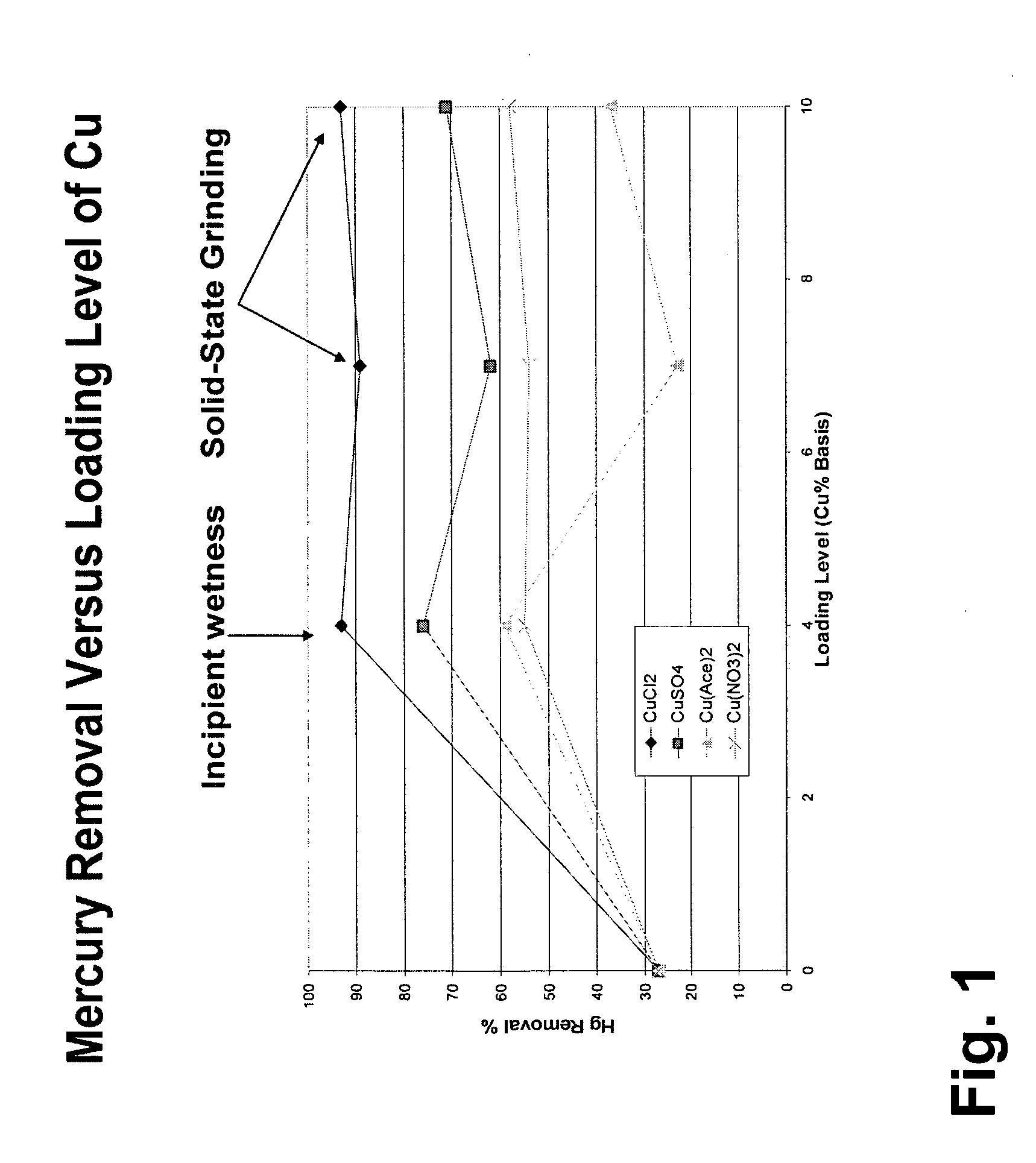
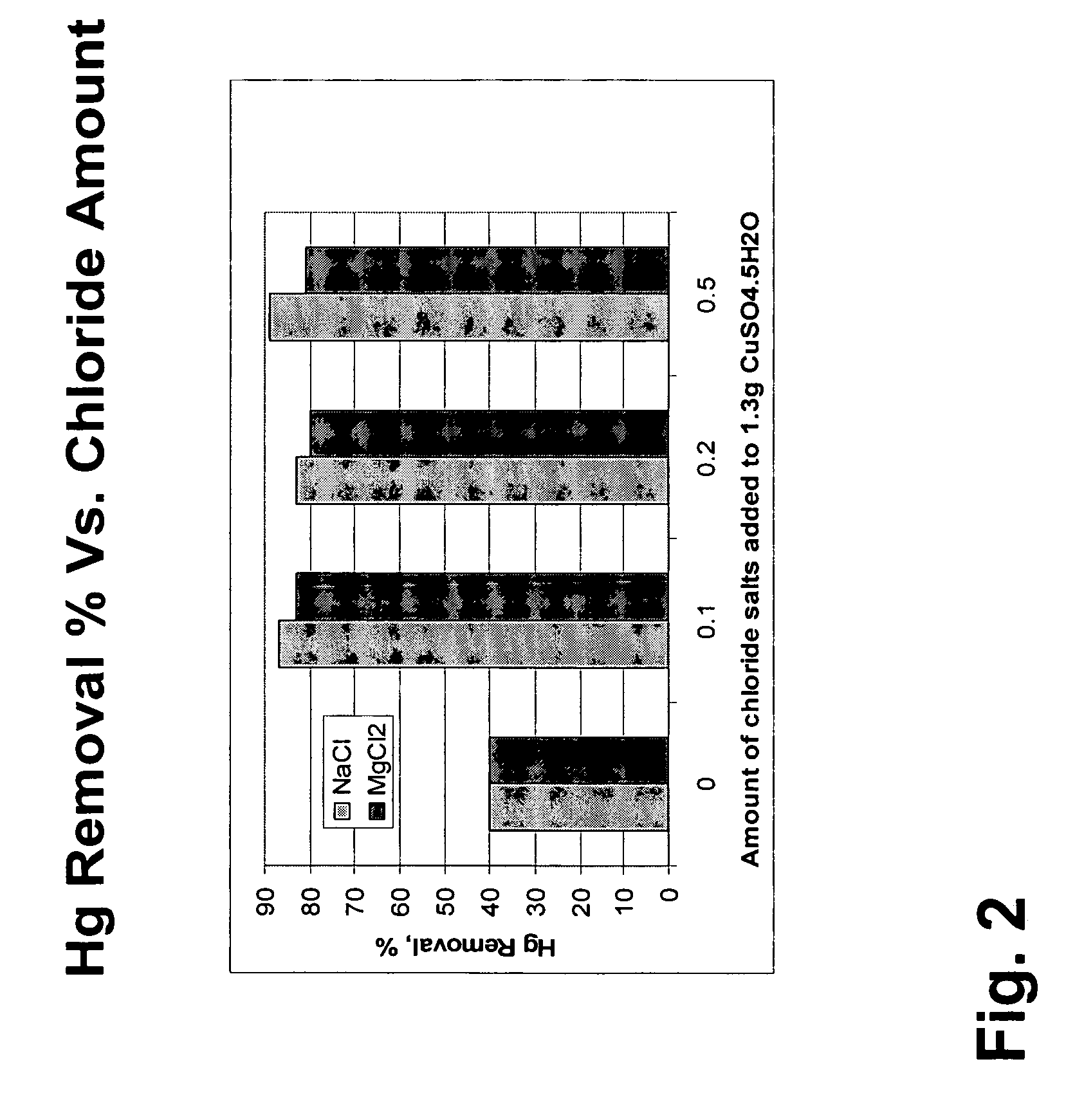
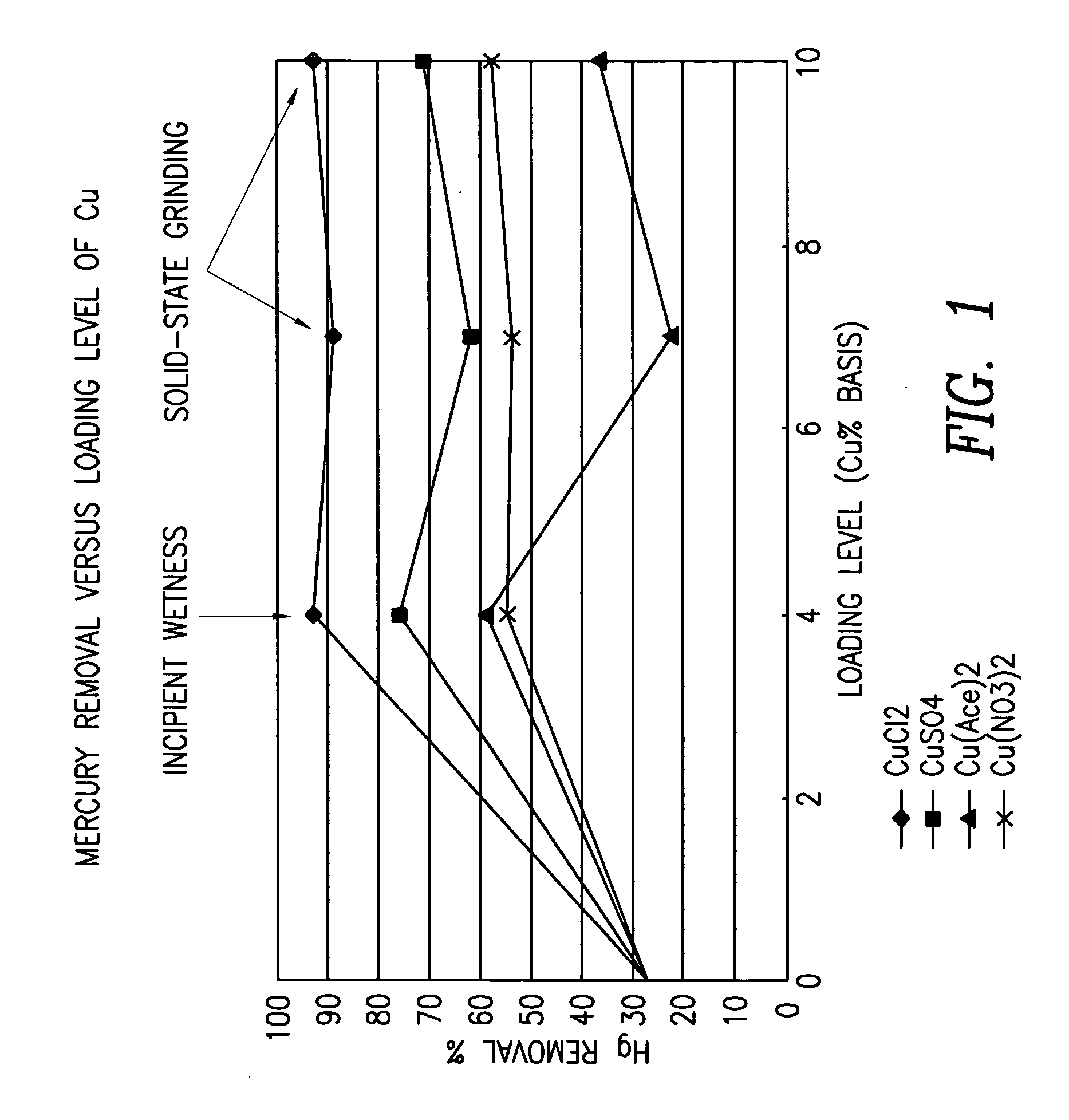
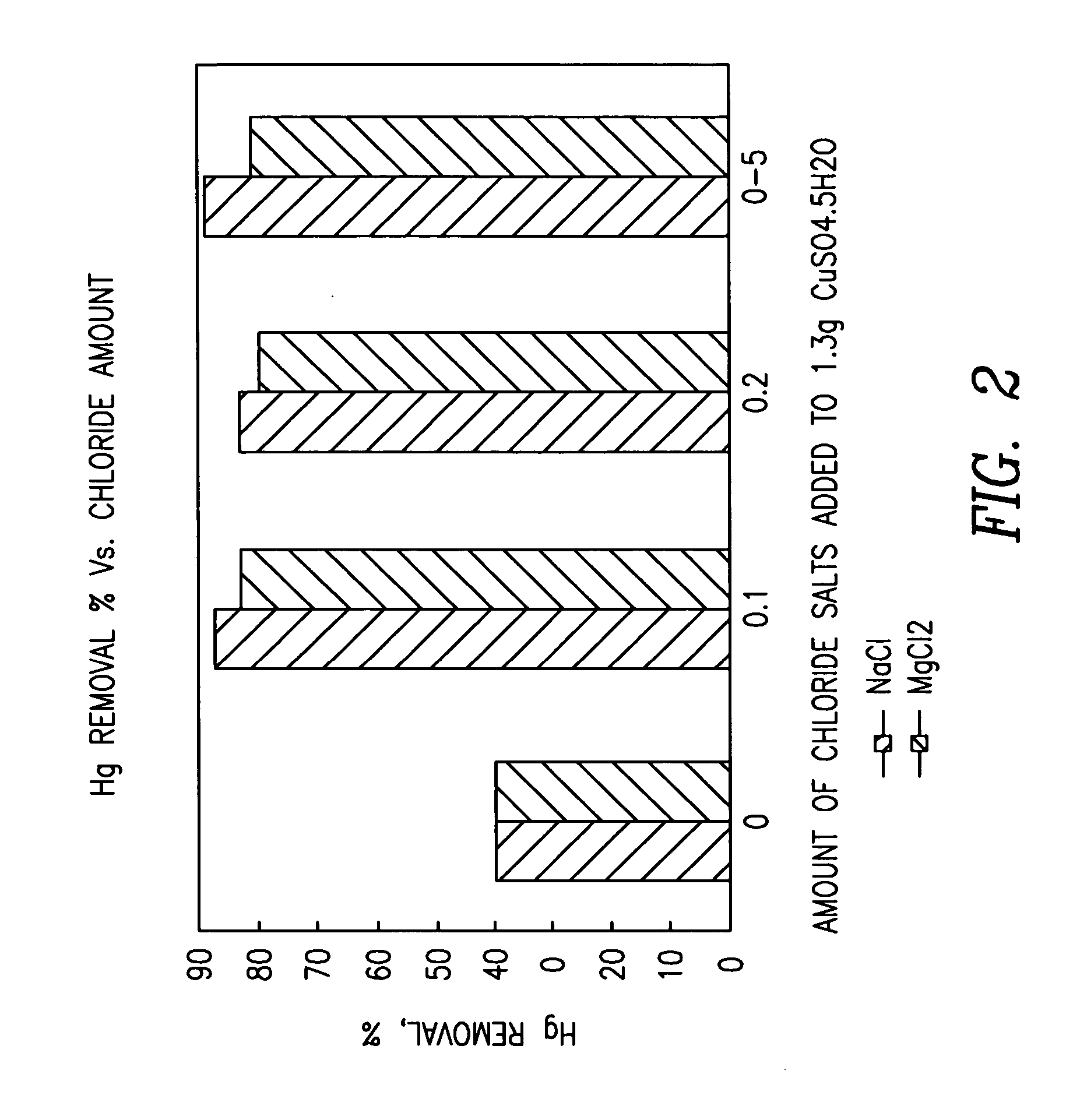
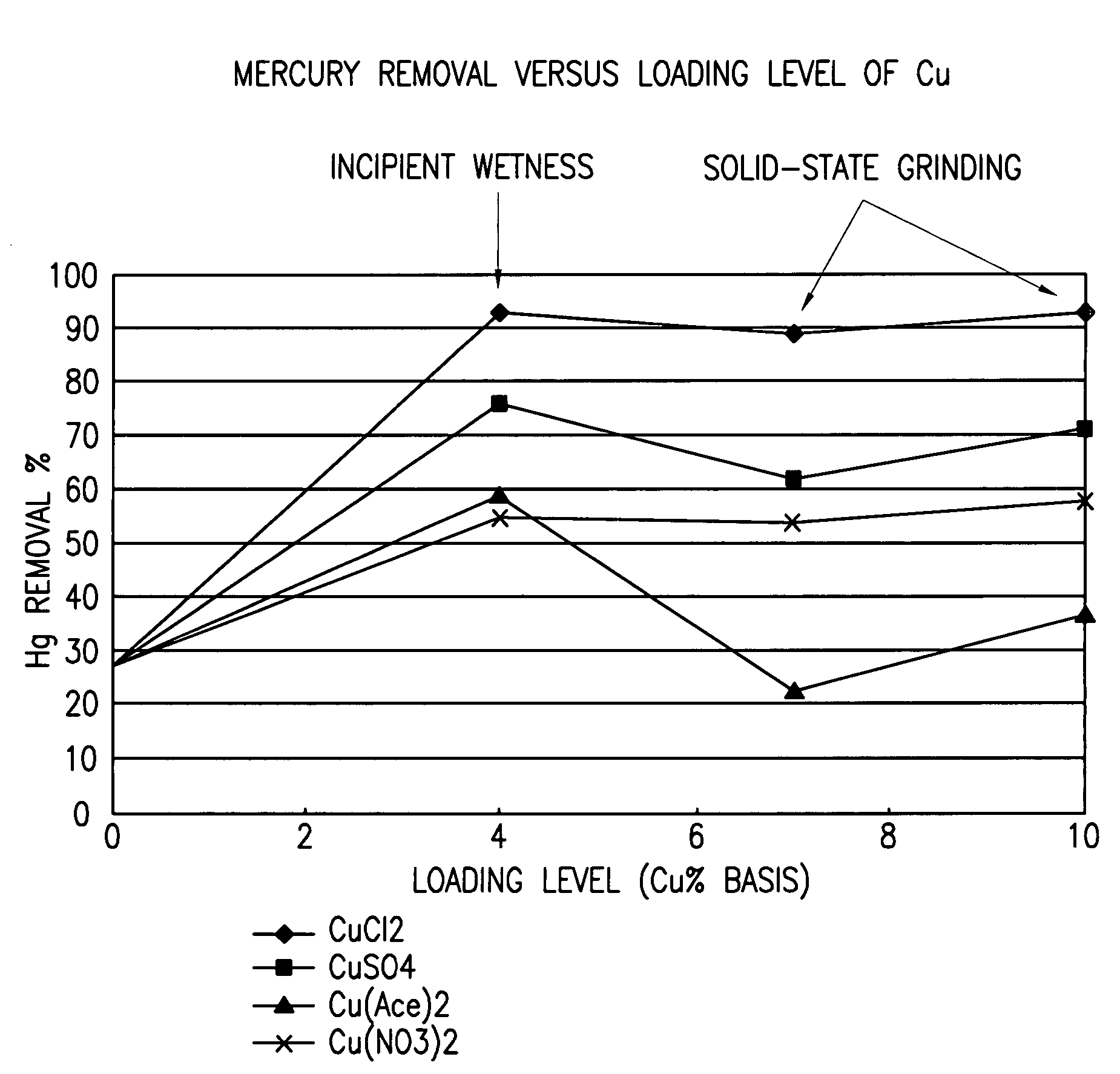
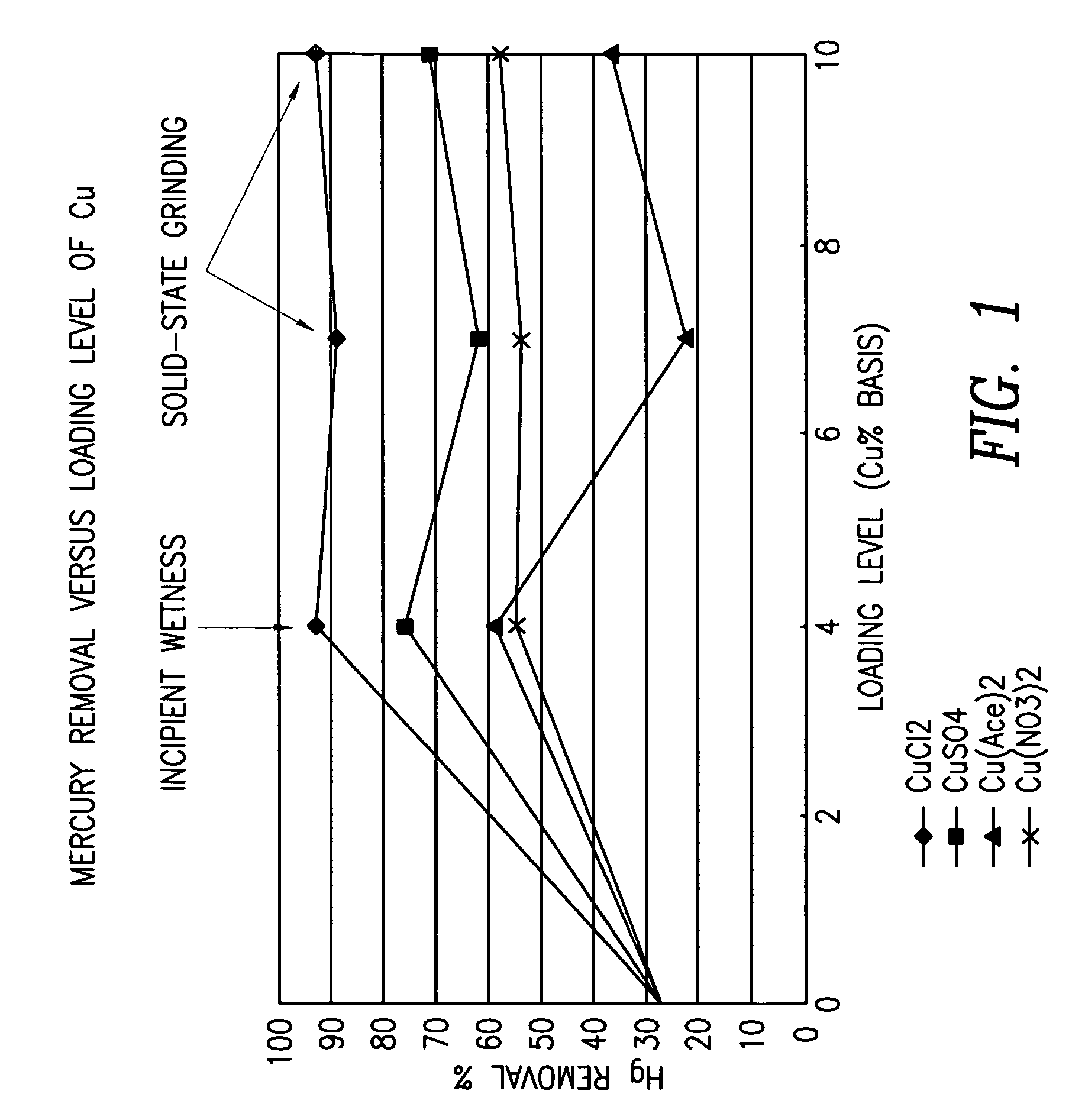
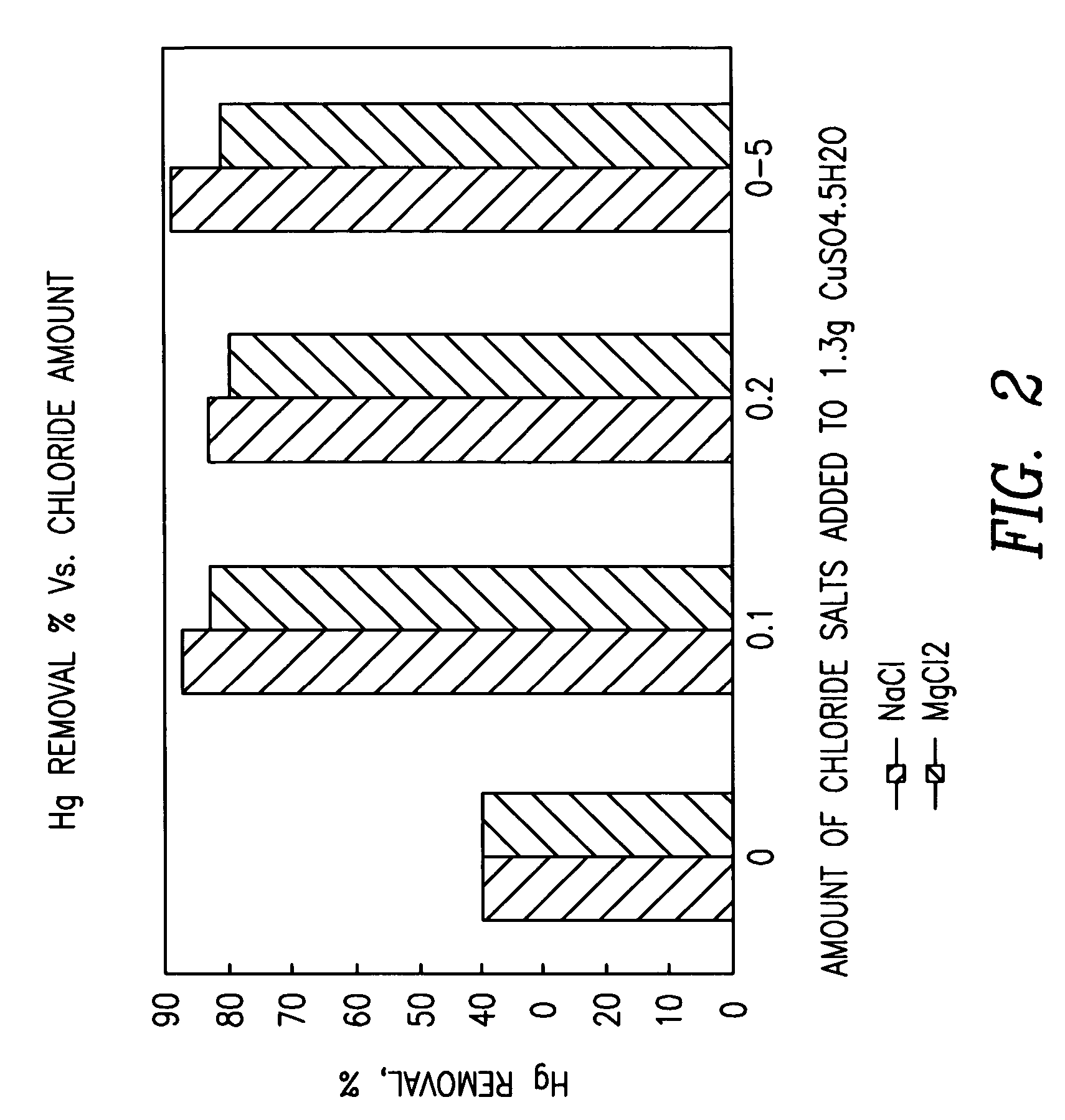
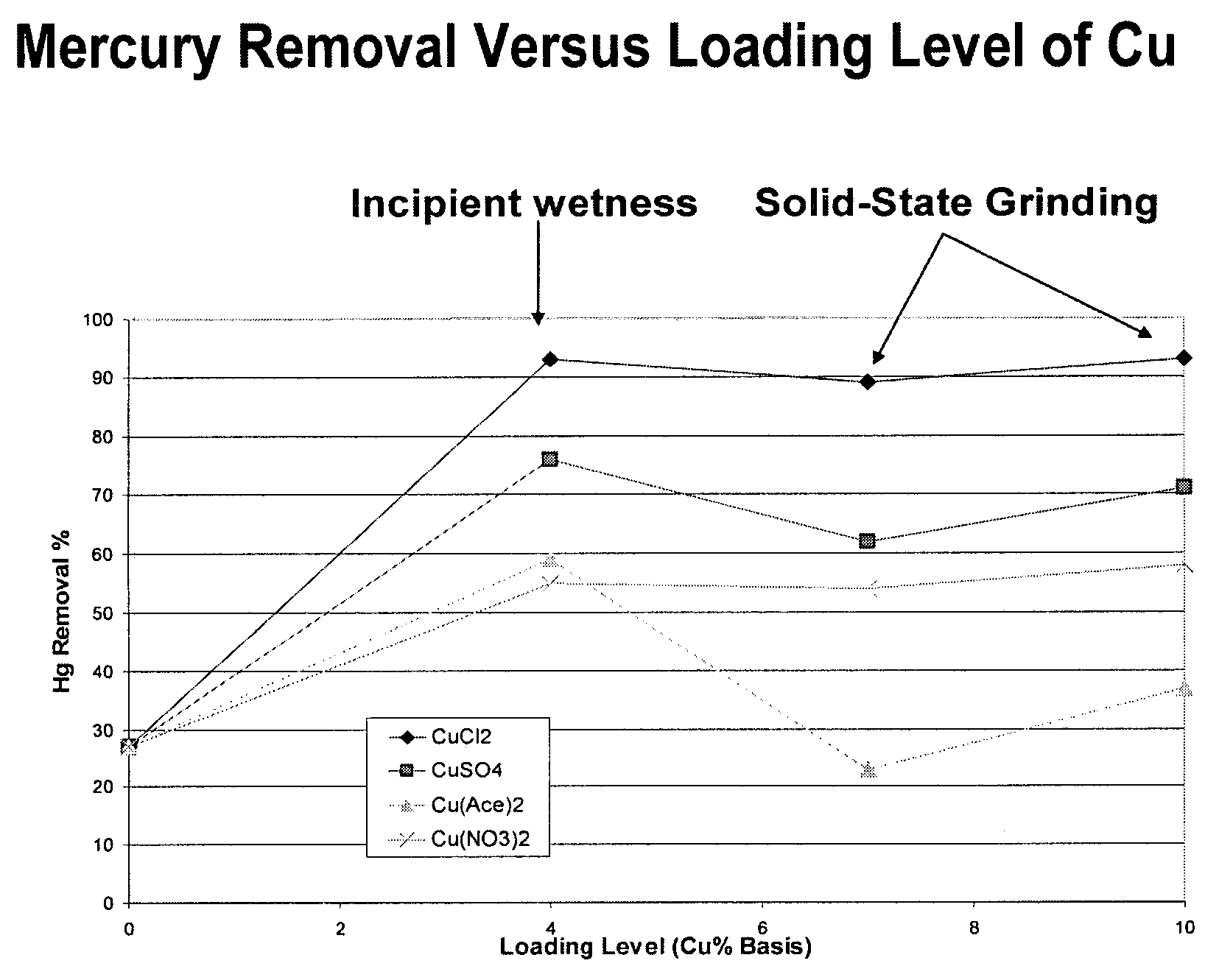
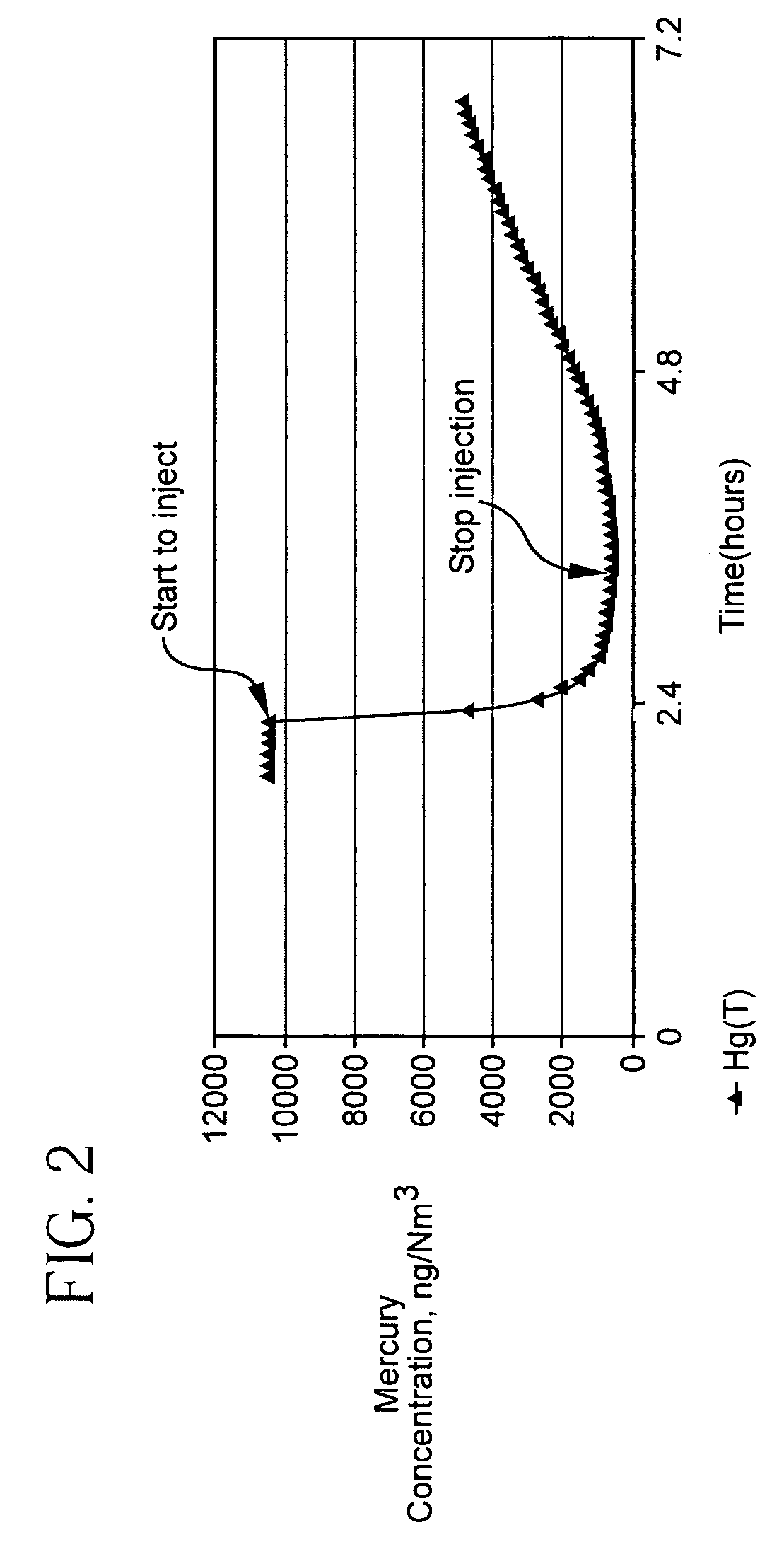
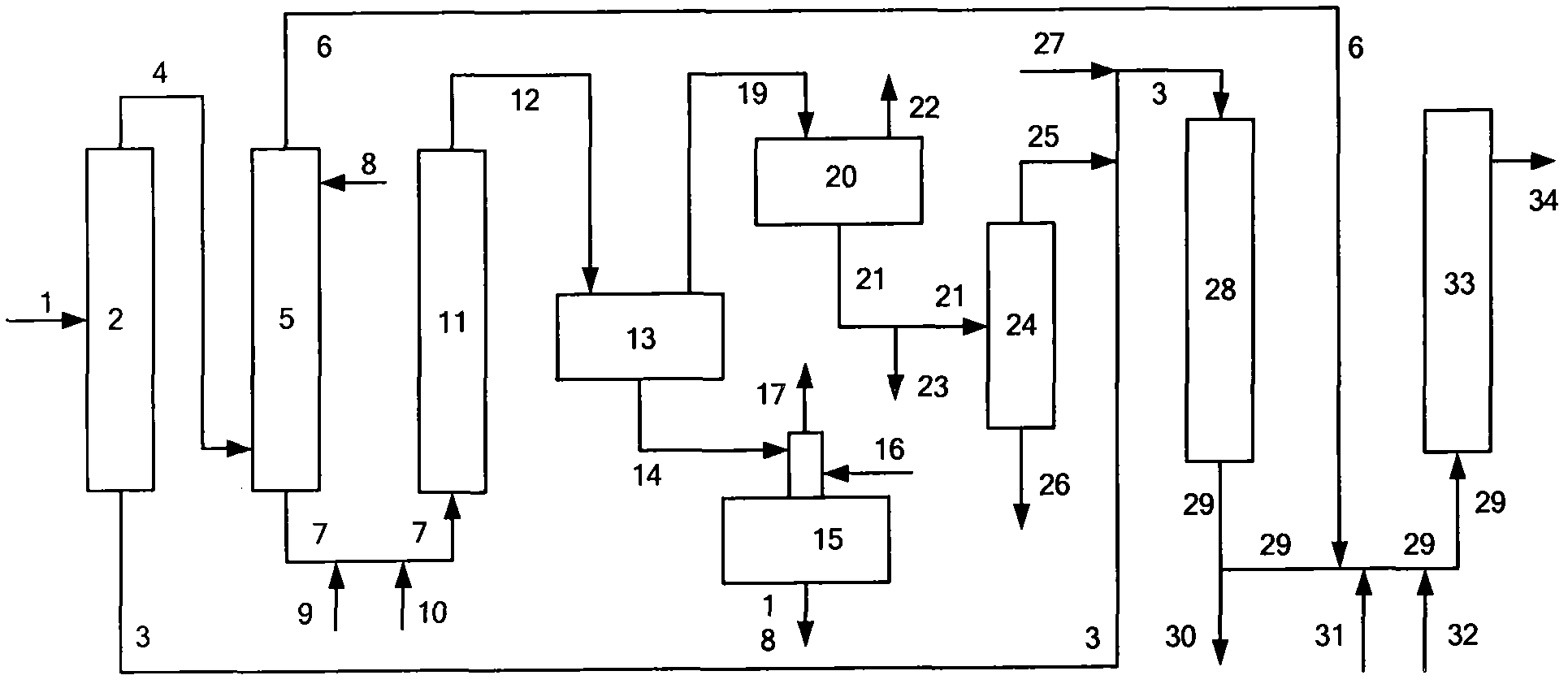
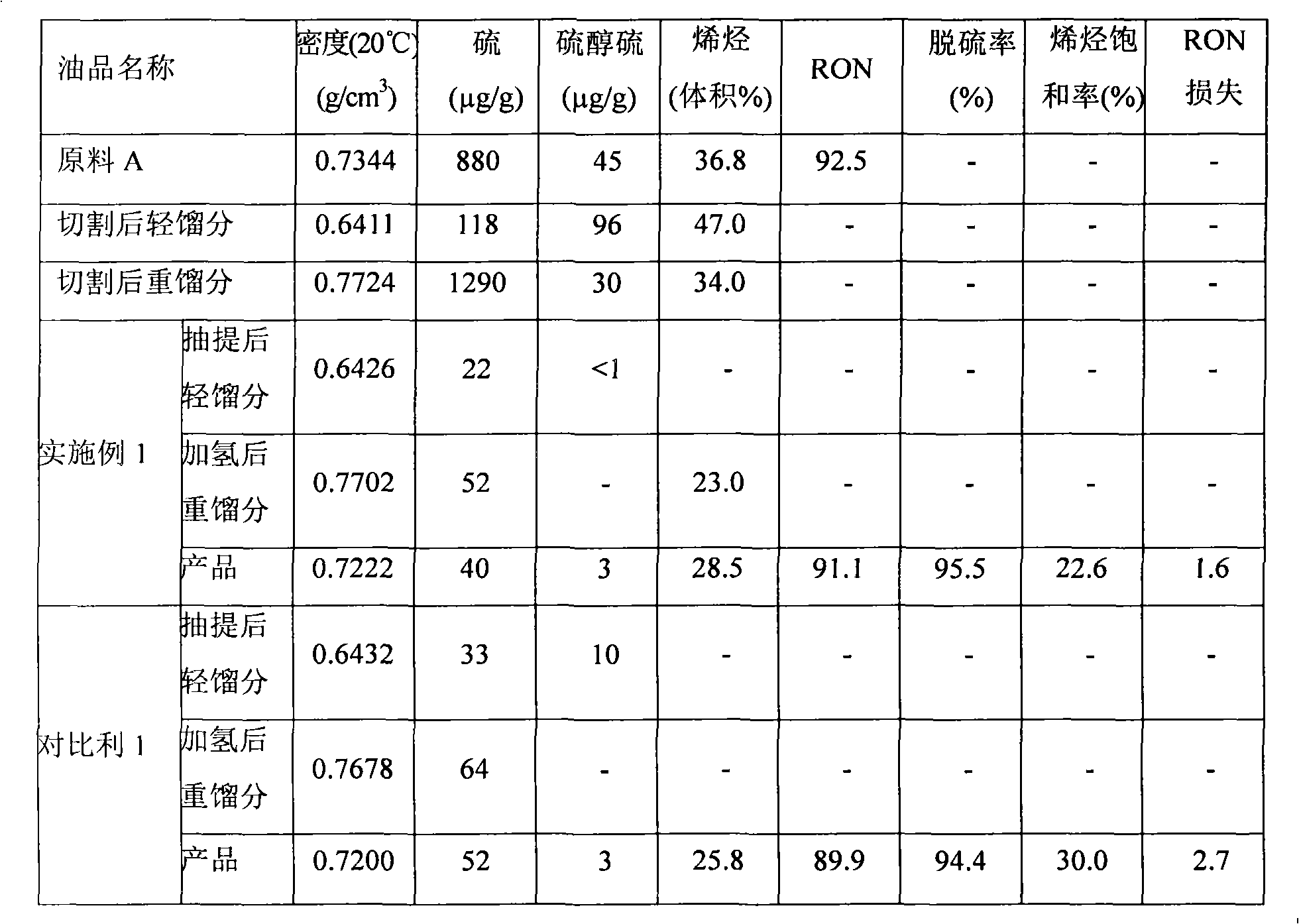
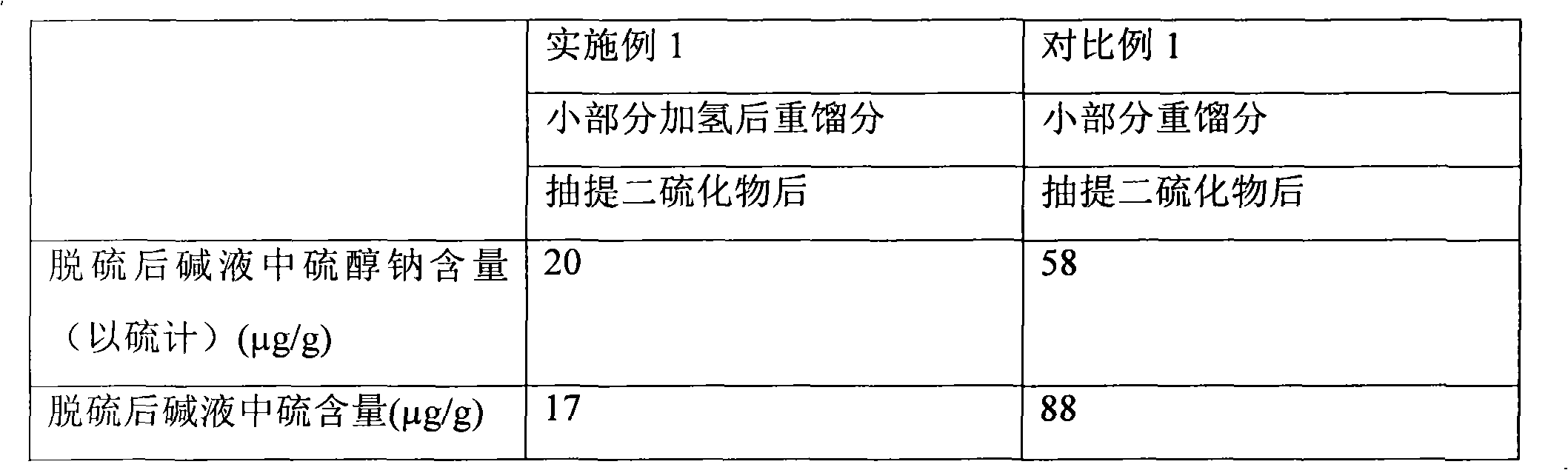
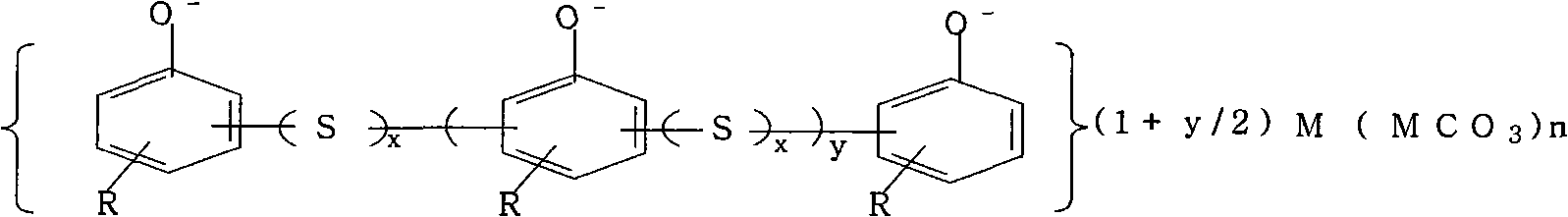Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Sulfide salt" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
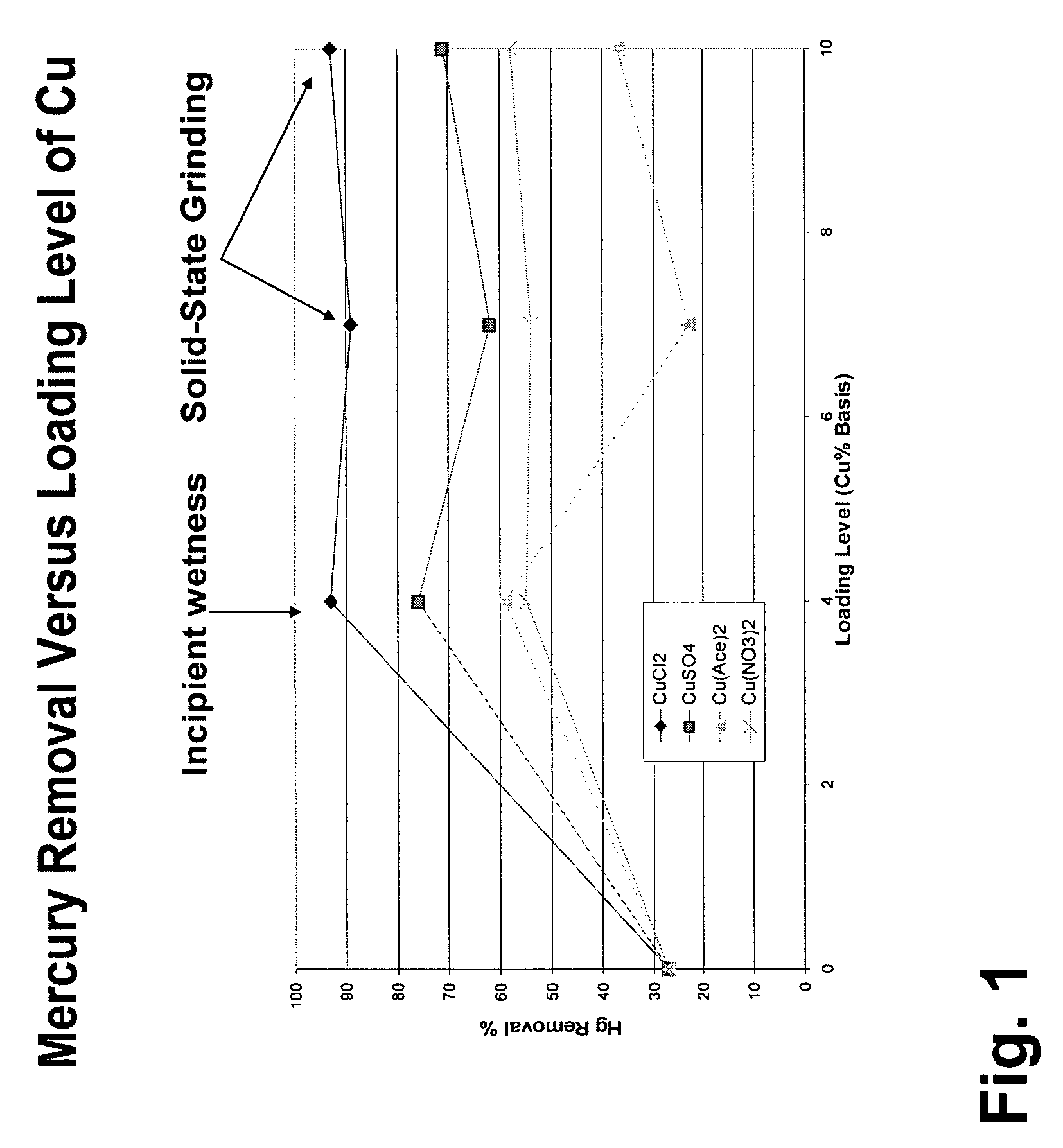
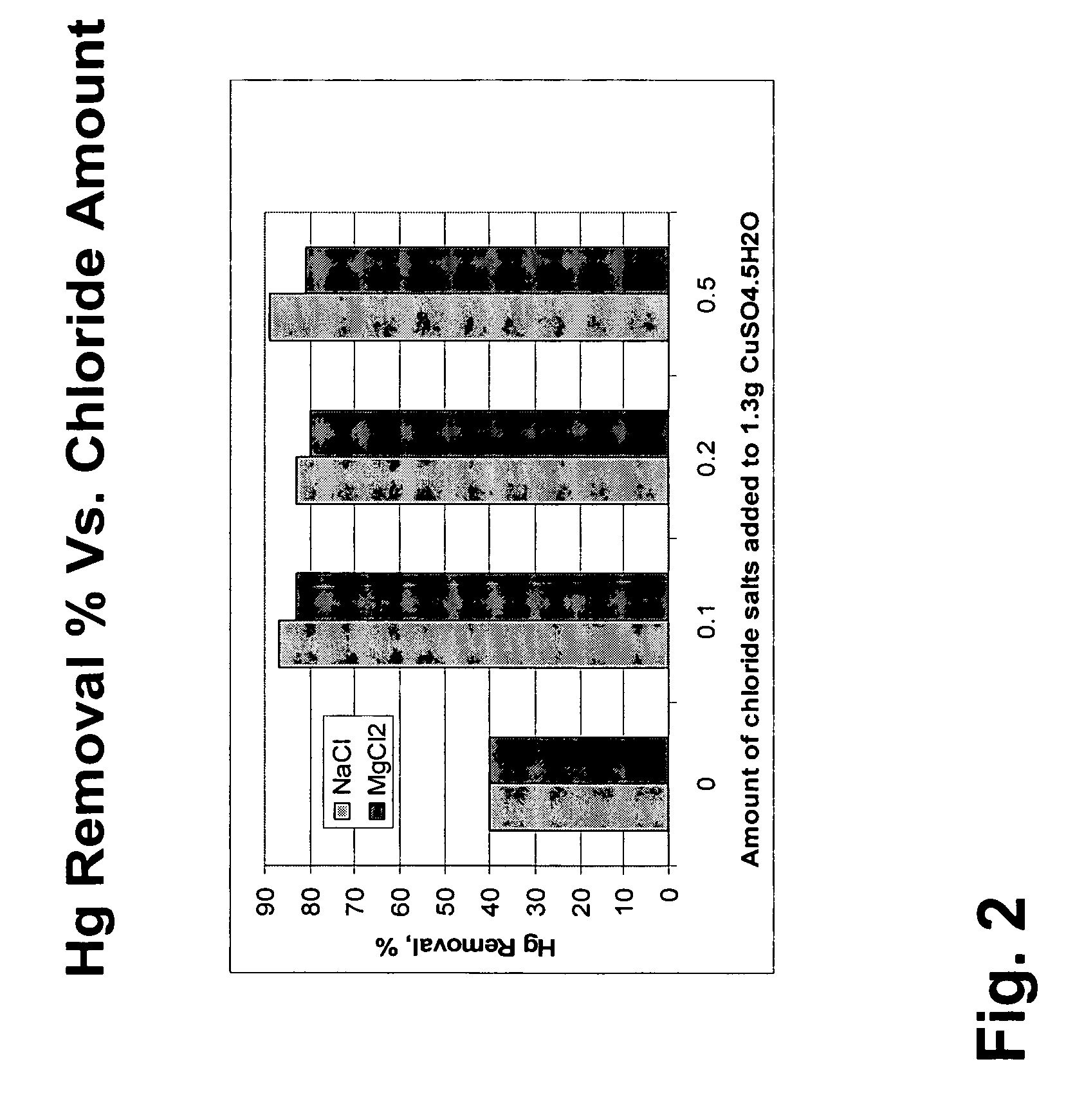
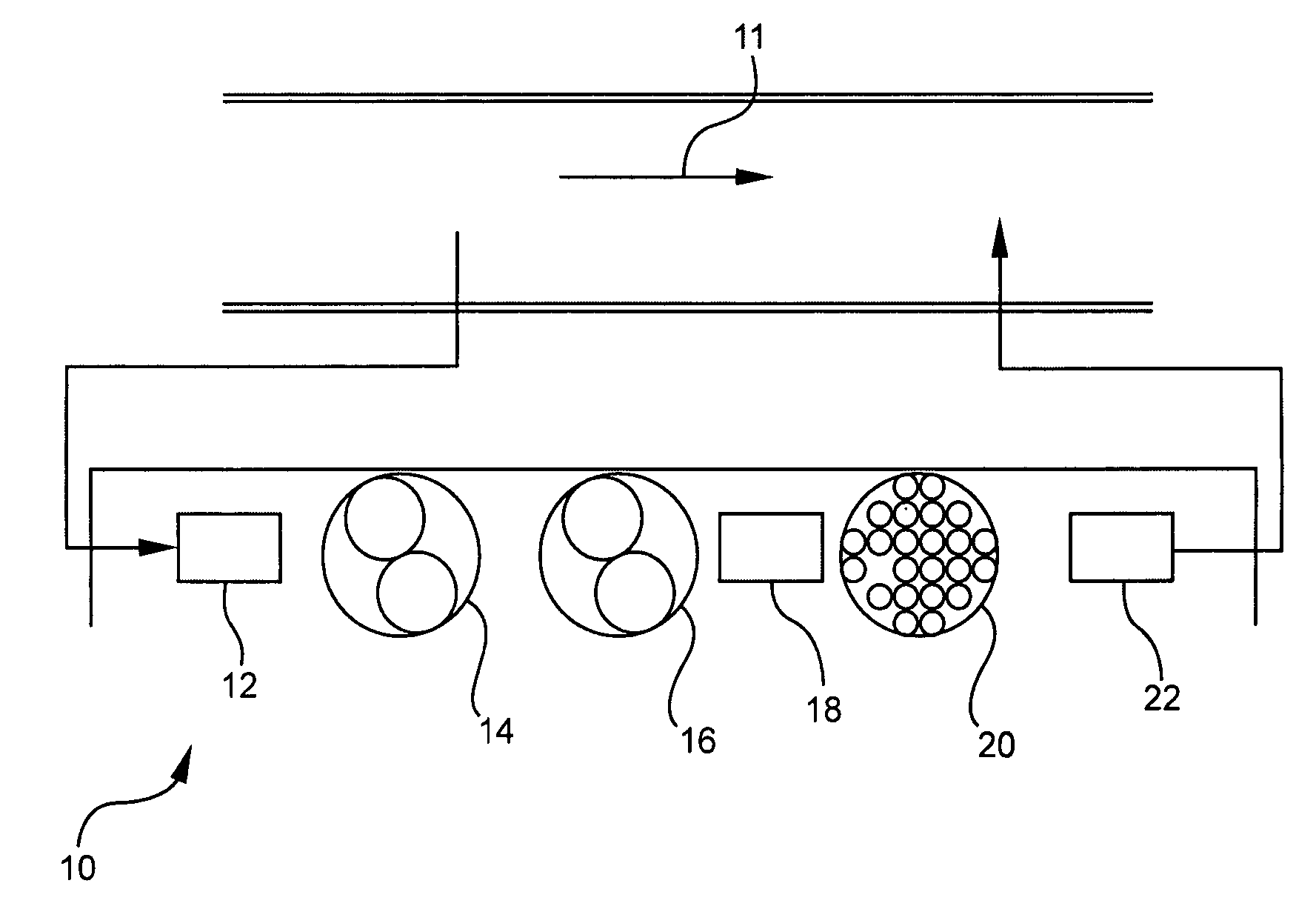
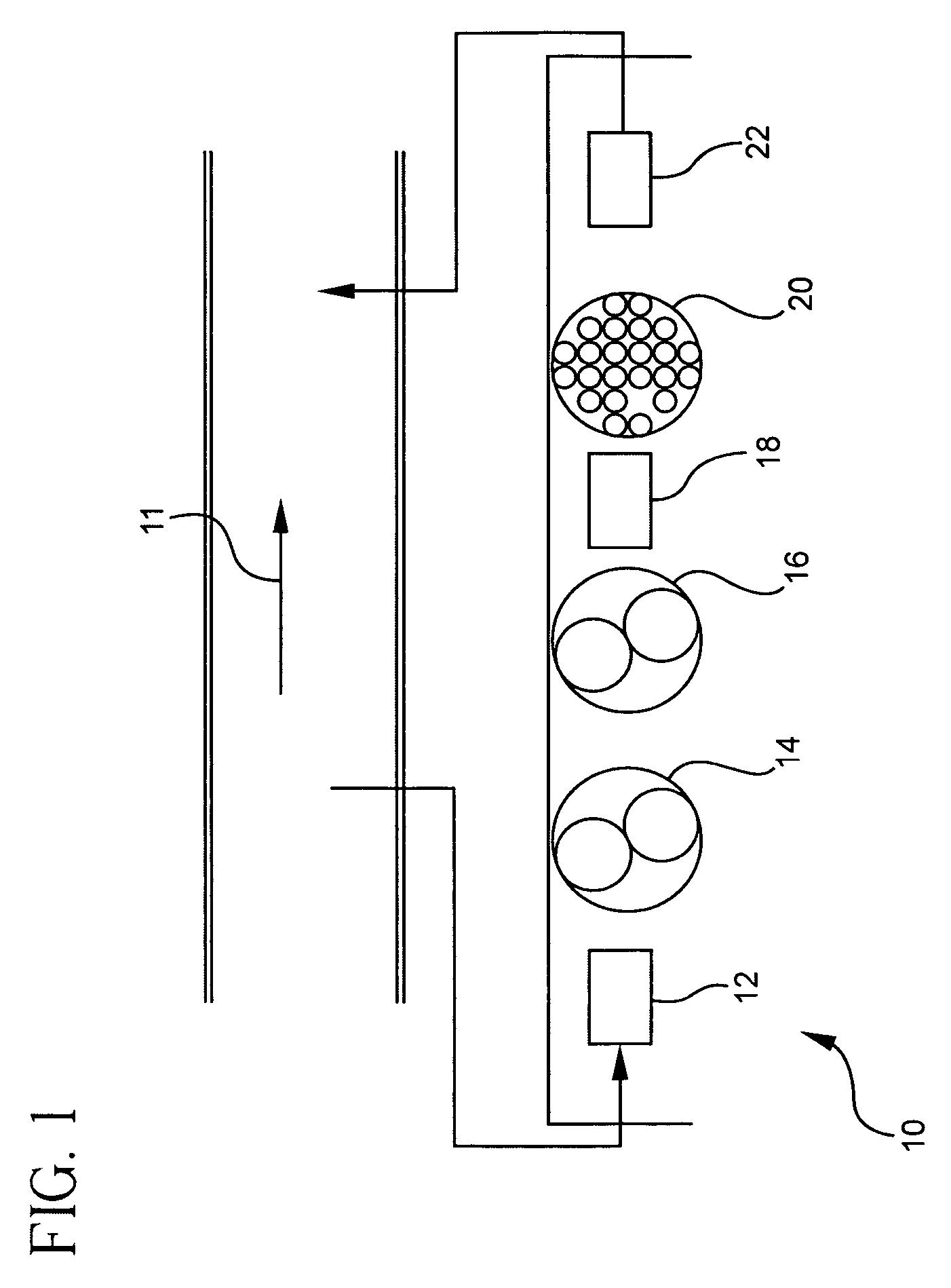
Pollutant emission control sorbents and methods of manufacture
InactiveUS20070122327A1Small particle sizePromotes Hg-captureGas treatmentOther chemical processesSorbentFlue gas
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing sorbent substrate particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the sorbent particles.
Owner:BASF CATALYSTS LLC
Methods of manufacturing bentonite pollution control sorbents
InactiveUS20070119300A1Small particle sizeLiquid surface applicatorsGas treatmentSodium BentoniteSorbent
Methods of manufacturing bentonite sorbents for removal of pollutants including mercury from gas streams, such as a flue gas stream from coal-fired utility plants are disclosed. The methods include mixing bentonite sorbent particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the bentonite sorbent particles.
Owner:BASF CATALYSTS LLC
Methods of manufacturing bentonite pollution control sorbents
InactiveUS7578869B2Small particle sizeGas treatmentLiquid surface applicatorsSodium BentoniteSorbent
Methods of manufacturing bentonite sorbents for removal of pollutants including mercury from gas streams, such as a flue gas stream from coal-fired utility plants are disclosed. The methods include mixing bentonite sorbent particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the bentonite sorbent particles.
Owner:BASF CATALYSTS LLC
Pollutant emission control sorbents and methods of manufacture
InactiveUS7704920B2Small particle sizePromotes Hg-captureGas treatmentOther chemical processesSorbentFlue gas
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing sorbent substrate particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the sorbent particles.
Owner:BASF CATALYSTS LLC
Pollutant emission control sorbents and methods of manufacture
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing fly ash particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the fly ash particles.
Owner:BASF CATALYSTS LLC
Gasoline desulfurization method
ActiveCN102851069AImprove the extraction effectAvoid inactivationTreatment with hydrotreatment processesHydrogenDistillation
The inventive gasoline desulfurization method includes performing cutting distillation on gasoline to obtain heavy fraction with high boiling range and light fraction having low boiling range; under selective hydrogenation desulfurization condition, contacting the heavy fraction and hydrogen gas with hydrogenation desulfurization catalyst to perform selective hydrogenation desulfurization, to obtain desulfurized heavy fraction; contacting the light fraction with alkali solution, to obtain sulfide-absorbed alkali solution and desulfurized light fraction; contacting the sulfide-absorbed alkali solution with oxidant, oxidation catalyst and a part of the desulfurized heavy fraction, to simultaneously perform alkali solution regeneration and desulfurization, so that sulfide salt in the alkali solution is oxidized into disulfide, which is extracted into the desulfurized heavy fraction; performing phase separation, and exhausting tail gas; returning at least part of the disulfide-absorbed heavy fraction to the heavy fraction after cutting distillation, to perform selective hydrogenation desulfurization; and mixing the desulfurized heavy and light fractions. The inventive method can obtain higher desulfurization rate and lower octane number loss.
Owner:CHINA PETROLEUM & CHEM CORP +1
High-performance composite stabilizer for mercury-contaminated soil and method for repairing mercury-contaminated soil
ActiveCN107384427AReduced ability to migrateReduced bioavailabilityContaminated soil reclamationOrganic fertilisersMultiple formsVulcanization
The invention discloses a high-performance composite stabilizer for mercury-contaminated soil and a method for repairing the mercury-contaminated soil. The composite stabilizer comprises an oxidizing agent, a composite stabilizer and a pH modifier; the method for repairing the mercury-contaminated soil comprises the steps of crushing and sieving the mercury-contaminated soil, taking screened mercury-contaminated soil granules; adding water to the mercury-contaminated soil granules for moisturizing, adding an oxidizing agent solution, stirring and heating for oxidizing reaction; adding a sulfide salt solution for vulcanization reaction; adding excessive ferrite sediments S2-, adding clay mineral and humus for adsorption, adding the pH modifier to adjust the pH value of the mercury-contaminated soil to be 6-7 and carrying out conservation for over 3 days. According to the method, mercury in multiple forms in the mercury-contaminated soil can be stably solidified at the same time, the stabilization effect is good, the period is short, and the drug is wide in raw material source, low in cost and beneficial to wide application.
Owner:AEROSPACE KAITIAN ENVIRONMENTAL TECH CO LTD
Lubricating oil composition of diesel engine
The invention relates to a lubricating oil composition of an engine, in particular to a lubricating oil composition for a heavy-load diesel engine, characterized by containing a great amount of hydrotreating base oil. The composition of the invention also contains suitable amounts of alkyl salicylates, sulfurized alkyl sulfide salts, sulphonate, macromolecular ashless dispersant, polyisobutylene-bis-succinimide ashless dispersant, zinc dialkyl dithiophosphate, phenolic antioxidant, amine-type antioxidant and sulfur-bearing antioxidant antiwear agent. The lubricating oil composition can satisfy the specification requirement of diesel engine oil CF-4, which is specified by GB11122-2006. The composition has simple prescription, low cost and convenient modulation.
Owner:PETROCHINA CO LTD
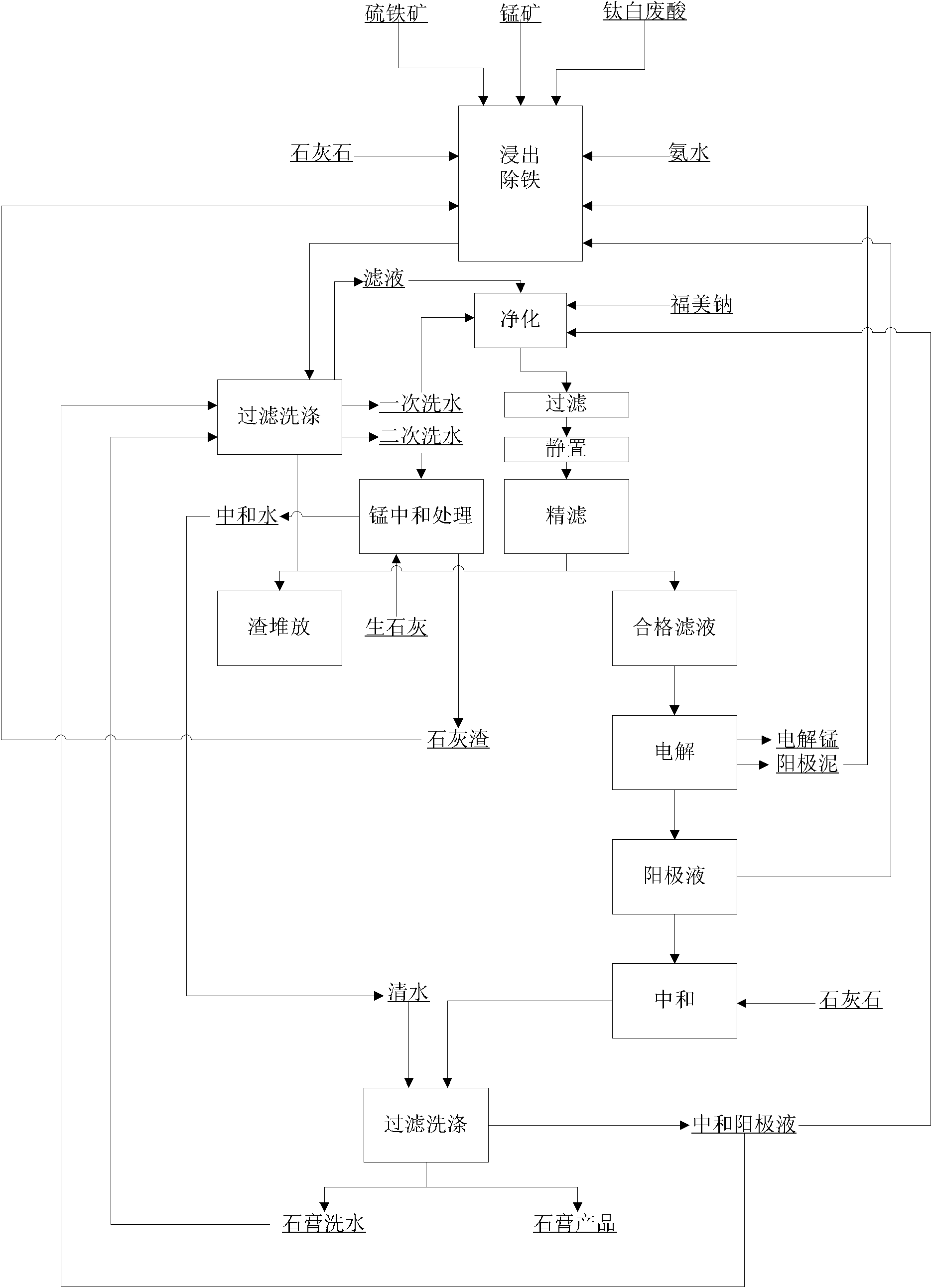
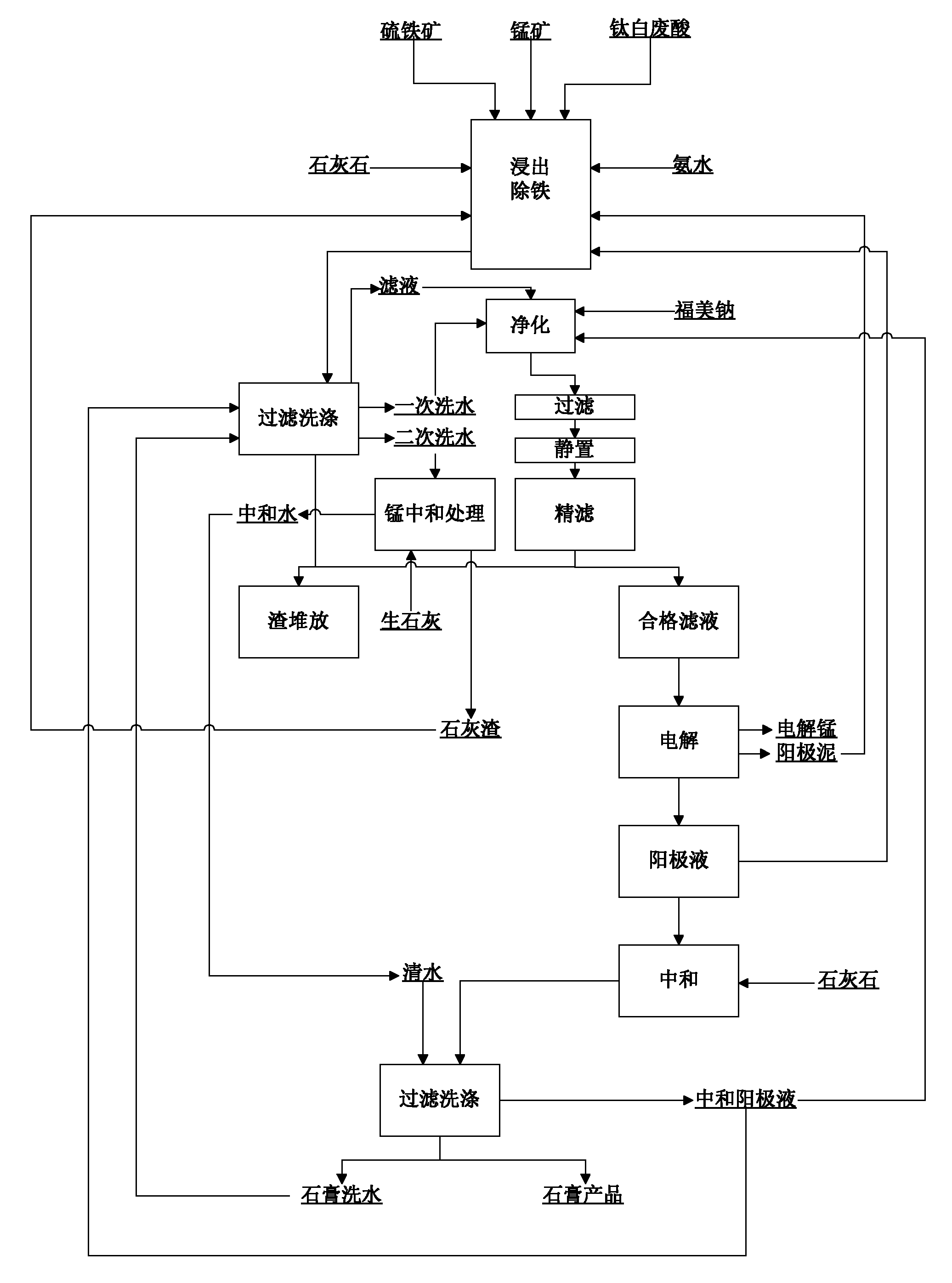
Method utilizing titanium white waste acid to prepare electrolytic manganese metal
ActiveCN101787546AReduce consumptionReduce the liquid-solid ratioPhotography auxillary processesProcess efficiency improvementElectrolysisManganese
The invention relates to a method utilizing titanium white waste acid to prepare electrolytic manganese metal, belonging to the field of metallurgical chemistry. The technical problem that the invention aims at solving is to provide the method with lower production cost for utilizing the titanium white waste acid to prepare the electrolytic manganese metal. The steps of the method are as follows: a. leaching: manganese ore containing manganese dioxide, pyrite ore and the titanium white waste acid are reacted in a reaction container after being heated to the temperature of 90 to 100 DEG C, water in proper quantity is added to make up for the evaporated quantity of water in the reaction process, CaCO3 is added when the pH value of solution is within 1.5 to 2 so as to neutralize the pH value of the solution to 4.8 to 5.2, and then ammonia water is added to adjust the pH value to 6.2 to 6.4; b. filtering: a reaction product of step a is filtered to obtain filtering solution and filtering residues; c. purifying and removing of heavy metal: sulfide salt is added to the filtering solution obtained in step b so as to remove the heavy metal, and the filtering solution is simply filtered and then filtered in a fine manner after being stewed for 24 to 48 hours so as to obtain the filtering solution that meets the electrolytic requirement; d. electrolyzing: the manganese metal and waste electrolytic solution namely anode solution containing sulfuric acid are obtained.
Owner:PANZHIHUA LIYU MINING
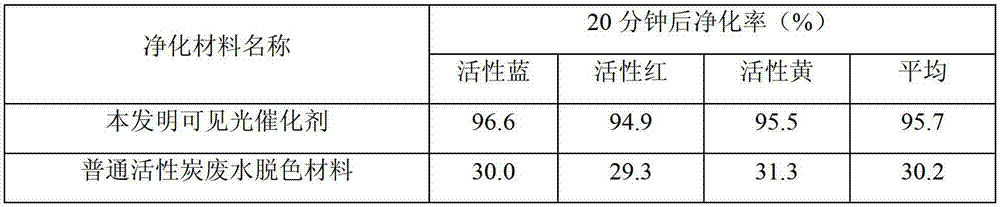
Preparation method of heterogeneous visible photocatalyst
InactiveCN103170353AImprove photocatalytic efficiencyParticle stabilizationPhysical/chemical process catalystsMicro nanoBismuth vanadate
The invention relates to a preparation method of a heterogeneous visible photocatalyst. The preparation method comprises the following steps of: adding bismuth nitrate and a metal chelating agent into solution, dripping mixed solution of metavanadate and polyethylene glycol (PEG), stirring and obtaining solution A; adjusting the pH value of the solution A to be 7.5-10 by using an alkaline agent, stirring under the temperature of 90-100 DEG C to obtain suspension liquid B; carrying out suction filtering, cleaning, drying and calcination on the suspension liquid B to obtain micro-nano particles of bismuth vanadate BiVO4; adding the micro-nano particles of bismuth vanadate BiVO4 into silver nitrate solution, carrying out ultrasonic stirring to obtain solution C; and dripping sulfide into the solution C, carrying out stirring, suction filtering and calcination to obtain the heterogeneous visible photocatalyst. The heterogeneous visible photocatalyst has a good effect to organic pollutants, cannot cause secondary pollution and can be used for a long time.
Owner:DONGHUA UNIV +1
Essentially insoluble heavy metal sulfide slurry for wastewater treatment
A product and method for the removal of pollutant heavy metals from aqueous solutions which precludes the end user from storing, handling, feeding and controlling hazardous soluble sulfide materials. The product is a slurry which includes a mixture of a liquid medium and an essentially insoluble salt wherein the salt is the reaction product of heavy metal ions, preferably selected from Mn++ ions, Fe++ ions, and Fe+++ ions, and sulfide ions derived from soluble sulfide sources such as sodium sulfide, hydrogen sulfide, and sodium hydrosulfide. Addition of the subject slurry to a wastewater stream will effect the precipitation of heavy metals with lesser equilibrium sulfide ion concentrations than that of the essentially insoluble salt. Solids collected by this method may be returned to subsequent wastewater streams for additional removal of heavy metals by any excess heavy metal sulfide salt.
Owner:SOUTHERN WATER TREATMENT
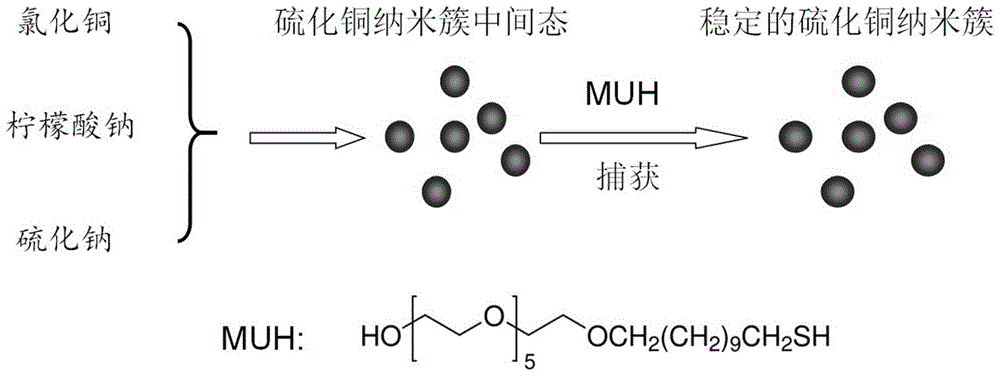
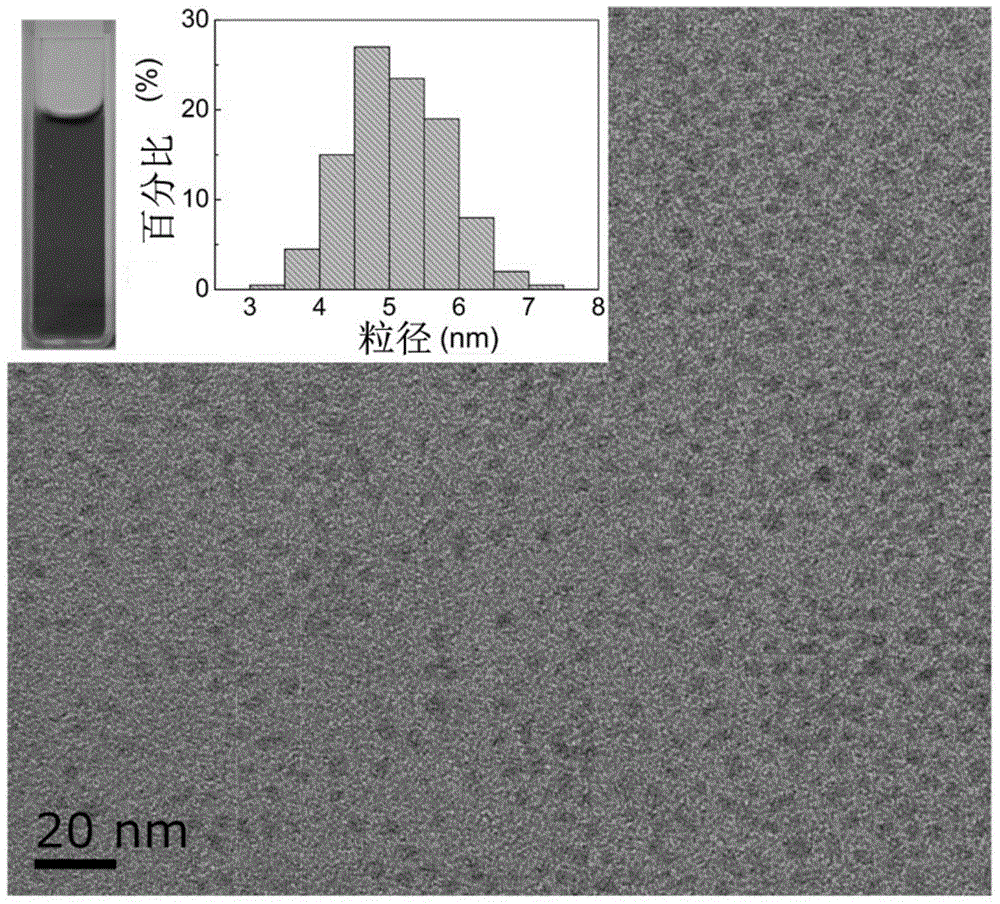
Method for preparing super-stable copper sulfide nano-cluster and application thereof
InactiveCN104609457AImprove stabilityGood dispersionAntibacterial agentsMaterial nanotechnologyDispersityHigh cell
The invention provides a method for preparing a super-stable copper sulfide nano-cluster. The method comprises the following steps: mixing a soluble copper salt solution and a protective agent at room temperature, mixing with a sulfurizing salt solution, thereby obtaining a copper sulfide nano-cluster reaction intermediate state solution; adding a trapping agent into the copper sulfide nano-cluster reaction intermediate state solution, stirring and reacting at room temperature, thereby obtaining a stable copper sulfide nano-cluster solution; dialyzing the stable copper sulfide nano-cluster solution in a dialysis bag, thereby obtaining the pure copper sulfide nano-cluster solution; or the method comprises the following steps: mixing a soluble copper salt solution, a protective agent, a sulfurizing salt solution and a trapping agent, stirring and reacting at room temperature, thereby obtaining MUH-stable copper sulfide nano-cluster solution by virtue of a one-pot process; and dialyzing the MUH-stable copper sulfide nano-cluster solution in a dialysis bag, thereby obtaining the pure copper sulfide nano-cluster solution. The copper sulfide nano-cluster prepared by the method has high stability, high dispersity and high cell and bacteria toxicity and can be used for developing anti-tumor drugs and antibacterial products.
Owner:SOUTHEAST UNIV
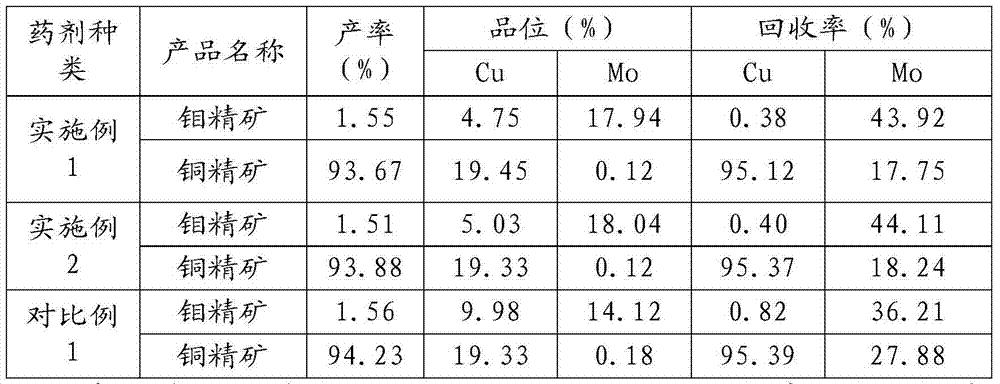
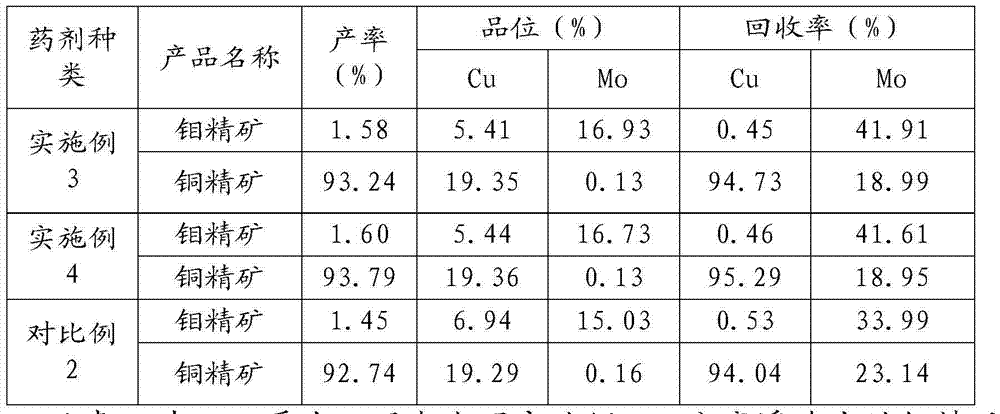
Copper molybdenum separation flotation agent and using method thereof
The invention relates to the technical field of non-ferrous metal processing, in particular to a copper molybdenum separation flotation agent and a using method thereof. The copper molybdenum separation flotation agent comprises inhibitor for inhibiting copper mineral and collecting agent for collecting molybdenum mineral, wherein the inhibitor comprises, by weight, 3 to 5 parts of sodium carbonate, 3 to 5 parts of sulfate, 10 to 15 parts of copper compounds, 15 to 20 parts of sulfide salt, and 15 to 20 parts of hydrosulphide salt; the collecting agent comprises, by weight, 1 to 3 parts of oleic acid, 15 to 25 parts of butyl octyl oleate, and 40 to 50 parts of oil pyrolysis products. The copper molybdenum separation flotation agent and the using method thereof reduce usage amount of the agent, can achieve a certain degree of degradation in waste water if the agents are mutually matched, and thus are more environmentally friendly.
Owner:TIBET HUATAILONG MINING DEV
Sulfur-doping graphene quantum dot, preparation method of sulfur-doping graphene quantum dot and application of lead ion detection
ActiveCN105670619AMonocrystallineApparent selective recognitionMaterial nanotechnologyFluorescence/phosphorescenceSingle crystalElectron microscope
The invention discloses a sulfur-doping graphene quantum dot, a preparation method of the sulfur-doping graphene quantum dot and application of lead ion detection. The preparation method comprises the following steps of dissolving a carbon source compound and a sulfur source compound into water; performing a hydro-thermal reaction under the alkaline condition; preparing the sulfur-doping graphene quantum dot, wherein the carbon source compound is 1,3,6-trinitro pyrene, and the sulfur source compound is soluble sulfide salt. The prepared sulfur-doping graphene quantum dot has excitation wavelength independence; when the excitation wavelength of 410 to 490nm is used for excitation, the fluorescence emission peak position is not changed; lattice lines can be obviously seen in a high resolution transmission electron microscope; the result proves that the sulfur-doping graphene quantum dot synthesized by the invention has single crystal performance; the fluorescence of the substance is quenched by lead ions; the result shows that the obvious selective recognition capability on lead ions is realized; the sulfur-doping graphene quantum dot is hopeful to be used for trace lead ion selective detection.
Owner:广州顺倬能源科技有限公司
Essentially insoluble heavy metal sulfide slurry for wastewater treatment
InactiveUS20050173350A1Water treatment parameter controlCell electrodesLiquid mediumSodium hydrosulfide
A product and method for the removal of pollutant heavy metals from aqueous solutions which precludes the end user from storing, handling, feeding and controlling hazardous soluble sulfide materials. The product is a slurry which includes a mixture of a liquid medium and an essentially insoluble salt wherein the salt is the reaction product of heavy metal ions, preferably selected from Mn++ ions, Fe++ ions, and Fe+++ ions, and sulfide ions derived from soluble sulfide sources such as sodium sulfide, hydrogen sulfide, and sodium hydrosulfide. Addition of the subject slurry to a wastewater stream will effect the precipitation of heavy metals with lesser equilibrium sulfide ion concentrations than that of the essentially insoluble salt. Solids collected by this method may be returned to subsequent wastewater streams for additional removal of heavy metals by any excess heavy metal sulfide salt.
Owner:SOUTHERN WATER TREATMENT
Preparation method and application of alumina-loaded nano ferrous sulfide composite material
InactiveCN106732330AGood removal effectEffectively distributes the load evenlyOther chemical processesWater contaminantsIron(II) oxideNanoparticle
The invention belongs to the technical field of water treatment, and in particular relates to a preparation method and application of an alumina-loaded nano ferrous sulfide composite material; the alumina-loaded nano ferrous sulfide composite material is mainly used for treating mercury-containing wastewater. The preparation method of the alumina-loaded nano ferrous sulfide composite material comprises the steps of preparing ferrous sulfide particle suspension liquid by taking a sulfide salt and a divalent ferric salt as raw materials and using a coprecipitation method in the N2 environment; after that, continuously adding alumina suspension liquid, which is taken as a carrier, into the ferrous sulfide particle suspension liquid, and enabling nano ferrous sulfide particles to completely cover the surface of alumina under the conditions of ultrasonic stirring and heating so as to obtain the alumina-loaded nano ferrous sulfide composite material. The alumina-loaded nano ferrous sulfide composite material has the beneficial effects that (1) the preparation method is simple, convenient and efficient, and the material can be synthesized in one step by using the coprecipitation method and is short in preparation period, low in cost and good in effect; (2) by enabling the alumina to be loaded in the composite material, ferrous oxide nanoparticles are effectively dispersed, and the defects that the ferrous oxide nanoparticles are easy to agglomerate and easy to oxidize are overcome.
Owner:ZHEJIANG UNIV
Method of producing beta-mercaptocarboxylic acids
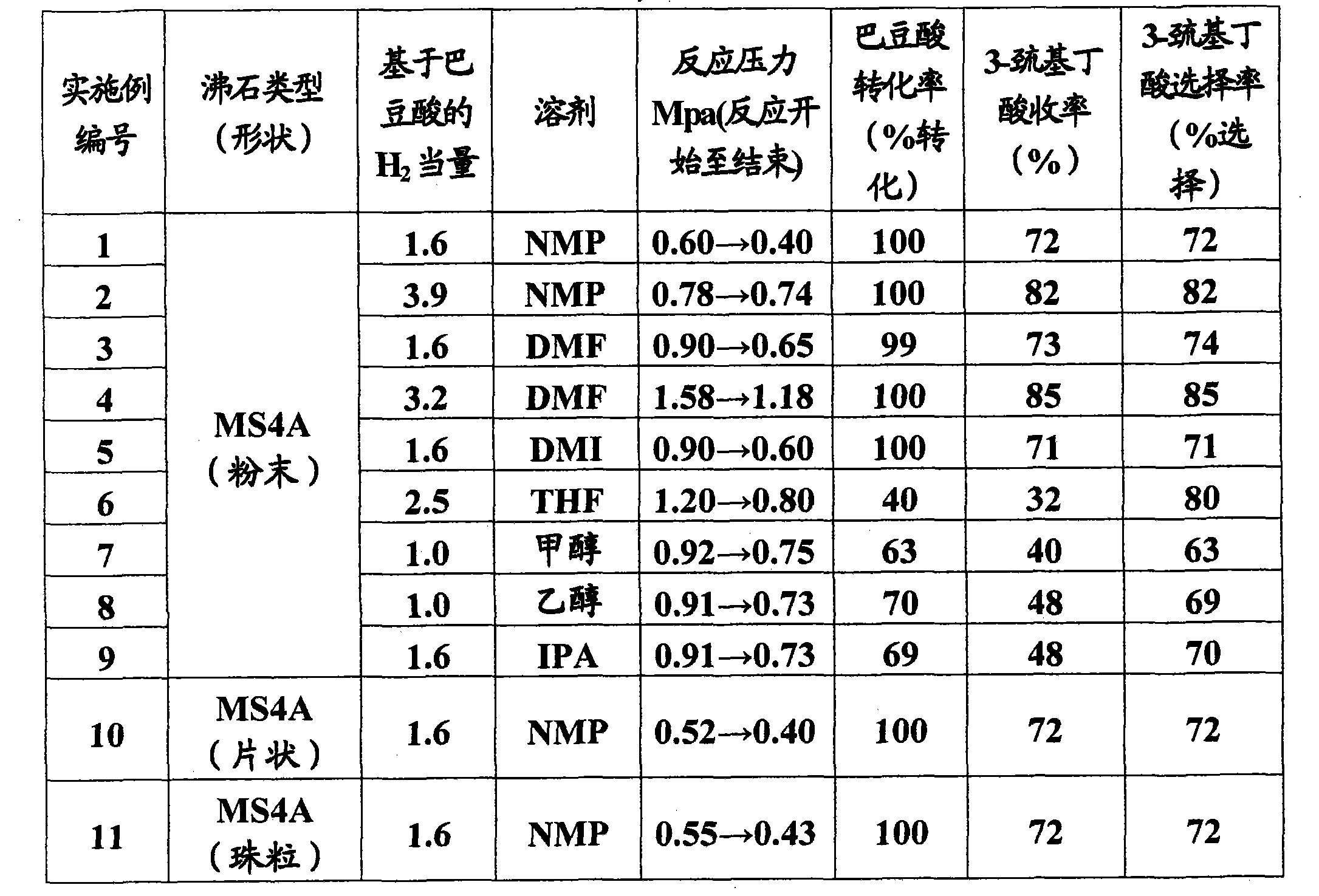
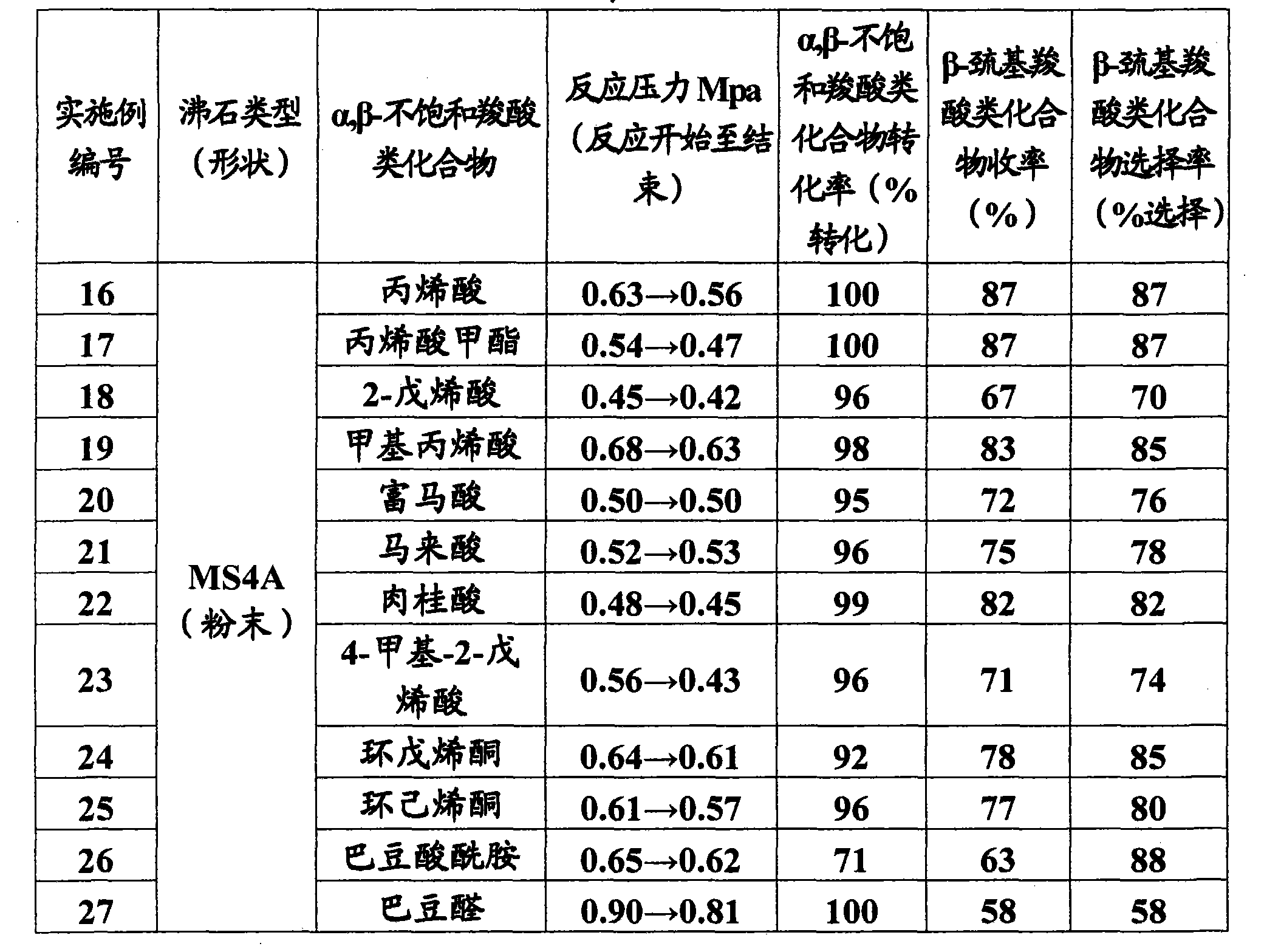
InactiveCN101801922AMercapto/sulfide group formation/introductionThiol preparationHydrogenSynthetic materials
The invention relates to a method for efficiently producing beta-mercaptocarboxylic acids using a solid acid catalyst such as zeolite, which product corresponds to respective starting materials selected from alpha, beta-unsaturated carboxylic acids (alpha, beta-unsaturated carboxylic acid, alpha, beta-unsaturated carboxylic acid ester, alpha, beta-unsaturated amide, alpha, beta-unsaturated aldehide and alpha, beta-unsaturated ketone) and hydrogen sulfides (hydrogen sulfide, sulfide salt and hydrosulfide salt), wherein a solvent compatible with water is used in the reaction. According to the invention, beta-mercaptocarboxylic acids which are useful as additives in synthetic materials for pharmaceutical or agricultural agents and in polymer compounds can be industrially produced efficiently by using easily available alpha, beta-unsaturated carboxylic acid (such as crotonic acid) at high yield.
Owner:RESONAC HOLDINGS CORPORATION
Separation of co2 and h2s using supported amines
Methods are provided for removing CO2 and / or H2S from a gas phase stream, such as a refinery flue gas stream, a coal-fired or petroleum-burning power plant, or a natural gas stream. A gas phase stream containing CO2 and / or H2S can be contacted under effective conditions with an aqueous slurry of supported amine particles. The CO2 and / or H2S can react with the supported amines to form bicarbonates, carbonates, carbamates, sulfide salts, or other species. Because the amine is part of, bonded to, or otherwise supported on a particulate substrate, the reaction product from the amine reaction can also remain bound to the particle. After reacting supported amines with CO2 and / or H2S captured from a gas stream, the supported amines particles can be separated from the aqueous slurry environment for regeneration of the supported amine and release of the CO2 and / or H2S.
Owner:EXXON RES & ENG CO
Z-type photocatalyst, preparation method and application thereof
ActiveCN109967110AImprove transfer efficiencyImprove photocatalytic performanceCatalyst activation/preparationCarbon layerQuantum dot
Relating to the field of photocatalysis technology, the invention specifically provides a Z-type photocatalyst, a preparation method and application thereof. The Z-type photocatalyst is a composite material, and includes C3N4, a carbon layer coated on the surface of C3N4 and a CdS quantum dot layer coated on the surface of the carbon layer. The preparation method includes: conducting coating treatment on a carbon source in a suspension of C3N4 to prepare a C3N4-carbon source; in an inert atmosphere, firstly adding mercapto acid and a cadmium salt into the C3N4-carbon source, then adding a sulfide salt to prepare C3N4-carbon source-CdS; and subjecting the C3N4-carbon source-CdS to calcinations treatment to convert the carbon source into a carbon layer, thus obtaining the Z-type photocatalyst. The Z-type photocatalyst provided by the invention has good performance of photodegradation of organic pollutants and photocatalytic hydrogen production.
Owner:TCL CORPORATION
Method for removing heavy metals in water by collaboration of dialysis through ion exchange membrane and chemical precipitation
InactiveCN102557195AReduce energy consumptionSimple structureWater/sewage treatment bu osmosis/dialysisWater/sewage treatment by flocculation/precipitationHigh concentrationPhysical chemistry
The invention provides a method for removing heavy metal ions in water. The technical principle is as follows: pollutants, namely the heavy metal ions in the water are permeated from a high-concentration side to a low-concentration side by utilizing the exchange role of the ion exchange membrane, and an alkaline or carbonate or sulfide salt solution is applied to the low-concentration side, so that the heavy metal ions can form an insoluble hydroxide or carbonate or sulfide salt for being precipitated and being further removed. According to the method disclosed by the invention, the processes of membrane separation and chemical precipitation are organically linked together, so that membrane dialysis and precipitation removal are synchronously performed. Compared with the traditional method, the method disclosed by the invention has the advantages that a reactor has a simple structure, the operation is easy and the application prospect is broad.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Method for removing foul odor of sulfur-containing organic wastewater
InactiveCN109354264ARealize clean governanceRealize resource processingWater treatment parameter controlWater treatment compoundsHigh concentrationAlkaline hydrolysis
The invention relates to the technical field of wastewater treatment, and discloses a method for removing foul odor of sulfur-containing organic wastewater. The method includes steps of (1), regulating the pH (potential of hydrogen) of the sulfur-containing organic wastewater until the sulfur-containing organic wastewater is acidic, adding metal salt and hydrogen peroxide into the sulfur-containing organic wastewater and carrying out advanced oxidation treatment on the sulfur-containing organic wastewater to obtain treatment fluid I; (2), regulating the pH of the treatment fluid I until the pHof the treatment fluid I reaches 10 at least, stirring the treatment fluid I and carrying out alkaline hydrolysis; (3), carrying out solid-liquid separation by means of filtering after alkaline hydrolysis is carried out so as to obtain filter residues and treatment fluid II, regulating the pH of the treatment fluid II and then carrying out follow-up treatment; (4), adding the filter residues intoacid solution, stirring the filter residues to obtain hydrogen sulfide gas and treatment fluid III, and reusing the treatment fluid III for regulating the pH of the sulfur-containing organic wastewater and supplementing metal ions at the step (1); (5), absorbing the hydrogen sulfide gas by alkaline liquor to obtain sulfide salt. The method has the advantages that high-salinity and high-concentration wastewater with a high sulfur content and organic matters can be treated by the aid of the method, and sulfur further can be recycled and reused.
Owner:ZHEJIANG QICAI ECO TECH CO LTD
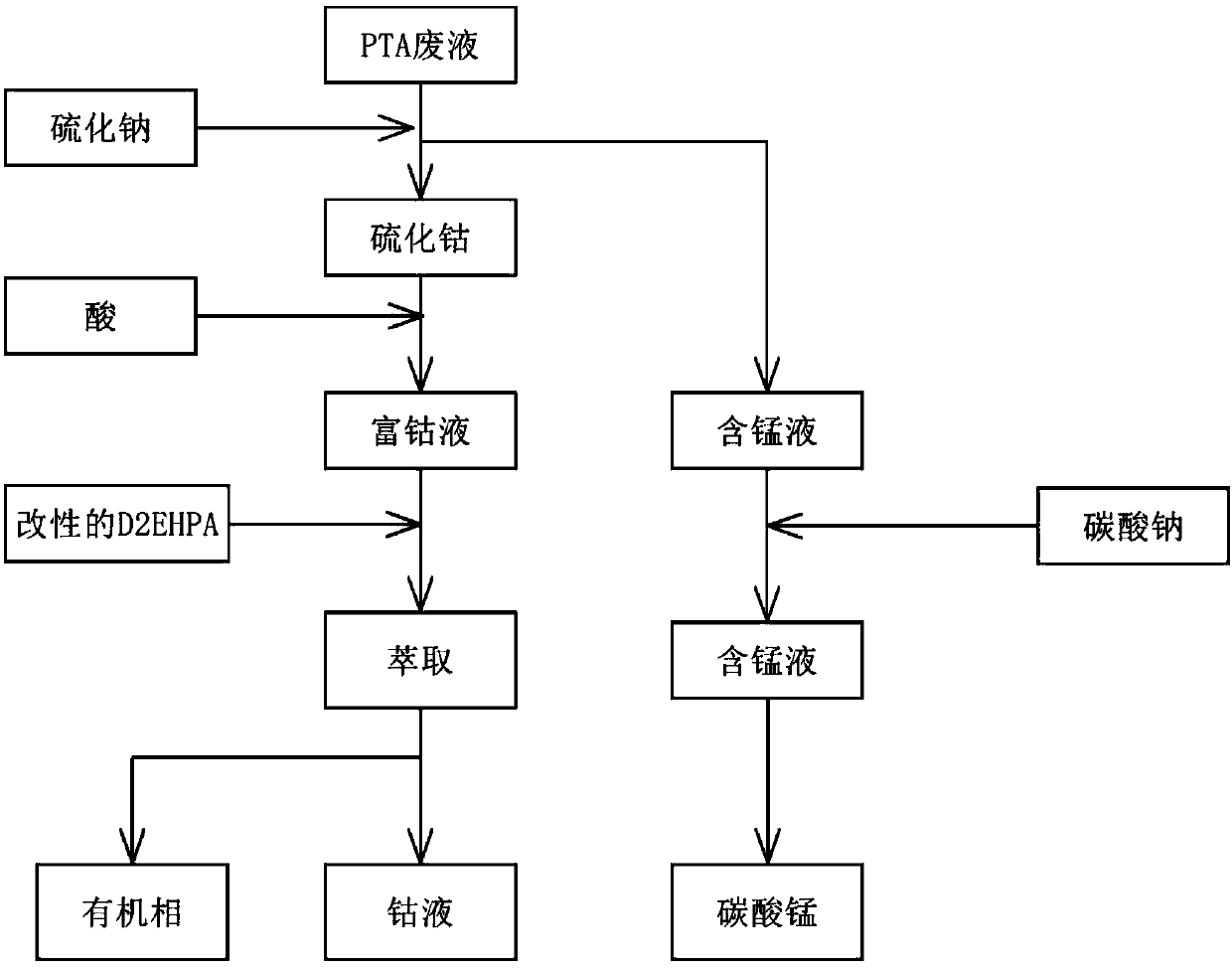
Method for separating cobalt and manganese in cobalt and manganese wastes
ActiveCN107904402AHigh recovery rateSimple processProcess efficiency improvementVulcanizationFiltration
The invention discloses a method for separating cobalt and manganese in a waste cobalt-manganese catalyst by a vulcanization precipitation and modified D2EHPA extraction combined technology. The method comprises the following steps: 1, Co-D2EHPA is prepared; 2, the pH value of wastes is controlled to be less than or equal to 3.5; 3, impurities are removed; 4, a sulfide salt solution is prepared; 5, the sulfide salt solution is added into waste liquid, after adding is completed, the reaction liquid reacts for 0.5-3 hours, and after the reaction is ended, filtering is carried out obtain a cobaltsulfide precipitate and a manganese-containing solution respectively; and 6, a reducing agent is added into the manganese-containing solution to react, suction filtration is conducted after ending, precipitate is washed and dried after suction filtration to obtain a manganese precipitate, and the manganese recovery is completed. The obtained cobalt sulfide precipitates through filtration are dissolved by acid, modified Co-D2EHPA is added into the solution, and multi-stage extraction and reverse extraction is carried out, so that the obtained solution contains only cobalt.
Owner:JIANGSU UNIV OF TECH
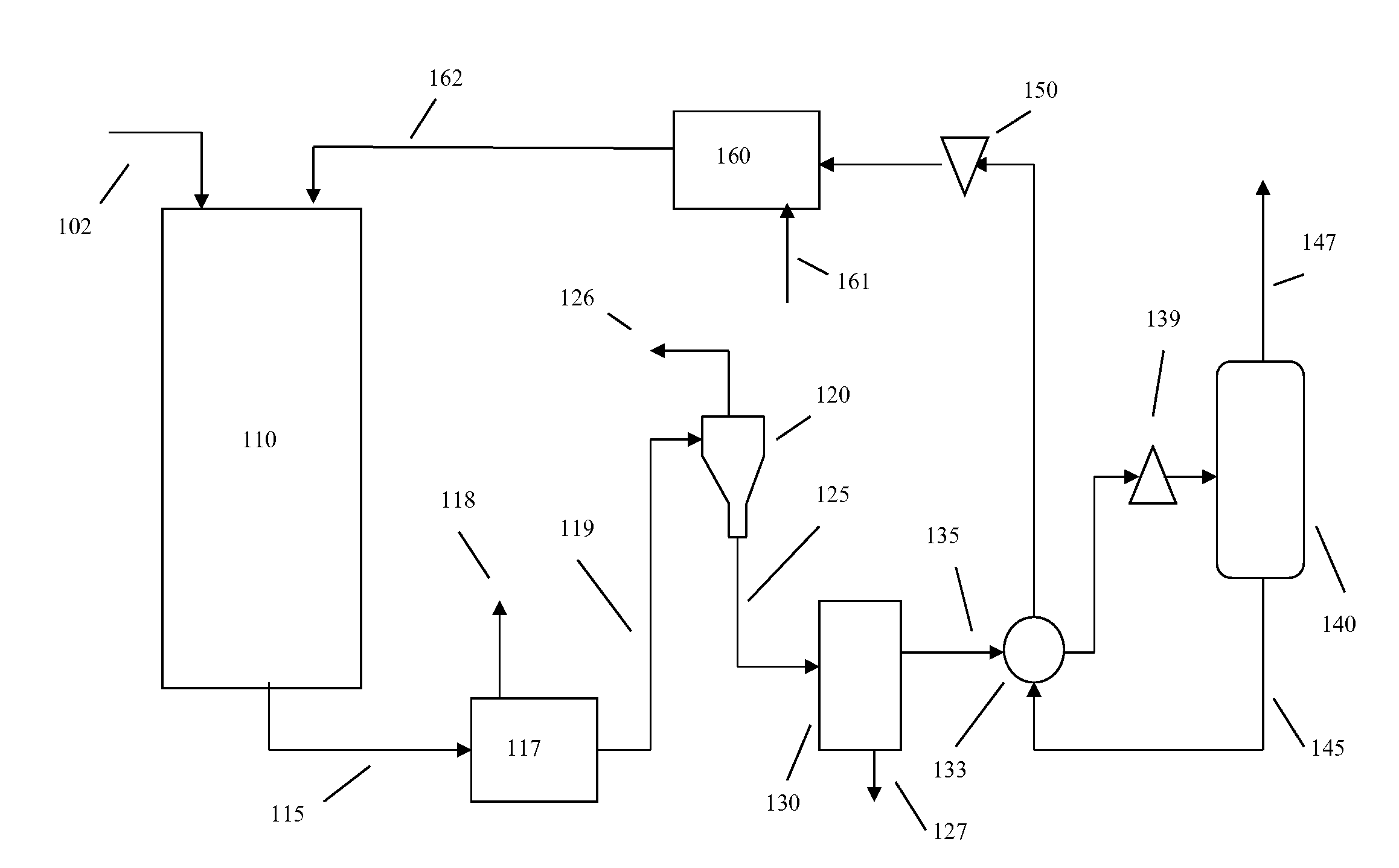
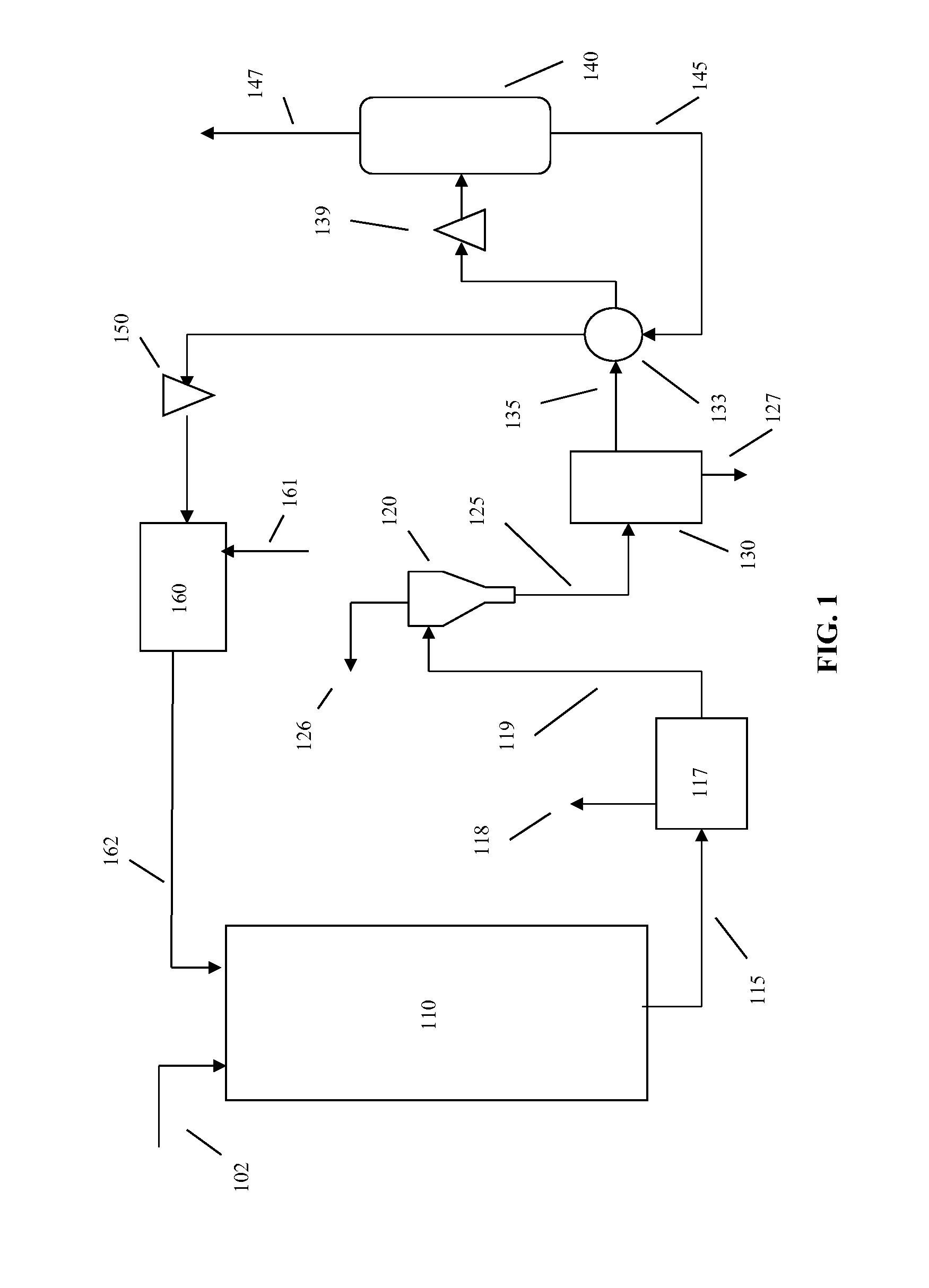
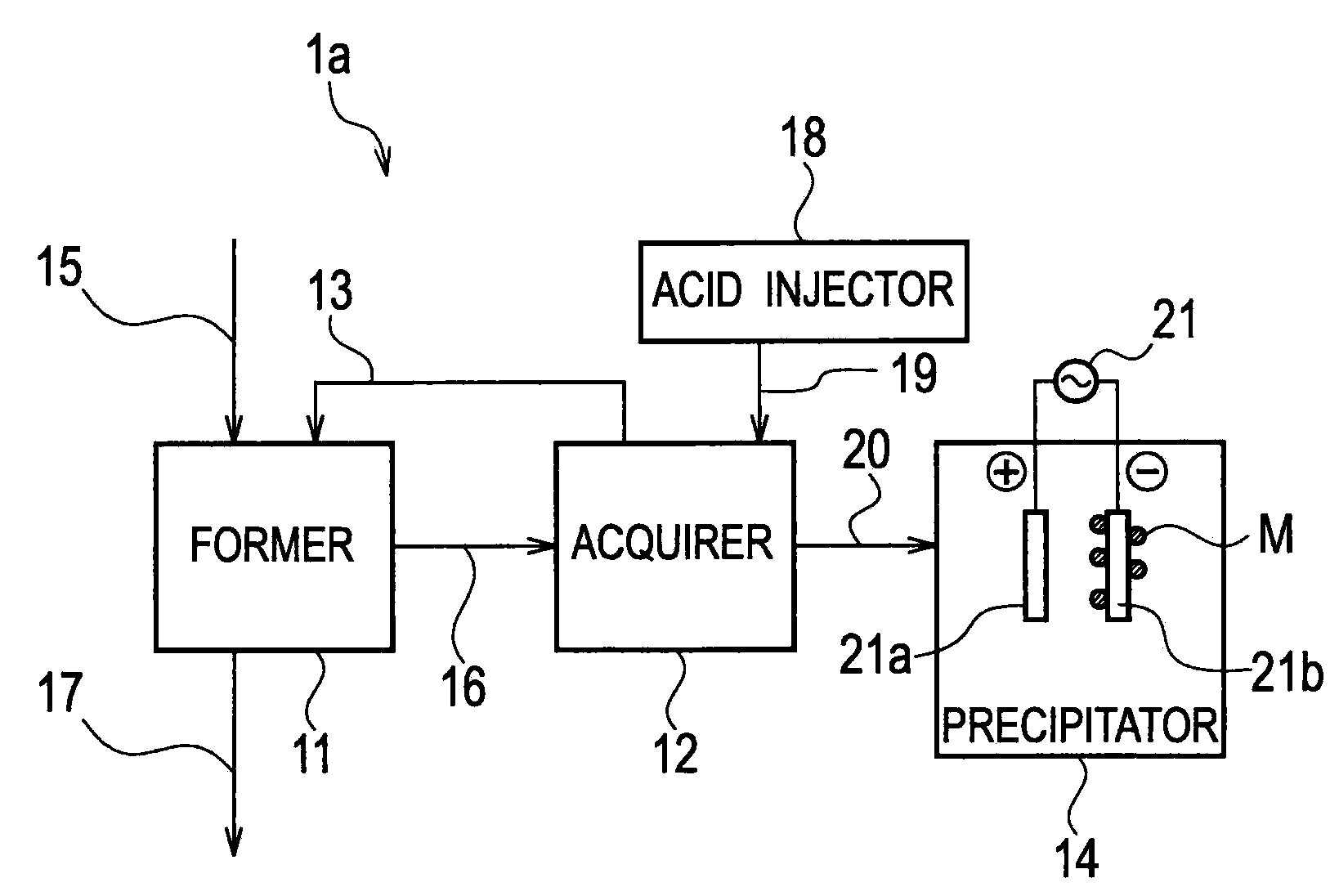
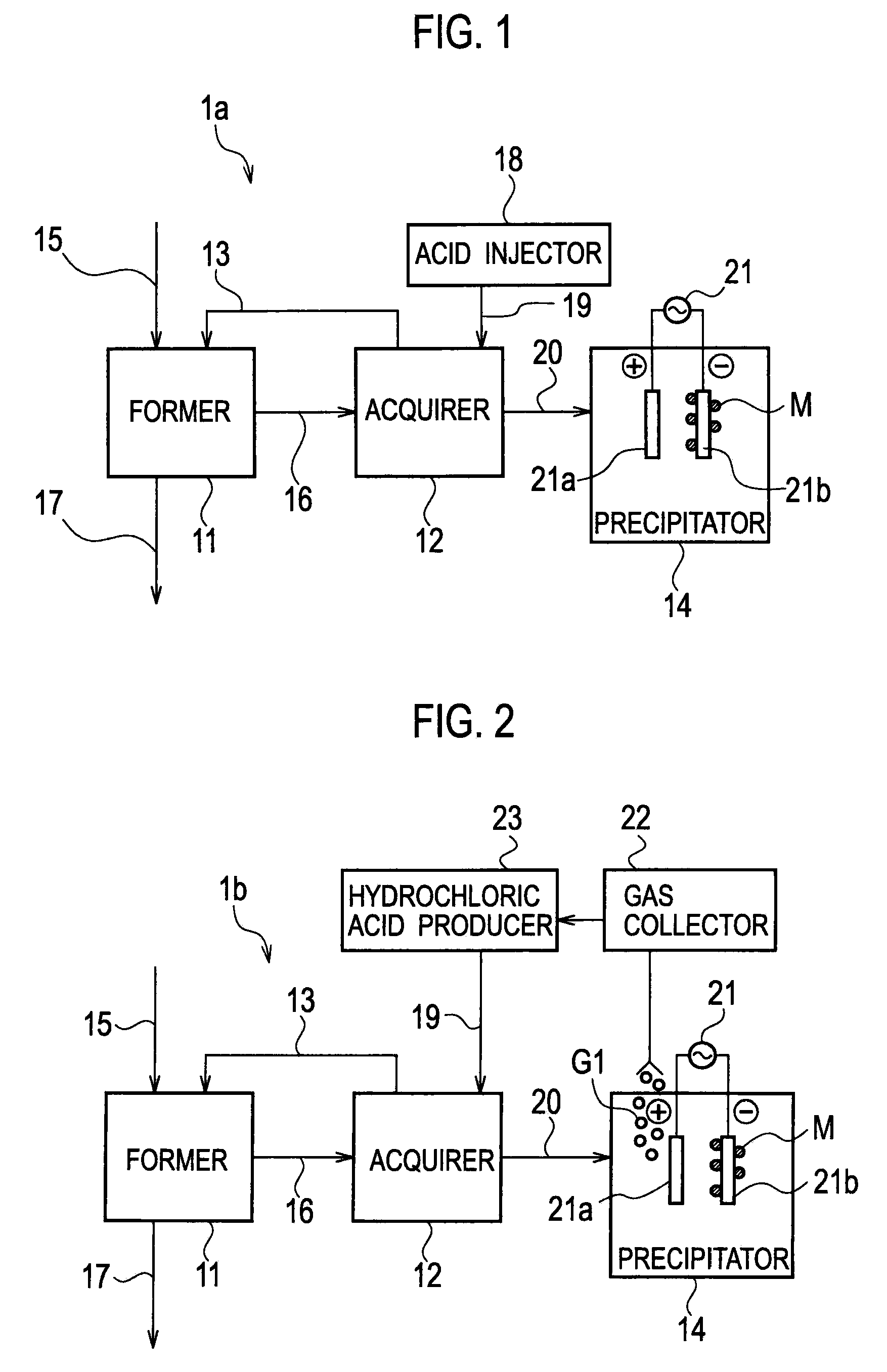
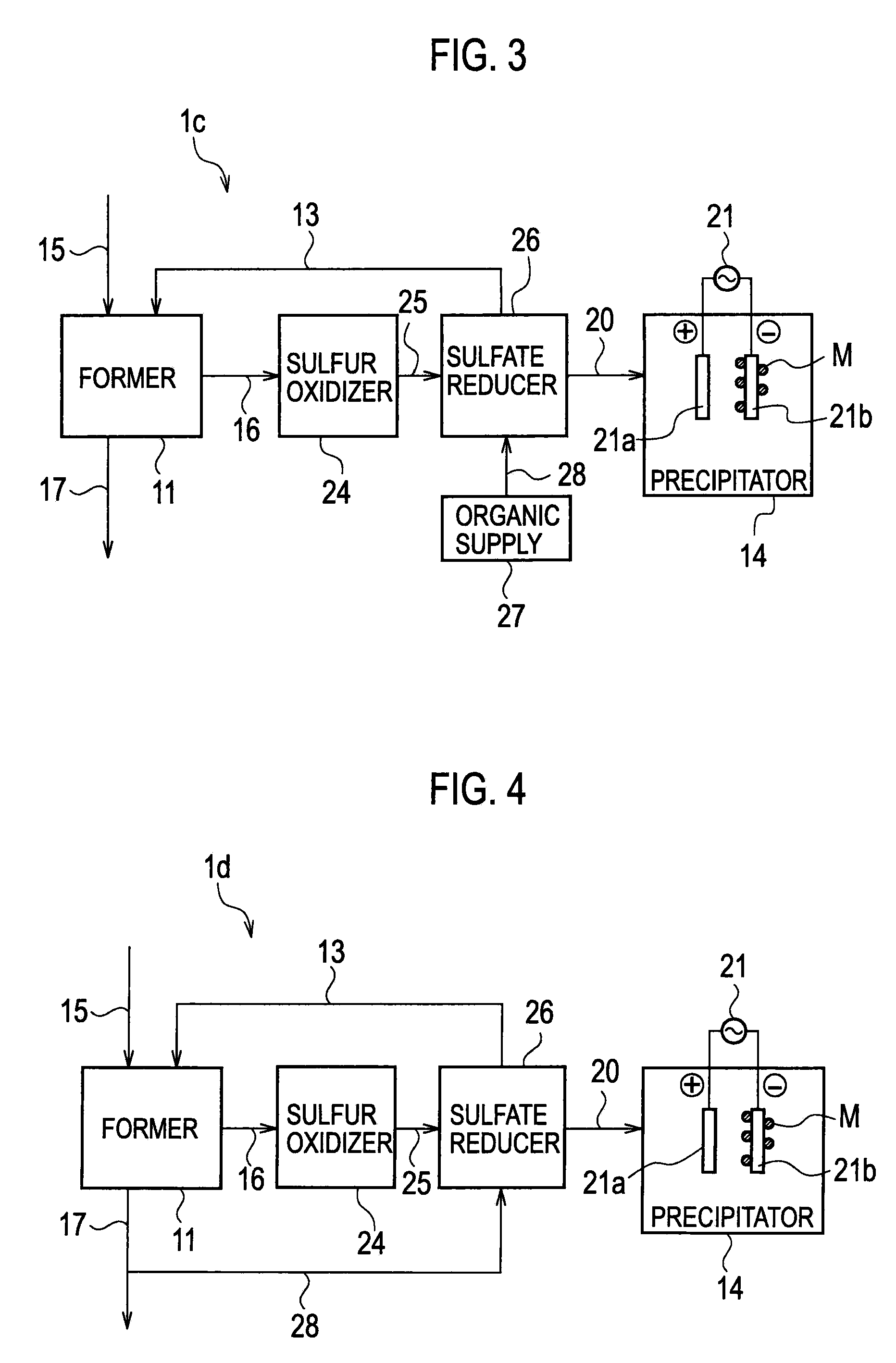
Heavy Metal Collection System and Heavy Metal Collection Method
InactiveUS20090065441A1Easy to useElectrostatic separatorsSludge treatmentCollection systemWastewater
A former (11) works, when supplied with wastewater containing heavy metals and hydrogen sulfide, to react heavy metals contained in wastewater with hydrogen sulfide to form sulfide salts, an acquirer (12) works, when supplied with sulfide salts and acid, to acquire hydrogen sulfide and heavy metal ions produced by reactions between sulfide salts and acid, a hydrogen sulfide supply line (13) supplies hydrogen sulfide acquired by the acquirer to the former, and a collection line (20) collects heavy metal ions.
Owner:KK TOSHIBA
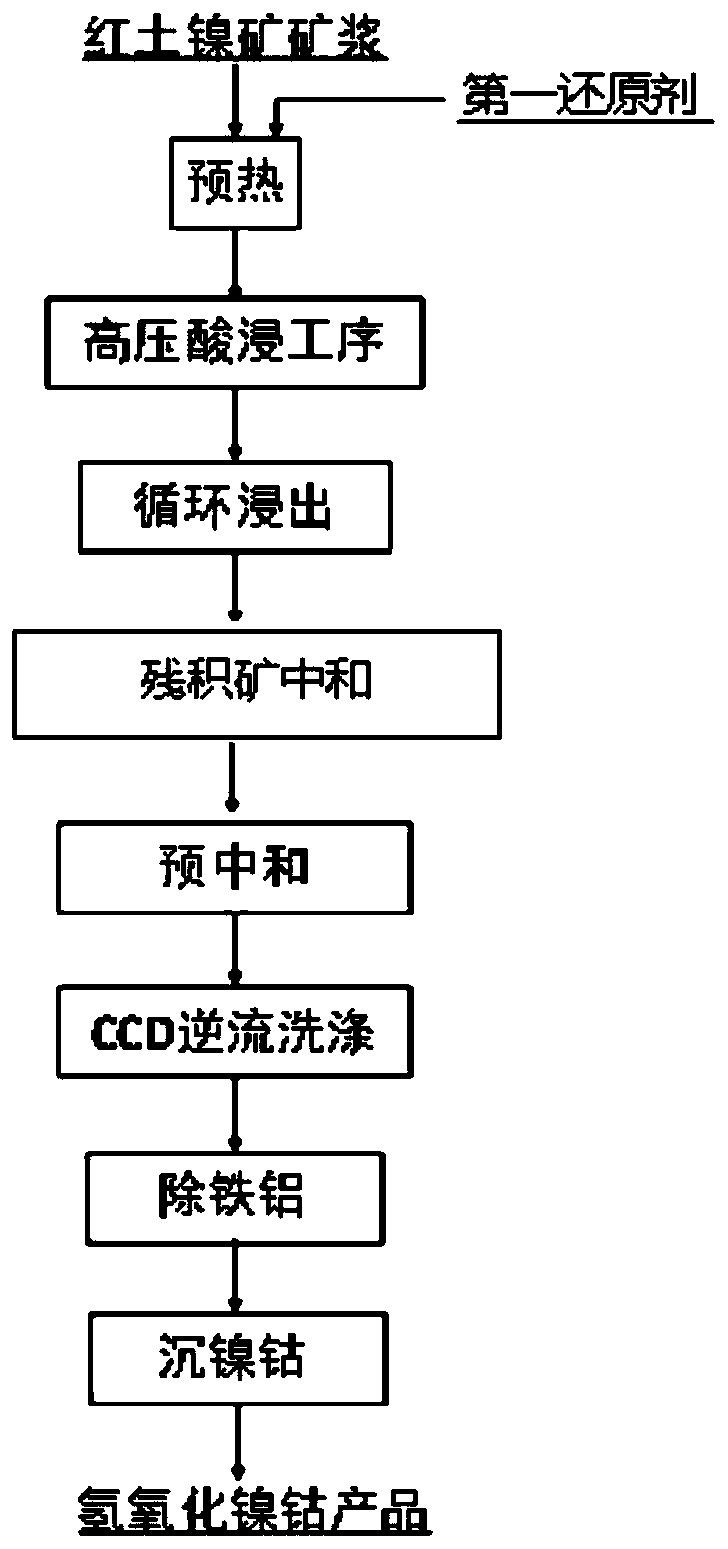
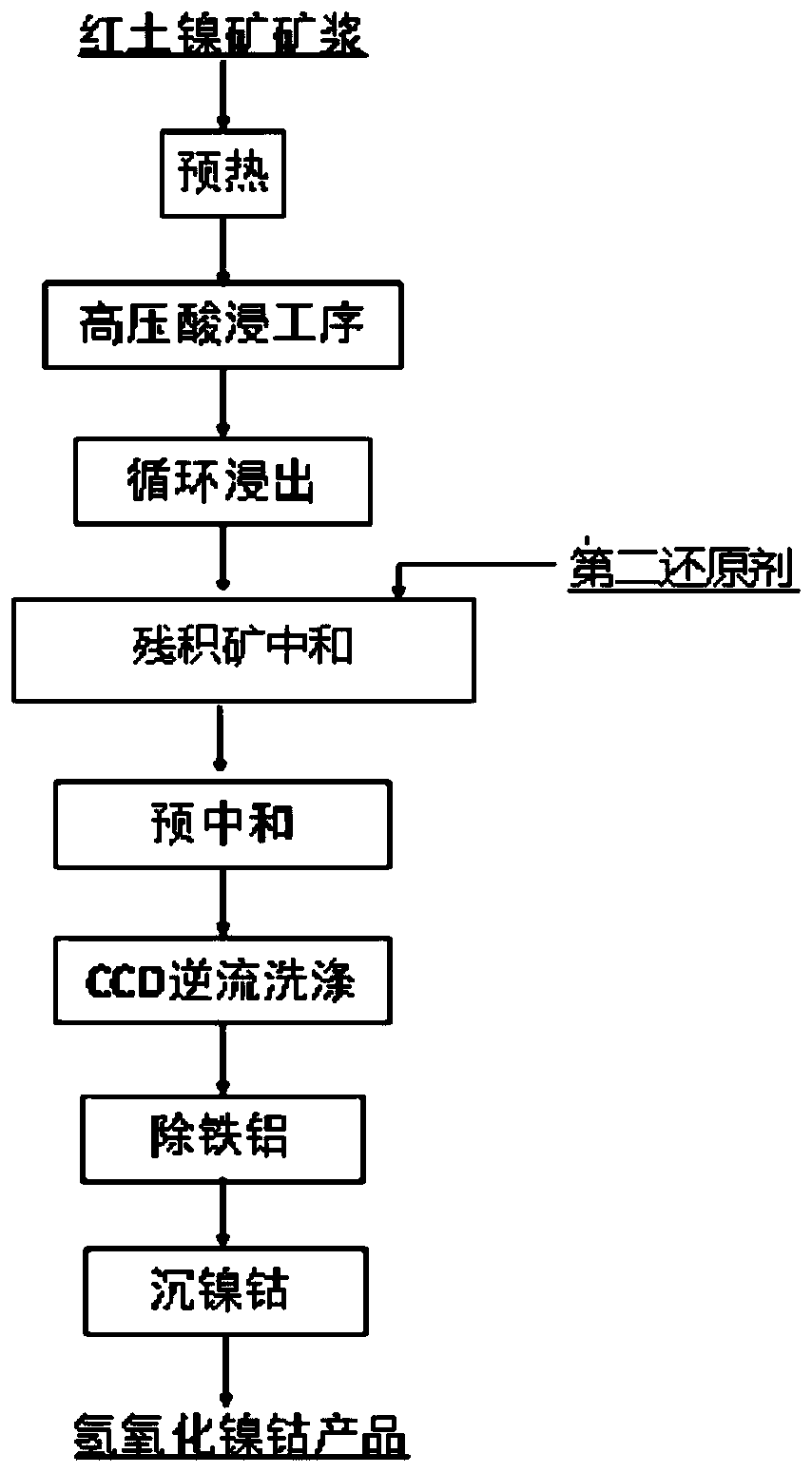
Method for removing hexavalent chromium in process of preparing cobalt nickel hydroxide from laterite-nickel ore
The invention provides a method for removing hexavalent chromium in the process of preparing cobalt nickel hydroxide from laterite-nickel ore. The method comprises the steps that before the high-pressure acid leaching step, laterite-nickel ore pulp is preheated, before the preheating step, a first reducing agent is added into the laterite-nickel ore pulp, and then the laterite-nickel ore pulp added with the first reducing agent is made to enter the high-pressure acid leaching step; wherein the first reducing agent is one or more of pyrite, sulfur and chromite, or organic wastewater; or, a second reducing agent is added into the leachate obtained in the cyclic leaching step, and then the residual ore neutralization step is executed; and the second reducing agent is one or more of sulfite, pyrosulfite, ferrite, thiosulfate, sulfide salt and bisulfite of alkali metal or alkaline-earth metal. By utilizing the method provided by the invention, hexavalent chromium can be removed from the source on the basis of not changing the high-pressure leaching procedure of the laterite-nickel ore, so that the nickel-cobalt hydroxide product is prevented from being carried out, and the original steps are not influenced.
Owner:CHINA ENFI ENGINEERING CORPORATION
Method for preparing copper nanocubes
The invention relates to a method for preparing copper nanocubes. A seeding growth method is used for preparing copper nanocubes of different side lengths; firstly, ethylene glycol is used as a solvent and reducing agent, PVP as a stabilizer, copper trifluoroacetate as a copper source, and hydrochloric acid and soluble sulfide salt as auxiliary agents to prepare copper cube seeds with an average side length of 35 nm; the ethylene glycol is used as the solvent and reducing agent, the PVP as the stabilizer, and the soluble copper salt as the copper source to obtain copper nanocubes with an average side length of 40 to 160 nm by controlling the relative using amount of seeds and copper salt. According to the method for preparing the copper nanocubes, operation is simple, the process is controllable, and stability of the obtained copper nanocubes is high, the method for preparing the copper nanocubes is an efficient copper nanocube synthesizing method and thus has broad market prospect.
Owner:黄帅
Manufacturing method and application of silver nano cubic particle
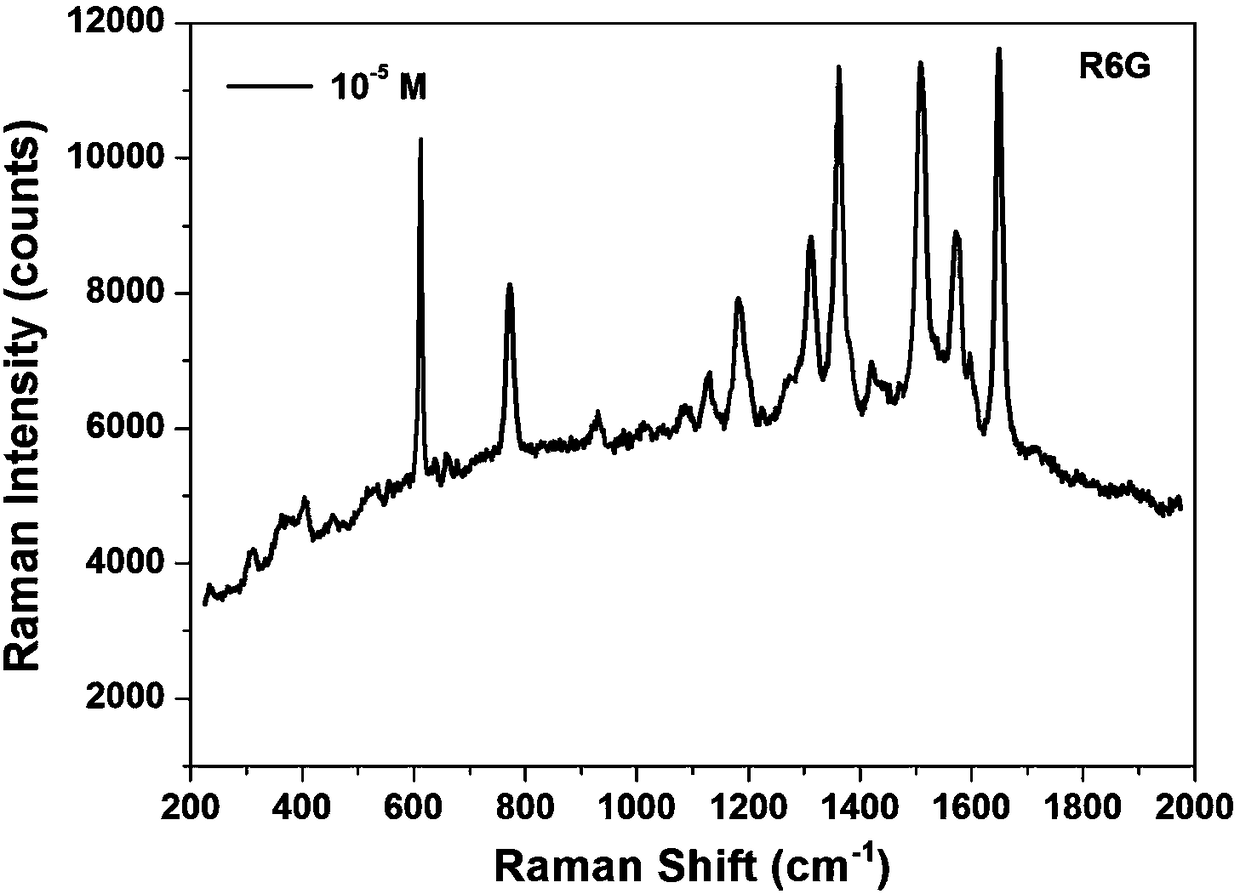
InactiveCN108465826ARegular structureRegular shapeRaman scatteringNanotechnologyHigh activitySulfide salt
The invention provides a preparation method and application of a silver nano cubic particle. The preparation method comprises the following steps of: 1) magnetically stirring and heating ethylene glycol for 1-3 hours at rotation speed being 200-300 rpm; 2) separately adding sulfide salt and polyvinylpyrrolidone into the ethylene glycol treated in step 1), thereby obtaining a 2-4 mM sulfide salt ethyl glycol solution and a 2-4 mM polyvinylpyrrolidone ethyl glycol solution; 3) under the stirring state, mixing the sulfide salt ethyl glycol solution with the polyvinylpyrrolidone ethyl glycol solution after dropwise adding to obtain a reaction solution; 4) adding silver nitrate into ethyl glycol treated in step 1) to obtain 40-50 mg / mL of silver nitrate ethyl glycol solution; and 5) under the stirring state, adding the silver nitrate ethyl glycol solution into the reaction solution in the step 3), and ending reaction when color of the reaction solution is changed to be red brown, thereby obtaining the silver nano cubic particle. The silver nano cubic particle prepared by the method is controllable in morphology, is stable in structure, and has very high activity in aspect of SERS.
Owner:JIANGHAN UNIVERSITY
Low-sulfur polysulfide silanes and process for preparation
An improvement on existing methods to desulfurize oligosulfide silanes generates no added raw materials or waste streams in addition to the sodium chloride which is generated as a byproduct in the current manufacturing process for polysulfide silanes. This is accomplished by using mercaptosilane salts as desulfurizing agents, which yield more product in addition to that already obtained from the desulfurized silane of higher sulfur rank. The process simply entails reacting an oligosulfide silane with a mercaptosilane salt to thereby obtain an oligosulfide silane whose average sulfur rank is less than the starting oligosulfide silane. Coproduct sulfide salts can be recycled back to the oligosulfide manufacturing process. The mercaptosilane salts are novel materials as well.
Owner:GENERAL ELECTRIC CO
Heavy metal repairing agent as well as preparation method and application thereof
ActiveCN114316994AImprove repair effectAchieve removalWater contaminantsContaminated soil reclamationReduction treatmentMetal particle
The invention provides a heavy metal repairing agent and a preparation method and application thereof, the heavy metal repairing agent is iron-based sulfurized polymetallic, and comprises a zero-valent iron core and ferrous sulfide and polymetallic particles covering the surface of the zero-valent iron core; the multi-metal particles comprise at least two types of metal elements other than iron. The preparation method comprises the following steps that (1) iron-containing waste residues are sequentially subjected to pretreatment, reduction treatment and acid pickling treatment, and iron-based multi-metal is obtained; (2) preparing a buffer solution, removing dissolved oxygen in the solution, and sealing a solution system; and (3) alkali metal sulfide salt, the iron-based multi-metal obtained in the step (1) and the buffer solution obtained in the step (2) are mixed, solid-liquid separation is conducted after vulcanization reaction, and the iron-based multi-metal sulfide, namely the heavy metal repairing agent, is obtained. According to the heavy metal remediation agent provided by the invention, efficient removal of various heavy metal pollutants is realized, meanwhile, the preparation cost is reduced, and the application prospect of the heavy metal remediation agent in the field of water and soil remediation is widened.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Gel polymer binder for lithium sulfur battery electrode material and preparation method thereof
ActiveCN104319404AEasy to operateImprove adhesionElectrode manufacturing processesPtru catalystPolyethylene glycol
The invention discloses a gel polymer binder for a lithium sulfur battery electrode material and a preparation method thereof. The preparation method comprises the following steps: (1) weighing 1,3-dichloro-2-propanol and polyethylene glycol, uniformly mixing, introducing N2 to deoxygenize, adjusting temperature, keeping constant temperature, adding a catalyst to accelerate a polymerization reaction, extracting a target product 1,3-dichloro-2-propyl alcohol polyethylene glycol ether, and then adjusting the pH value of the solution with an alkaline solution; (2) weighing a sulfosalt, completely dissolving the sulfosalt in distilled water, adjusting temperature, keeping constant temperature, and adding elemental sulfur to prepare a polysulfide salt; (3) mixing a product 1,3-dichloro-2-propyl alcohol polyethylene glycol ether in the step (1) and a product polysulfide salt in the step (2), adjusting temperature, keeping constant temperature, taking out a gel-like substance, and rotatably evaporating at a certain temperature to obtain a gel polymer binder for a lithium sulfur battery. The gel polymer binder prepared by the invention has the characteristics of easiness in operation, good bonding effect, high electrode active material utilization rate, good battery rate capability and the like.
Owner:CHANGZHOU UNIV
Desulfurization control method and device, storage medium and electronic equipment
ActiveCN111140319AExtend regeneration timeTo achieve the effect of desulfurization in advanceGas treatmentInternal combustion piston enginesSulfidationProcess engineering
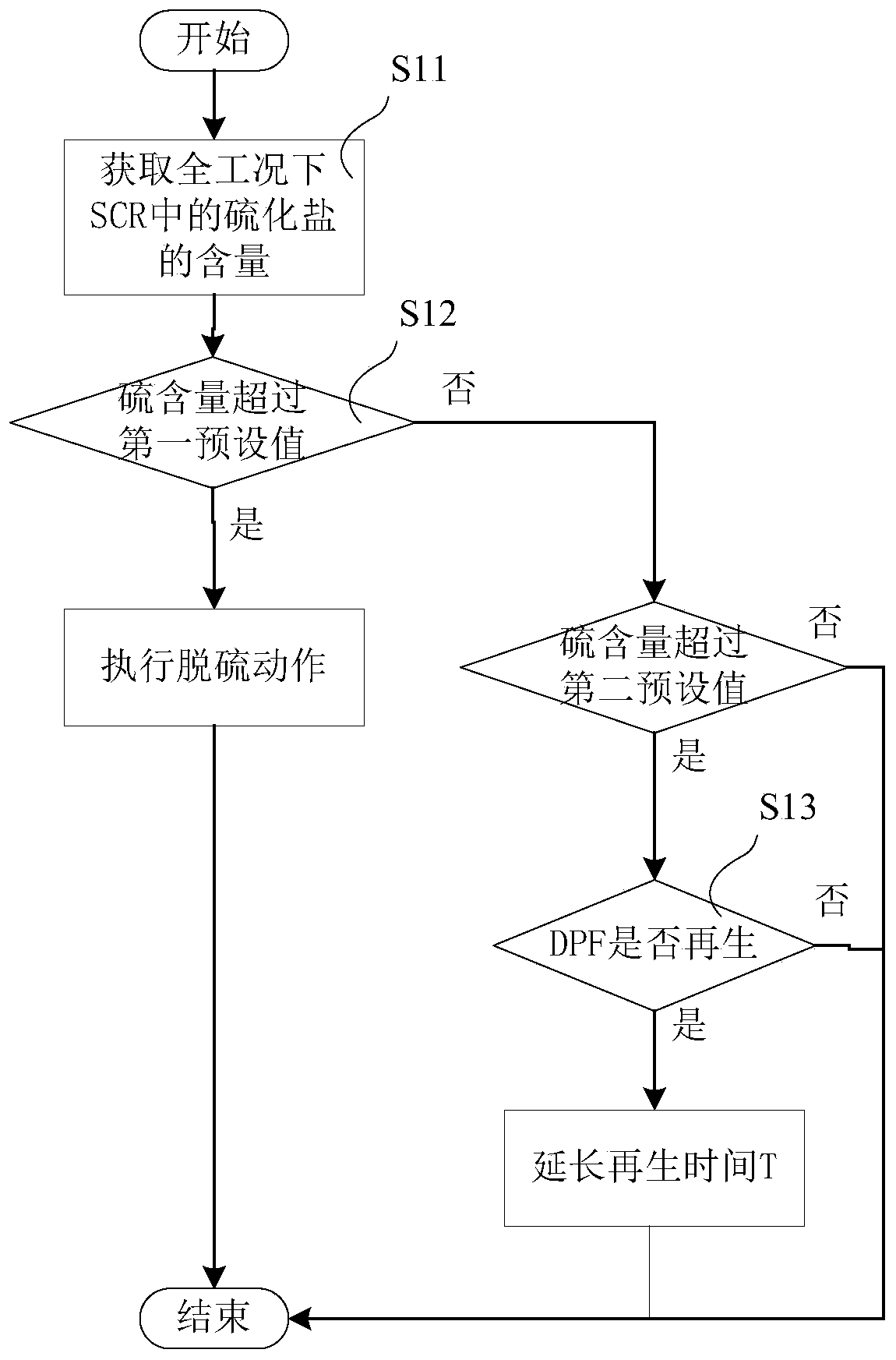
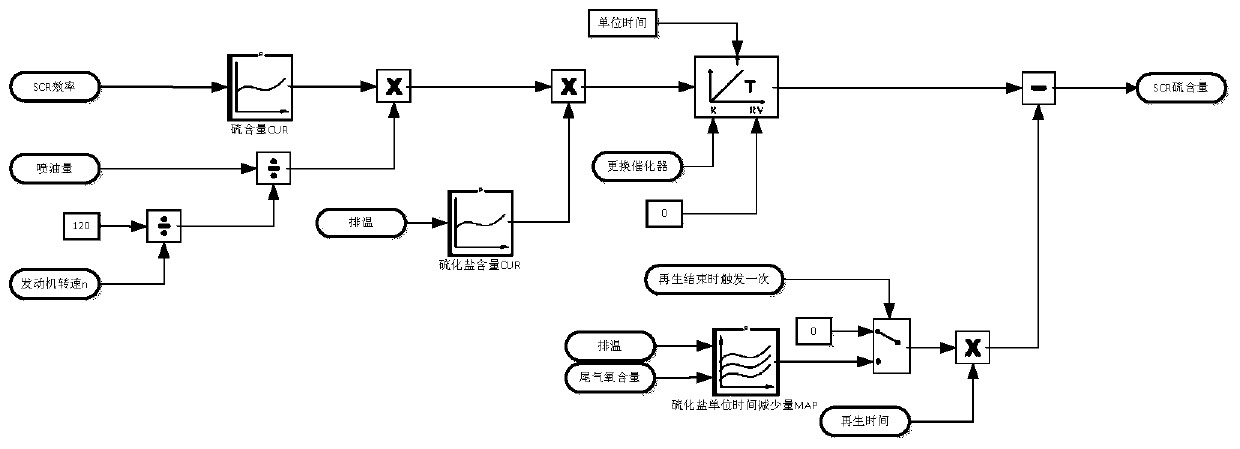
The embodiment of the invention provides a desulfurization control method and device, a storage medium and electronic equipment. The method comprises the following steps that firstly, the content of sulfide salt in SCR is obtained under all working conditions; whether the content of the sulphide salt is greater than a first preset value or not is judged, if so, a desulfurization action is executed, if not, whether the content of the sulphide salt is greater than a second preset value or not is judged, if so, a regeneration state of DPF is obtained, and if the regeneration state is regeneration, the regeneration time is controlled to be a third preset value, wherein the first preset value is greater than the second preset value, the third preset value is greater than the control duration during the normal regeneration, therefore, according to the scheme, when the content of the sulfide salt is greater than the first preset value, the desulfurization action is executed, and when the content of the sulfide salt is less than the first preset value and greater than the second preset value, the regeneration time is prolonged, so that the accumulated degree of sulfur can be judged in advance, thereby achieving the effect of desulfurization in advance.
Owner:WEICHAI POWER CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com