thereof
InactiveCN103950989AMild reaction conditionsHigh yieldMaterial nanotechnologyIron sulfidesCapacitanceSupercapacitor
The invention discloses a FeS nano-material and a preparation method thereof. The FeS nano-material has a sheet structure, wherein the thickness of a sheet is 20-100 nanometers, and the length of the sheet is 0.2-5 microns. The preparation method comprises the steps of (1) dissolving potassium ferrocyanide and sodium sulfide into water, then adding a surfactant, and uniformly mixing; (2) reacting for 3-50 hours under hydrothermal conditions at 140-220 DEG C, and naturally cooling to room temperature; (3) centrifuging, washing and drying an obtained product to obtain a sheet FeS capacitor electrode material. The FeS nano-material disclosed by the invention is simple and efficient, and is suitable for large-scale production; a prepared super-capacitor material has a relatively large specific surface area, relatively high specific capacitance and potential application values.
Owner:ANHUI NORMAL UNIV
Synthesis of lithium iron sulphides and their use as cathodes
A process for the production of a lithium transition metal sulphide such as lithium iron sulphide, the process comprising reacting a transition metal sulphide with lithium sulphide in a solvent comprising a molten salt or a mixture of molten salts. Lithium transition metal sulphides obtained using this process are useful in the production of electrodes, in particular for rechargeable lithium batteries.
Owner:QINETIQ LTD
Preparation method of graphene oxide/iron disulfide composite nano particles
InactiveCN102910615AUniform particle sizeGood dispersionMaterial nanotechnologyCarbon compoundsHydration reactionPolyvinyl alcohol
The invention relates to nano FeS2 materials, and particularly relates to a preparation method of graphene oxide / iron disulfide composite nano particles. According to the preparation method of the graphene oxide / iron disulfide composite nano particles, ferrous chloride tetrahydrate (FeCl2.4H2O) and sulfur powder are used as raw materials, are dissolved in water together with surfactant polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP), and then mixed with graphene oxide under a certain pH (potential of hydrogen) condition; the obtained mixed solution is arranged in a reaction kettle and sealed, is held for 12 hours to 24 hours in a constant-temperature box at 180 DEG C to 200 DEG C by using a hydrothermal method, is cooled to normal temperature, stood and separated, centrifugally washed and dried, finally the graphene oxide / iron disulfide composite nano particles can be obtained.
Owner:JIANGSU UNIV
Process for making nano-sized and micro-sized precipitate particles
Owner:XIAMEN NANOTECH
Cathode active substance for lithium ion battery, cathode material containing cathode active substance and lithium ion battery
InactiveCN102701160AHigher quality than capacityImprove conductivityTin compoundsCell electrodesAluminium-ion batterySodium-ion battery
The invention discloses a cathode ternary compound CuxMySz active substance for a lithium ion battery, a cathode material containing the substance and the lithium ion battery using the cathode material as a cathode. M in the chemical formula CuxMySz belongs to an IVB group, a VB group, a VIB group, a VIII group, an IIB group and an IVA group in a fourth period and a fifth period in periodic table of chemical element and is any one in metal elements of an IIIA group in third, fourth, fifth and sixth periods, such as Ti, Cr, Mo, Fe, Al, Ga, In, Tl, Sn and the like. The active substance has higher charge-discharge capacity which is about two times that of commonly used graphite material in the charge-discharge capacity, thereby being a novel lithium ion battery cathode material with good application prospect and high capacity.
Owner:EAST CHINA UNIV OF SCI & TECH
Production of positive active material of lithium battery
The method includes selecting pyrite: purity is 90-99.44%; sulfur content is 48.1-53.15%; iron content is 42.09-46.50. Impurity content: Sio <0.1-1%; Mgo is less than or equal to 0.1-1%; Al2O3<0.15-1%; Cao is less than or equal to 0.1-1%; acid soluble iron 0.38-1%. The selected pyrite is through breaking, screen sizing (75% passes through 200 mesh screen, 25% passes through 325 mesh screen). Then it will be heat treated at certain condition. After heat treatment it will be cooled until it is below 80deg.C.
Owner:HUIZHOU CITY DESAY LITHIUM BATTERY S & T CO LTD
Method for removing toxic substances in water
Arsenic and TOC are removed from drinking water or wastewaters by use of finely-divided metallic iron in the presence of powered elemental sulfur or other sulfur compounds such as manganese sulfide, followed by an oxidation step. A premix may be produced for this process, by adding the iron, sulfur and oxidizing agent to water in a predetermined pH range. The iron and sulfur are mixed for a period of time dependent upon the temperature and pH of the water and the presence of complexing or sequestering minerals and organic acids in the water. An oxidizing agent is added to the mixture and agitating is continued. In a preferred embodiment the oxidizing agent is hydrogen peroxide. Water is decanted from the mixture after a sufficient reaction time, to produce a concentrated premix. This premix can be added to water intended for drinking or to industrial effluents containing toxic materials. Use of various gradations and mixtures of this sulfur-modified iron (SMI) premix have been successfully demonstrated to remove the following toxic substances from water: arsenic (arsenite and arsenate); disinfection byproducts and precursors; copper; chrome VI; sulfate; and chlorinated solvents including trichloroethene. Metals removed may be present in the untreated water in either the dissolved state or as a fine particulate. SMI has been fabricated using sulfur in the amount of up to 50% of the weight of the iron. SMI premix has been manufactured using a wetted but non-fluid mix at room temperature and at elevated temperature. SMI has been successfully demonstrated in pressure and gravity contact beds in both upflow and downflow modes. It has been prepared in uniformly-graded media similar in size and gradation to commercially-available filter media. Spent SMI can be recycled as a non-hazardous material as feed material to a steel production facility.
Owner:SANTINA PETER F
Essentially insoluble heavy metal sulfide slurry for wastewater treatment
A product and method for the removal of pollutant heavy metals from aqueous solutions which precludes the end user from storing, handling, feeding and controlling hazardous soluble sulfide materials. The product is a slurry which includes a mixture of a liquid medium and an essentially insoluble salt wherein the salt is the reaction product of heavy metal ions, preferably selected from Mn++ ions, Fe++ ions, and Fe+++ ions, and sulfide ions derived from soluble sulfide sources such as sodium sulfide, hydrogen sulfide, and sodium hydrosulfide. Addition of the subject slurry to a wastewater stream will effect the precipitation of heavy metals with lesser equilibrium sulfide ion concentrations than that of the essentially insoluble salt. Solids collected by this method may be returned to subsequent wastewater streams for additional removal of heavy metals by any excess heavy metal sulfide salt.
Owner:SOUTHERN WATER TREATMENT
Ultra-small metal chalcogenide compound nano crystal and biological synthesis method and application thereof
InactiveCN106315663AMild reaction conditionsEasy to operateMaterial nanotechnologyEnergy modified materialsSynthesis methodsFluorescence
The invention discloses an ultra-small metal chalcogenide compound nano crystal and a biological synthesis method and application thereof. In one embodiment, the synthesis method is as follows: a protein with the isoelectric point PI lower than 9.0 and a metal salt are mixed with water evenly to form a mixed solution, the mixed solution is adjusted to alkaline, a reducing agent and a sulfur source are added for reaction to form the ultra-small metal chalcogenide compound nano crystal with the particle size less than 10nm. The method has the characteristics of mild reaction conditions, simple operation, direct use of particles without additional surface modification in biological photothermal therapy, and the like, the prepared ultra-small metal chalcogenide compound nano crystal is uniform in size, good in dispersion and high in stability, and the prepared ultra-small metal chalcogenide compound nano crystal has very good bio-compatibility without any surface modification, also has the photothermal effect, and is a great-application-prospect nano material used for photothermal therapy and biological fluorescence imaging.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
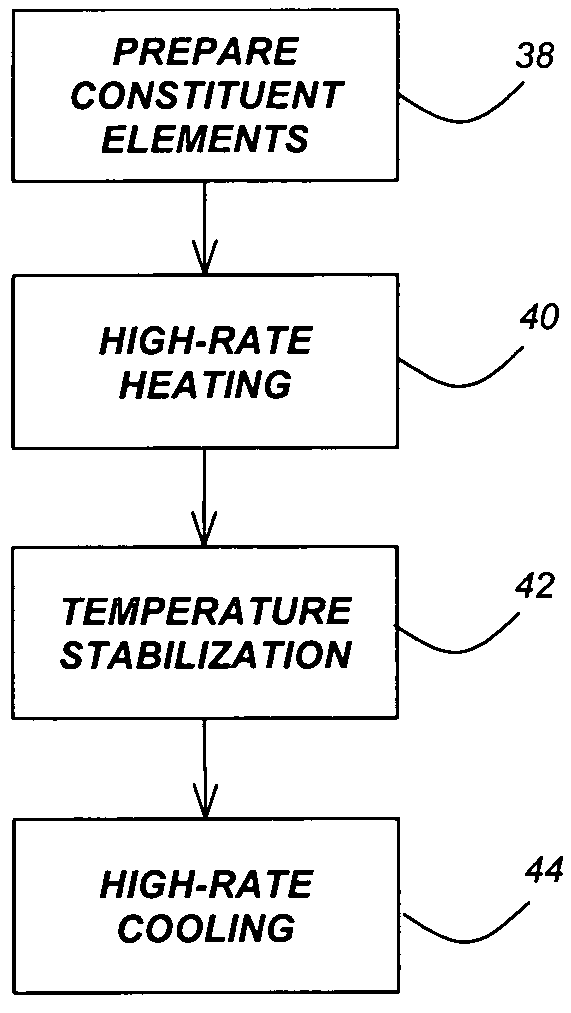
Nanoparticles and systems and methods for synthesizing nanoparticles through thermal shock
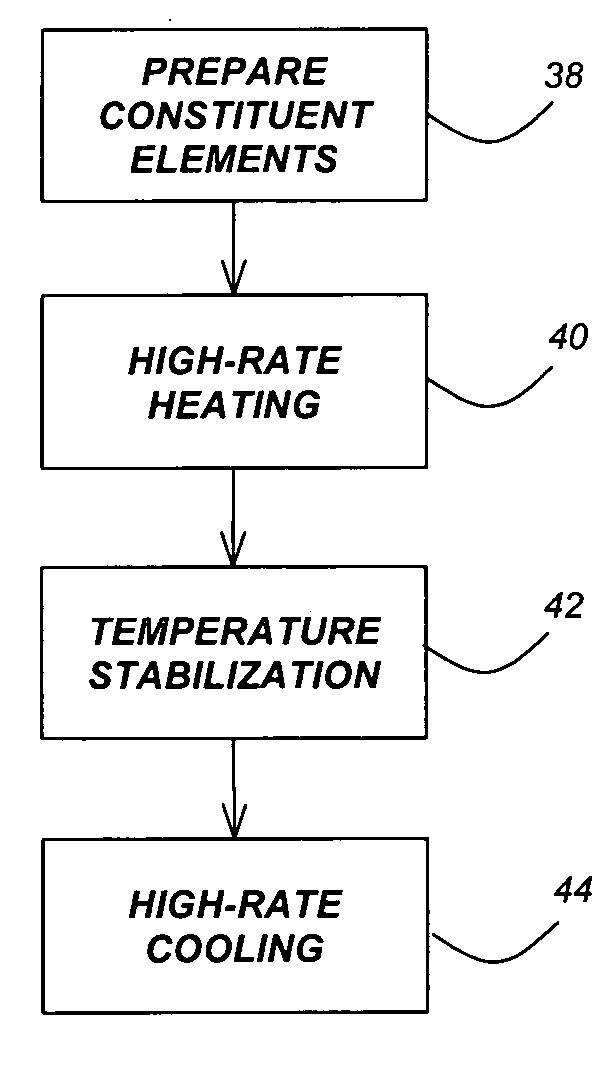
ActiveUS20180369771A1Increase temperatureHeat treatmentsMaterial nanotechnologyThermal energyThermal shock
Systems and methods of synthesizing nanoparticles on substrates using rapid, high temperature thermal shock. A method involves depositing micro-sized particles or salt precursors on a substrate, and applying a rapid, high temperature thermal pulse or shock to the micro-sized particles or the salt precursors and the substrate to cause the micro-sized particles or the salt precursors to become nanoparticles on the substrate. A system may include a rotatable member that receives a roll of a substrate sheet having micro-sized particles or salt precursors; a motor that rotates the rotatable member so as to unroll consecutive portions of the substrate sheet from the roll; and a thermal energy source that applies a short, high temperature thermal shock to consecutive portions of the substrate sheet that are unrolled from the roll by rotating the first rotatable member. Some systems and methods produce nanoparticles on existing substrate. The nanoparticles may be metallic, ceramic, inorganic, semiconductor, or compound nanoparticles. The substrate may be a carbon-based substrate, a conducting substrate, or a non-conducting substrate. The high temperature thermal shock process may be enabled by electrical Joule heating, microwave heating, thermal radiative heating, plasma heating, or laser heating.
Owner:UNIV OF MARYLAND
Modified FeS nano-particle as well as preparation method and application thereof
InactiveCN104478004AHigh reactivityLarge specific surface areaMaterial nanotechnologyIron sulfidesCross-linkWastewater
The invention provides a modified FeS nano-particle as well as preparation method and application thereof, wherein the modified FeS nano-particle comprises aFeS nano-particle and cross-linked polyvinylpyrrolidone; the cross-linked polyvinylpyrrolidone wraps on the surface of the FeS nano-particle to form a shell-core structure; the mass ratio of the cross-linked polyvinylpyrrolidone to the FeS nano-particle is (0.1-0.5):1.1. The preparation method of the modified FeS nano-particle c comprises the following step: importing N2 to stir and mix a cross-linked polyvinylpyrrolidone solution and a FeCl2.4H2O solution, dropwise adding an Na2F.9H2O solution under the condition of importing N2 and stirring to obtain the modified FeS nano-particle. The modified FeS nano-particle provided by the invention is high in reaction activity and large in specific surface area; both the Fe2+ and the S2- of the FeS nano-particle have reducing property and can be applied to the treatment of chromate wastewater, so that compared with the present widely applied zero-valent iron nano-particles, the modified FeS nano-particle is better in treatment effect.
Owner:HUNAN UNIV
Preparation method and application of one-dimensional rod-like CuFeS2 compound
ActiveCN104362343AImprove cycle performanceLower synthesis costCell electrodesIron sulfidesFerrous saltsAlloy
The invention relates to a preparation method and application of a one-dimensional rod-like CuFeS2 compound, and relates to an alloy type transition metal sulfide. The preparation method comprises the following steps of: dissolving water-soluble cupric inorganic salt and water-soluble ferrous inorganic salt into deionized water, then adding sulfur powder, and stirring into yellow green turbid liquid; carrying out hydrothermal reaction in a hydrothermal kettle, and then centrifuging, cleaning and drying to obtain a grey black final product, namely the one-dimensional rod-like CuFeS2 compound. The one-dimensional rod-like CuFeS2 compound prepared through the method disclosed by the invention can be used as a secondary battery electrode material. The preparation method disclosed by the invention can be used for preparing the one-dimensional rod-like CuFeS2 compound by utilizing a hydrothermal synthesis method, is simple in synthetic method and low in raw material cost, and adopts the water-soluble cupric salt, ferrous salt (ferrous ion) and the deionized water as reaction solvents. The synthesized material as a positive material or negative material of a secondary battery has good circulating property. A secondary lithium ion battery which takes the synthesized material as a positive pole has the advantages of excellent circulating property and good capacity retention ratio.
Owner:XIAMEN UNIV
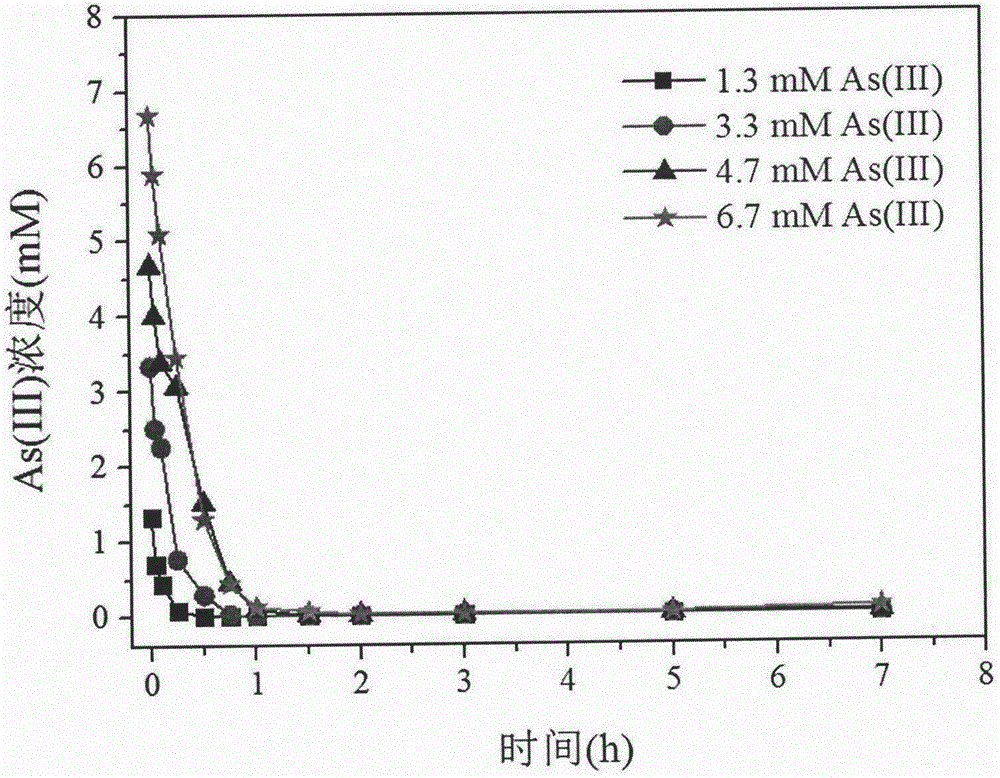
Method for removing high-concentration As (III) in water body through oxidation dissolution of ferrous sulfide (FeS)
InactiveCN106830275ANo subsequent arsenic releaseGood and stable arsenic removal effectWater contaminantsIron sulfidesHigh concentrationArsenate
The invention provides a method for removing high-concentration As (III) in a water body through oxidation dissolution of ferrous sulfide (FeS). According to the method, amorphous and high-activity FeS is synthesized in an anaerobic glove box by virtue of a simple chemical precipitation method, As (III) in the water body is removed by virtue of the oxidation dissolution of FeS, arsenic is removed by virtue of the adsorptive property of a Fe (III) iron mineral formed by FeS reaction and produced iron arsenate, a removal effect of As (III) is very good, a subsequent arsenic release phenomenon is avoided, and therefore, the arsenic removal effect is good and stable.
Owner:TIANJIN POLYTECHNIC UNIV
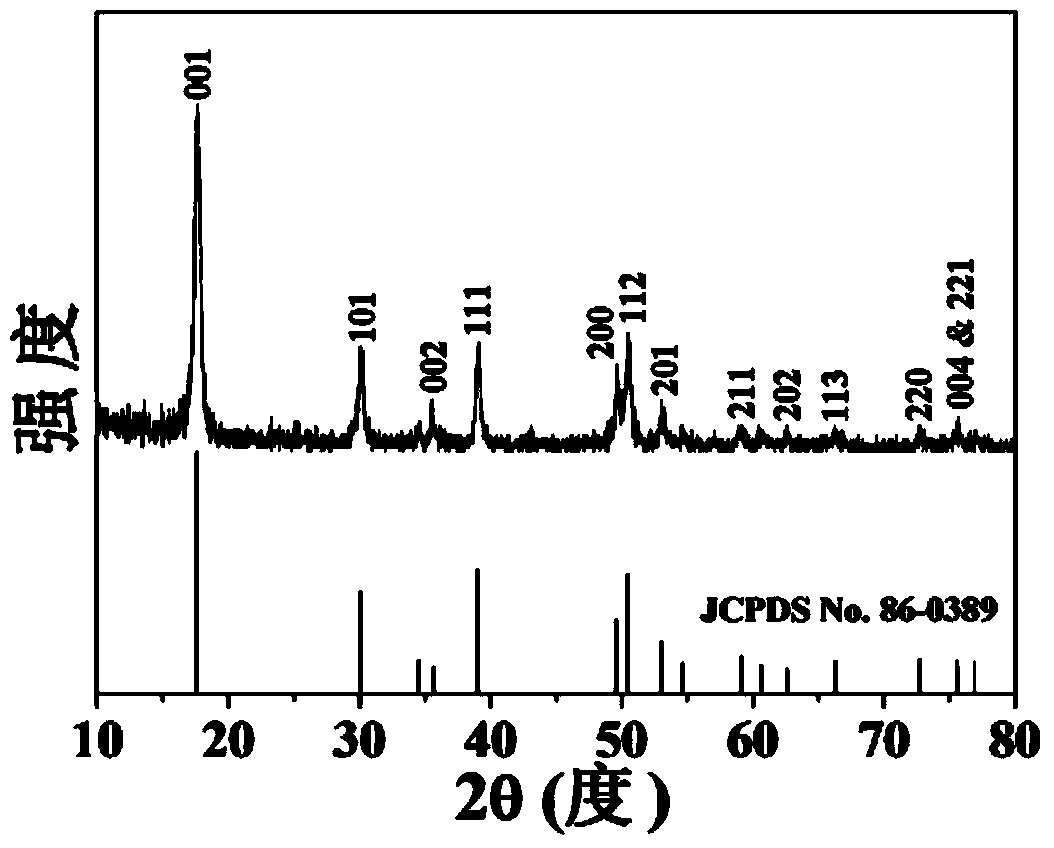
Pyrite nano mineral material and preparation method thereof
ActiveCN102320668ARegulatory structure stateRegulate physicochemical propertiesSulfur preparation/purificationNanotechnologyChemical reactionRoom temperature
The invention discloses a pyrite nano mineral material and a preparation method thereof. The pyrite nano mineral material uses pyritic sulfur and sulphide concentrate as raw materials, the mass percent of pyrite in the pyritic sulfur is not smaller than 50%, and the mass percent of the pyrite in the sulphide concentrate is not smaller than 90%. The preparation method comprises the following stepsof: crushing pyritic sulfur ores, sieving with a sieve of 100 meshes to obtain powder or directly selecting the sulphide concentrate with the particle diameter which is smaller than 0.165mm as powder, adding a bonder to the powder, pelletizing and molding to obtain particles with the particle diameter of 1-3mm, calcinating the particles at a temperature of 500-1000DEG C for 0.5-30min under anaerobic conditions, separating gas from solids, condensating the gas, recovering sulfur, and cooling the solids under oxygen isolation conditions to a room temperature to obtain a finished product. The material has the advantages of large specific area, strong magnesium, high chemical reaction activity, simple preparation technology, low cost and wide raw material resources.
Owner:HEFEI UNIV OF TECH
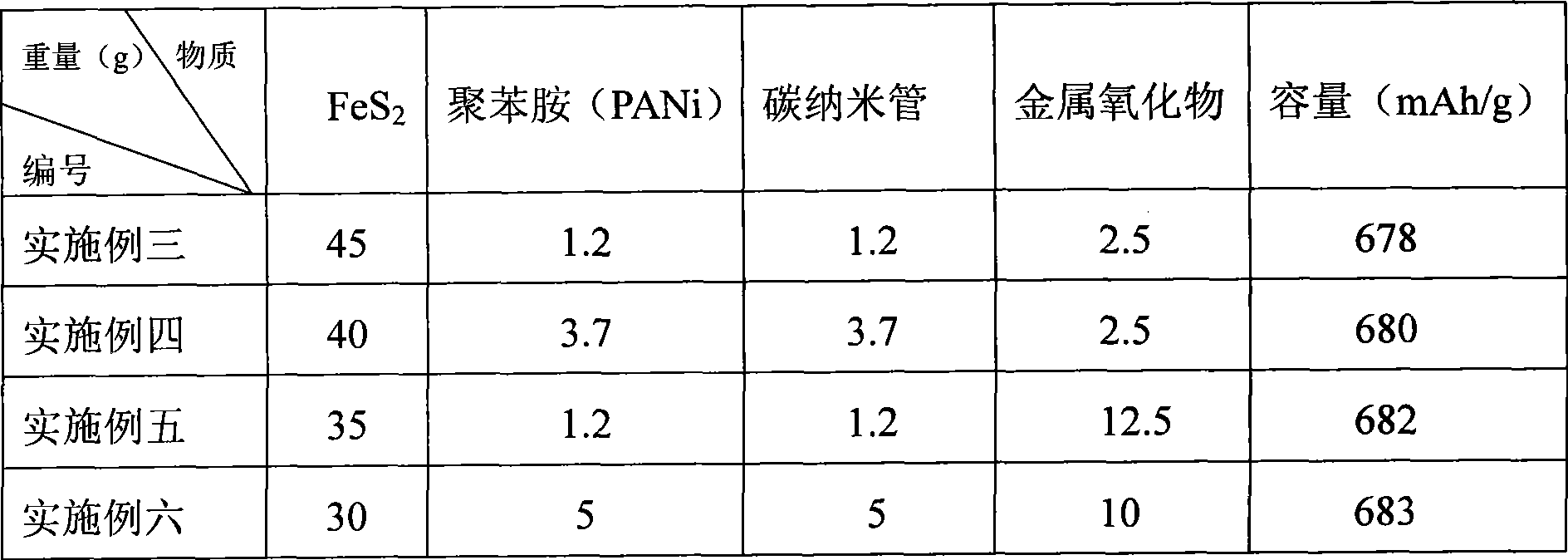
Iron-based sulfide electrode material, preparation method and application in solid state battery
ActiveCN106784815AImprove electrochemical performanceIncrease energy densityMaterial nanotechnologyCell electrodesIron(II,III) sulfideSolid-state battery
The invention provides an iron-based sulfide electrode material, a preparation method and application in a solid state battery, and belongs to the technical field of secondary lithium batteries. The iron-based sulfide electrode material which is based on conversion reaction is selected from one or more of ferrous sulfide, ferriferous sulfide and hepta-ferous octa-sulfide. The preparation method of the iron-based sulfide electrode material has the advantages of being simple and short in time.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
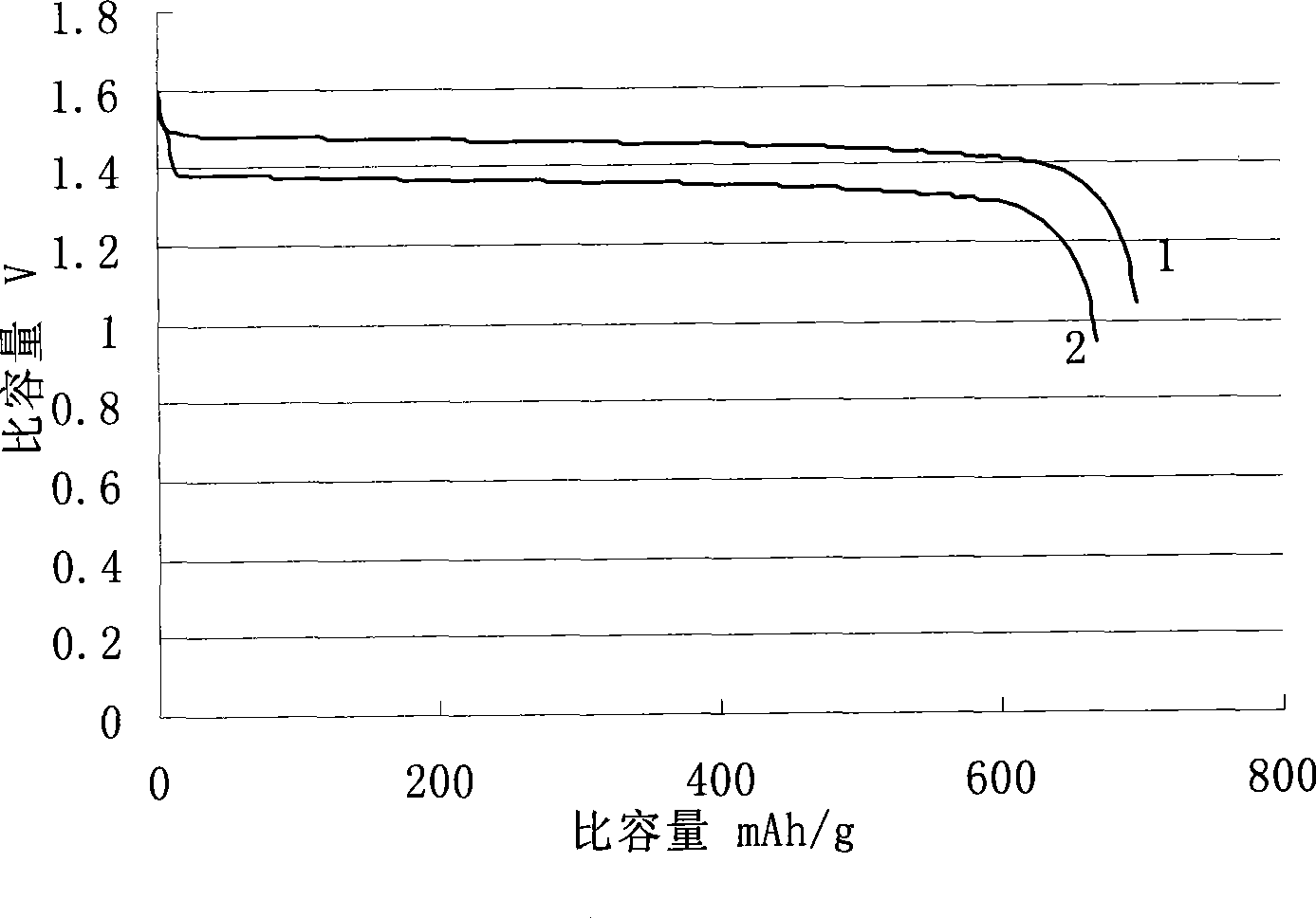
Anode material and anode plate of lithium-iron disulfide battery and method for preparing same
InactiveCN101521279ALower open circuit voltageHigh conductivityElectrode manufacturing processesActive material electrodesIron disulfideBattery cell
The invention discloses an anode material and an anode plate of a lithium-iron disulfide battery and a method for preparing the same, wherein the anode material comprises iron disulfide which is an iron disulfide nanometer crystal, and FeSO4, (NH2)2CS and S taken as reaction raw materials and PVP taken as a dispersing agent react under the acid or alkali condition to prepare the iron disulfide. The obtained iron disulfide is an N-shaped crystal, of which the purity is as high as 100 percent; compared with the iron disulfide used in the prior art, the iron disulfide of the invention can greatly reduce the open circuit voltage of the lithium-iron disulfide battery, make the voltage platform more stable, improve the conductive activity of the battery, improve the discharging performance of heavy current, improve the discharging depth of the battery and make the lithium-iron disulfide battery have higher actual values.
Owner:广州市天球实业有限公司 +1
Processes for Producing Synthetic Pyrite
ActiveUS20090087374A1Increase surface areaMaterial nanotechnologyOxide/hydroxide preparationPowder mixtureIron powder
Process of making high purity, synthetic FeS2, and an electrochemical battery employing such synthetic FeS2 in the positive electrode. Synthetic FeS2 may be prepared by a sulfidation process comprising reacting ferric oxide, hydrogen sulfide, and elemental sulfur at a temperature above the melting point of element sulfur. Synthetic FeS2 may also be produced by a milling process that comprises (i) milling iron powder and sulfur powder in the presence of a milling media and a processing agent to provide a homogenous powder mixture, and (ii) treating the powder mixture to form FeS2. In the milling process, the powder mixture may be treated to form FeS2 by heating the powder mixture or subjecting the powder mixture to a subsequent milling operation.
Owner:ENERGIZER BRANDS
Method for preparing general nano metal sulphide
InactiveCN101857276AHigh yieldUniform sizeNanostructure manufactureZinc sulfidesCarbamateMetallic sulfide
The invention discloses a method for preparing general nano metal sulphide. The method is characterized in that: diethyldithio carbamate is adopted as a single-source reaction precursor of the sulphide, and is thermally decomposed, nucleated and grown in the mixed solution of surfactants with different coordination properties to prepare appearance and size-controllable sulphide nanocrystals in a pure state by one step. In the method, the thermal decomposition of the reaction precursor in the high-boiling mixed solution of the surfactants and components of the solution are controlled, and the nucleation and growth process of the nanocrystals is adjusted, so the prepared metal sulphide has the characteristics of pure phase state, uniform size, high yield, relatively higher dispersibility in non-polar organic solvents, easily controlled reaction conditions and large-scale production.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Preparation method of pyrite-type ferrous disulfide micron/nano crystalline material with controllable morphology
InactiveCN102786098AEffective particle size controlEffective control of morphologyMaterial nanotechnologyIron sulfidesThio-Active agent
The invention relates to a preparation method of a pyrite-type ferrous disulfide micron / nano crystalline material with a controllable morphology. The method comprises the following steps of: (1) respectively adding solvent dimethyl sulfoxide into compounding agent thioglycolic acid and surfactant polyvinylpyrrolidone under the condition of agitating and leading in nitrogen or argon, and obtaining a solution A by mixing and agitating; (2) orderly adding an iron source and a sodium thiosulfate water solution into the solution A, leading in the nitrogen or argon and vigorously stirring to obtain a solution B; (3) transferring the solution B into a reaction kettle and reacting for 4-12 hours at 120-180 DEG C after uniformly agitating, to obtain pyrite-type ferrous disulfide suspension liquid; (4) centrifugally separating, washing for a plurality of times and drying the pyrite-type ferrous disulfide suspension liquid in vacuum to constant weight to obtain the pyrite-type ferrous disulfide micron / nano crystalline material. The product obtained by the method has controllable particle size and morphology, good process repeatability and stable quality, and is expected to be applied to the fields such as photovoltaic conversion and lithium ion battery materials.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com