Gel polymer binder for lithium sulfur battery electrode material and preparation method thereof
A gel polymer, lithium-sulfur battery technology, applied in the direction of battery electrodes, electrode manufacturing, circuits, etc., can solve the health hazards of operators, the reduction of the proportion of sulfur active materials, poor rate performance and other problems, to improve the migration speed, improve Excellent magnification performance and good magnification performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
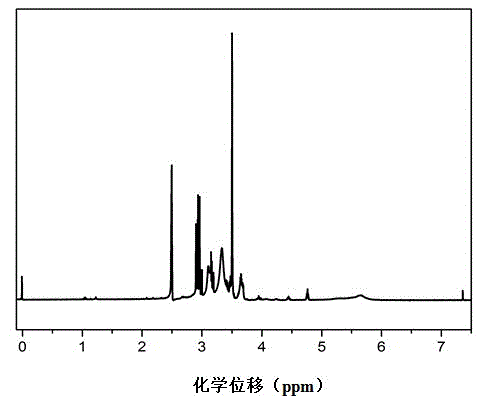
[0030] Example 1 Preparation of gel polymer binder for lithium-sulfur batteries
[0031] (1) Measure 5.16g of 1,3-dichloro-2-propanol and 20.8g of polyethylene glycol 400 (PEG400), mix them evenly, and inject N 2 Exhaust oxygen for 60 minutes, adjust the temperature to 120 degrees Celsius, add catalyst concentrated sulfuric acid and then react at constant temperature for 3 hours, the amount of concentrated sulfuric acid is 10% of the total weight of 1,3-dichloro-2-propanol and polyethylene glycol, and extract the target product , 3-dichloro-2-propanol polyethylene glycol ether (P(DCP-PEG400)), and then adjust the pH value of the solution to 6.5 with sodium hydroxide solution.
[0032] (2) Weigh 12.09g of sodium sulfide and completely dissolve it in distilled water, add 1.92g of sublimed sulfur, adjust the temperature to 80 degrees Celsius and keep the temperature constant for 5 hours to prepare a sodium disulfide solution.
[0033] (3) Mix the product P (DCP-PEG400) of step...
Embodiment 2
[0035] Example 2 Preparation of lithium-sulfur battery electrode sheet without adding lithium salt.
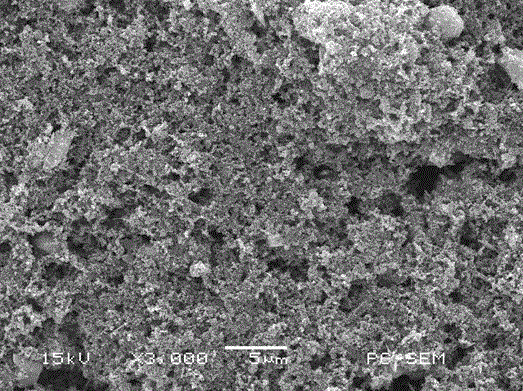
[0036] Take the gel polymer binder prepared in Example 1, dissolve in N-methylpyrrolidone solvent in a certain proportion with the carbon-sulfur compound of the positive electrode material of the lithium-sulfur battery and the conductive agent, and apply it on the current collector by ultrasonication for 10 minutes. On the aluminum foil, dry in vacuum at 50 degrees centigrade, make lithium-sulfur battery electrode sheet; Wherein, what used in the present invention is the carbon-sulfur composite of core-shell structure prepared by solution method, and the composition of electrode sheet is 10% gel Polymer binder, 85% carbon-sulfur composite of lithium-sulfur battery cathode material and 5% conductive agent.
[0037] The surface morphology of the electrode sheet prepared by the above method was characterized, and the SEM image is as follows: figure 2As shown, according to the...
Embodiment 3
[0038] Example 3 Preparation of lithium-sulfur battery electrode sheet, adding lithium salt.
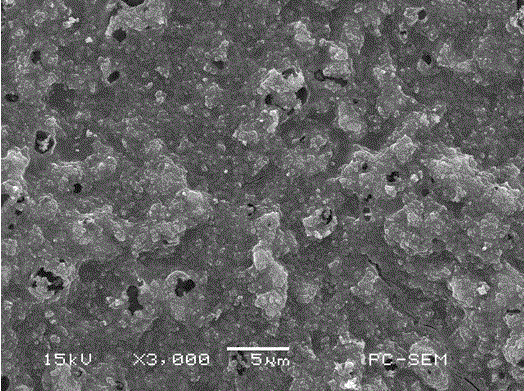
[0039] Take the gel polymer binder prepared in Example 1, and dissolve it in N-methylpyrrolidone solvent in a certain proportion with the carbon-sulfur compound of lithium-sulfur battery cathode material, lithium salt, and conductive agent, ultrasonically for 10 minutes, and apply Distributed on the aluminum foil of the current collector, and dried in vacuum at 50 degrees Celsius, the lithium-sulfur battery electrode sheet is obtained; wherein, the carbon-sulfur compound with the core-shell structure prepared by the solution method is used in the present invention, and the lithium salt is selected from perchloric acid Lithium, the composition of the electrode sheet is 9% gel polymer binder, 77% carbon-sulfur composite of lithium-sulfur battery cathode material, 9% lithium salt and 5% conductive agent.
[0040] The surface morphology of the electrode sheet prepared by the above meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com