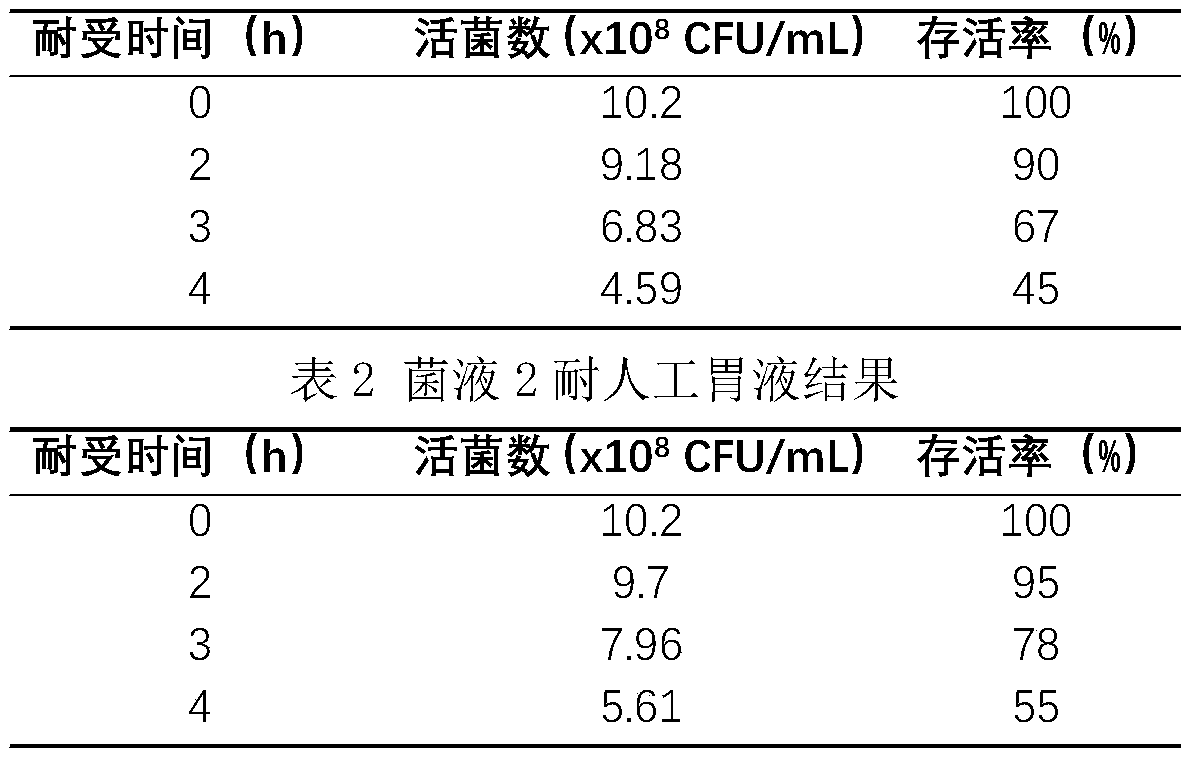
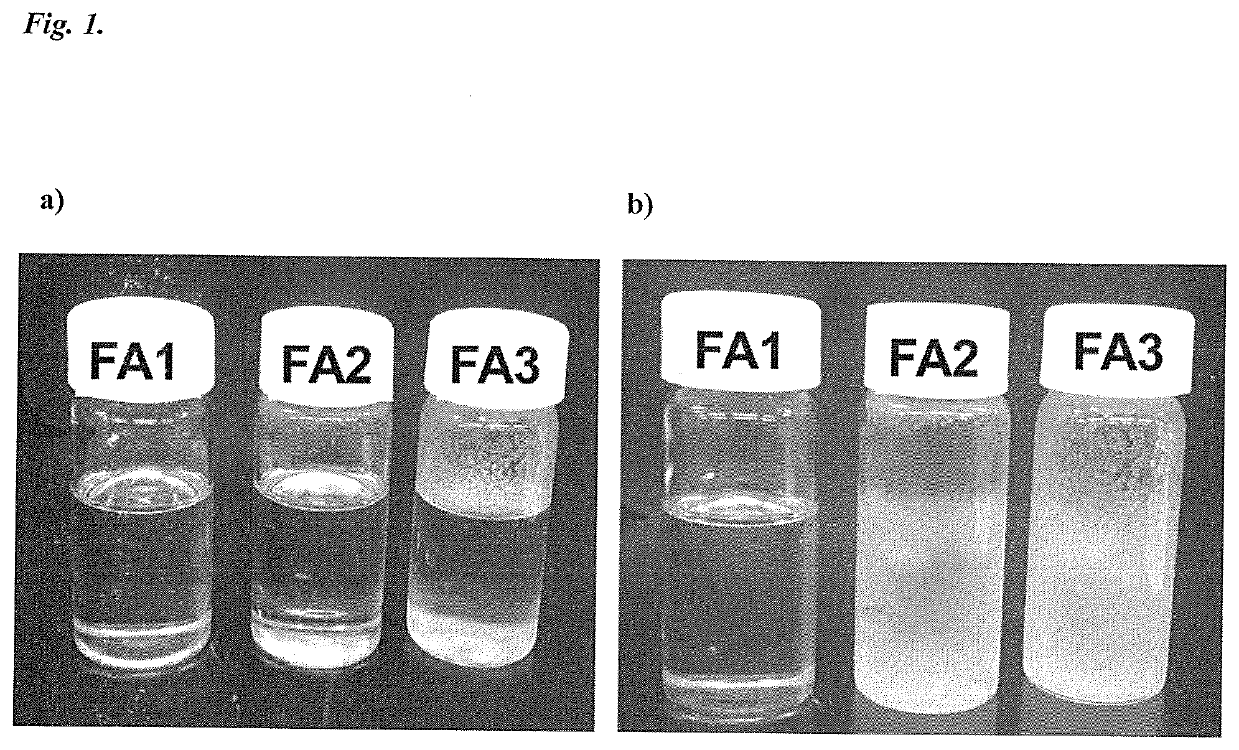
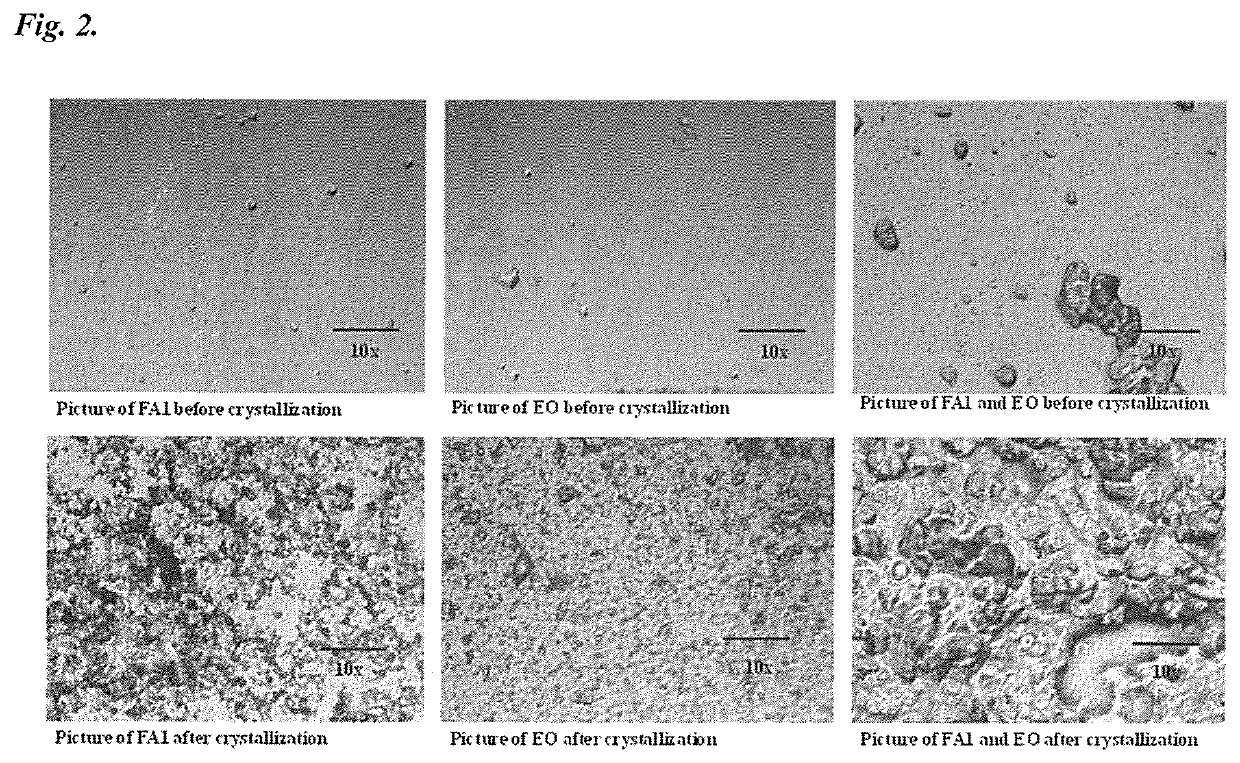
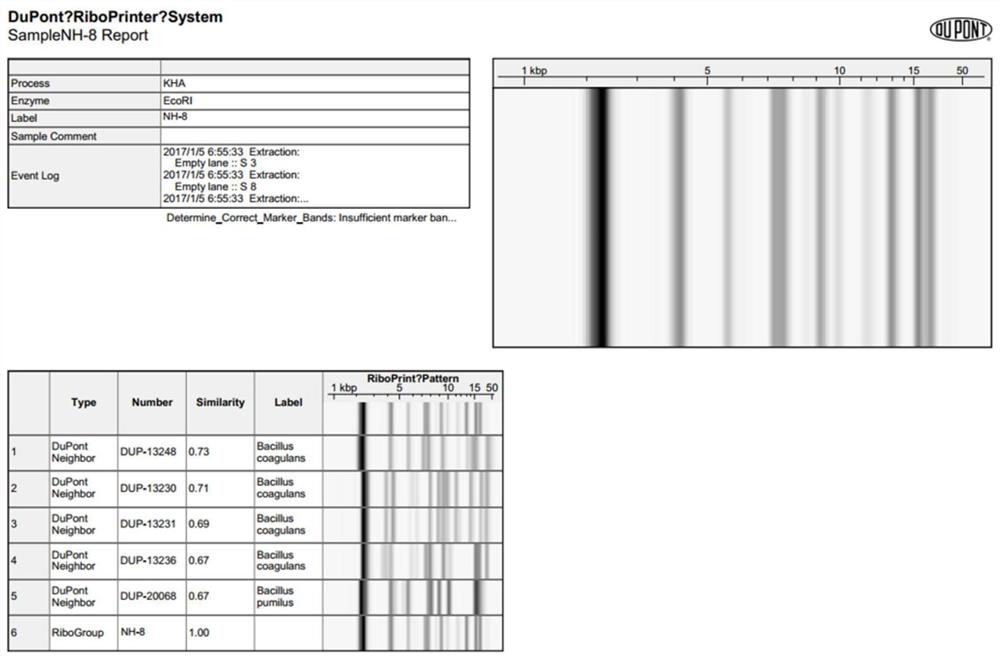
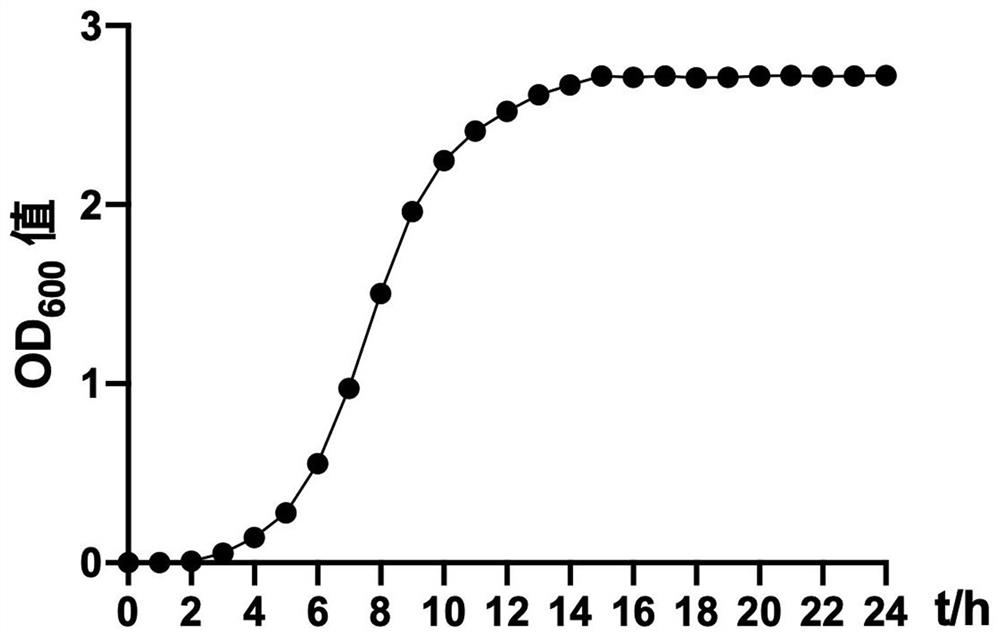
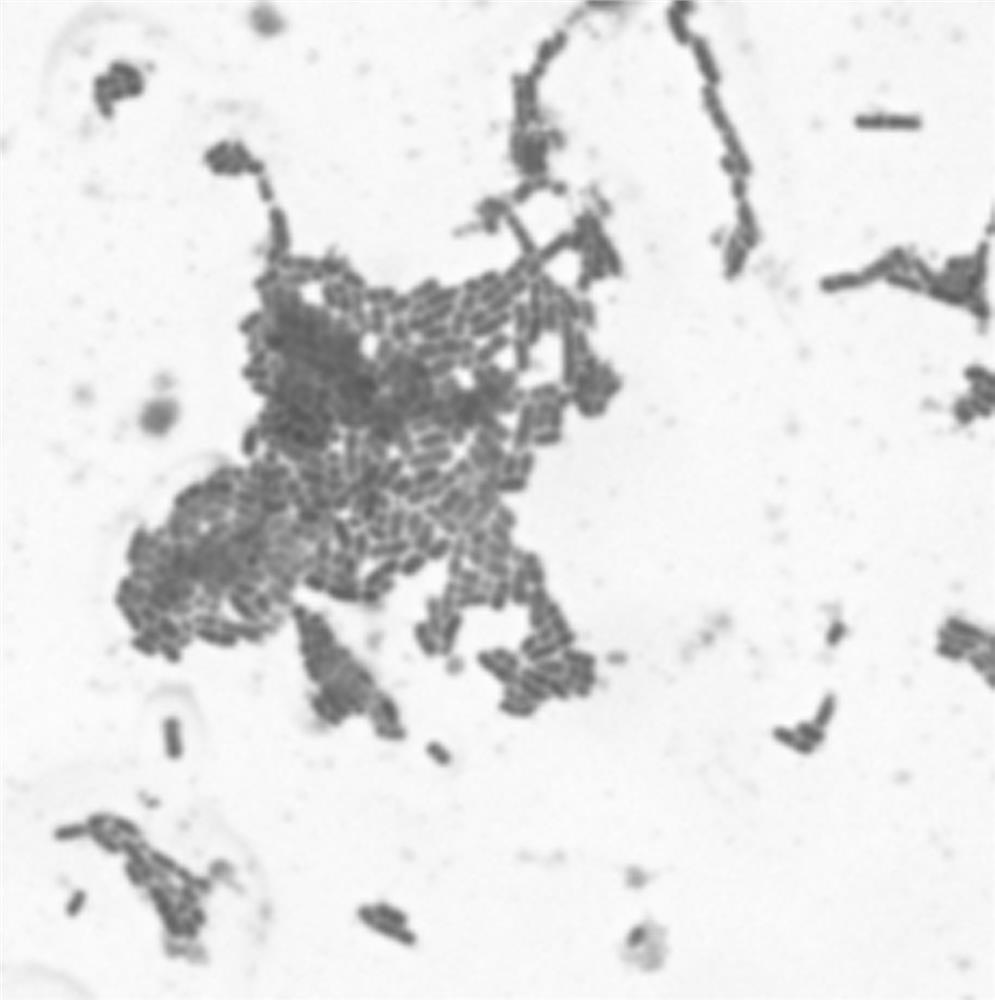
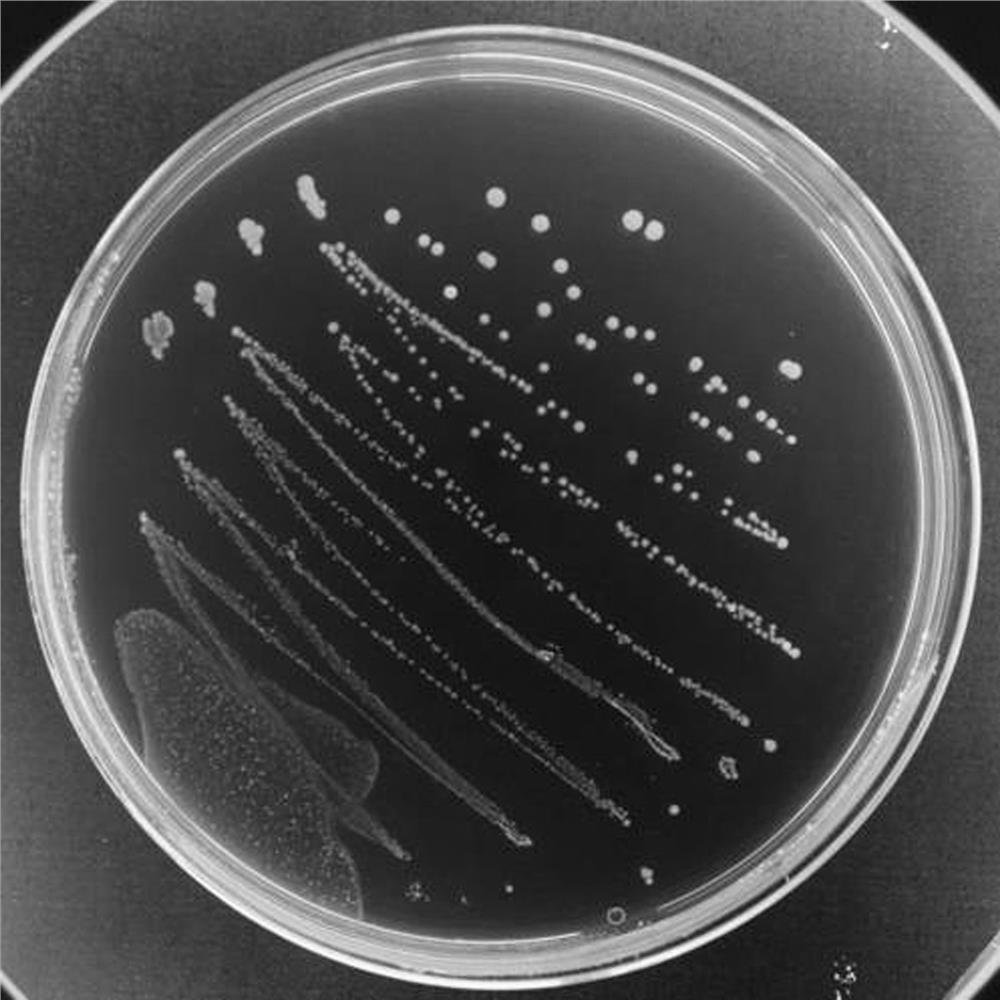
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Succus entericus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Succus entericus An antiquated, nonspecific term for the sum total of intestinal secretions. succus (suk'kus) plural.succi [L. succus, juice]
Bacillus subtilis shou003, anti-vibrio protein and preparation method and applications of bacillus subtilis shou003 and anti-vibrio protein
The invention provides bacillus subtilis (Bacillussubtilis) shou003, an anti-vibrio protein and a preparation method and applications of the bacillus subtilis shou003 and the anti-vibrio protein. The preparation method of the anti-vibrio protein comprises the following steps: screening out bacillus subtilis shou003 (preserved in China Center for Type Culture Collection with a number of CCTCC No.: M2013571 on November 13, 2013) from intestinal tract of a healthy large yellow croaker; and then extracting the anti-vibrio protein from the fermentation broth of bacillus subtilis shou003, wherein the amino acid sequence of the anti-vibrio protein is shown as SEQ ID No.: 1. The bacillus subtilis shou003 and the anti-vibrio protein show good effects on inhibiting aquatic pathogenic bacteria, in particular pathogenic vibrio; in addition, the bacillus subtilis shou003 shows outstanding tolerance to temperature, NaCl, gastric juice, intestinal juice and cholate and can be widely applied to the prevention of germs during aquaculture; on that basis, the optimal fermentation culture method of the bacillus subtilis shou003, and a preparation method of the anti-vibrio protein are provided.
Owner:SHANGHAI OCEAN UNIV
Probiotics microcapsule and preparation method thereof
ActiveCN105105144ADefinite protectionImprove stabilityFood ingredient functionsFood preparationBiotechnologyEmbedding rate
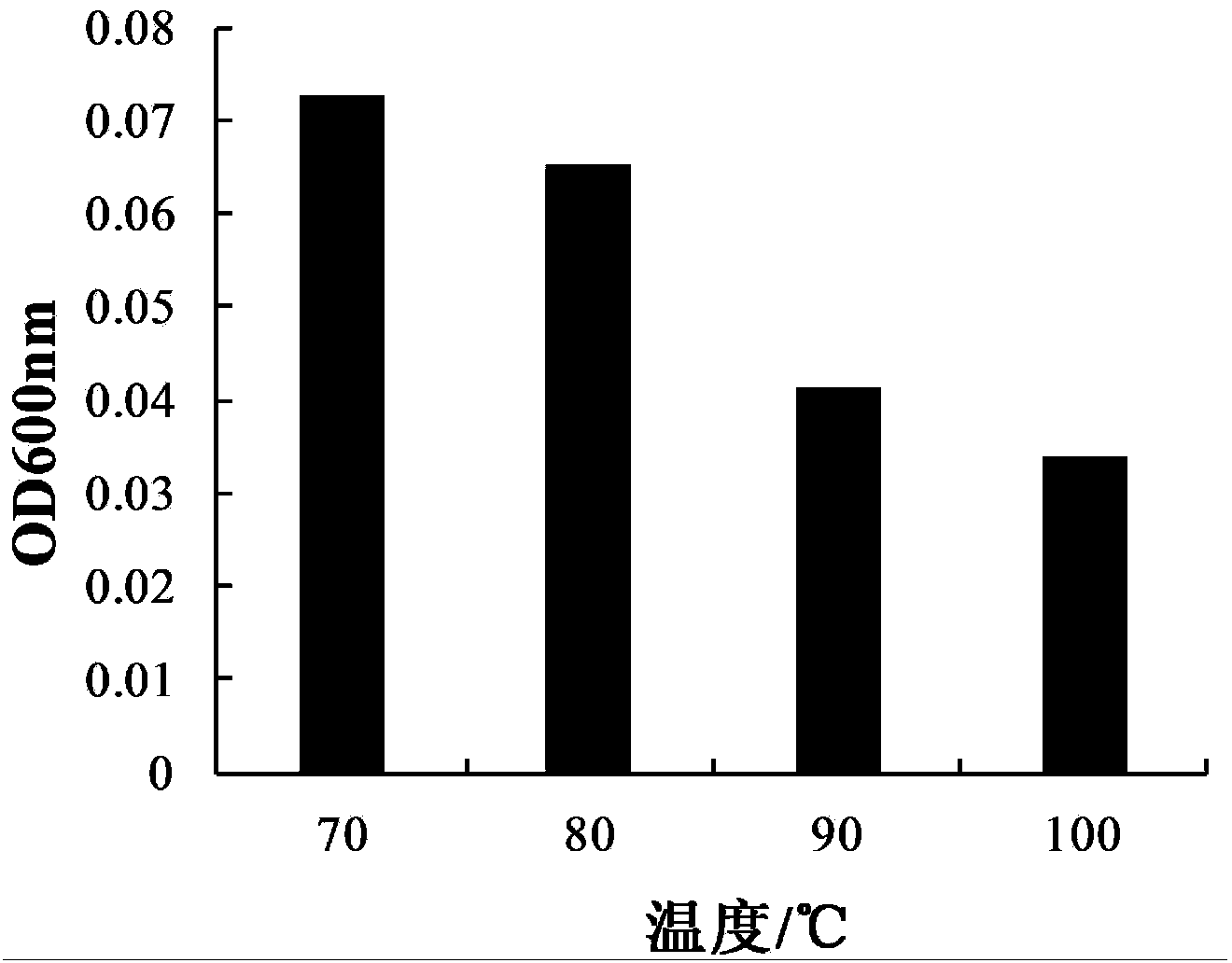
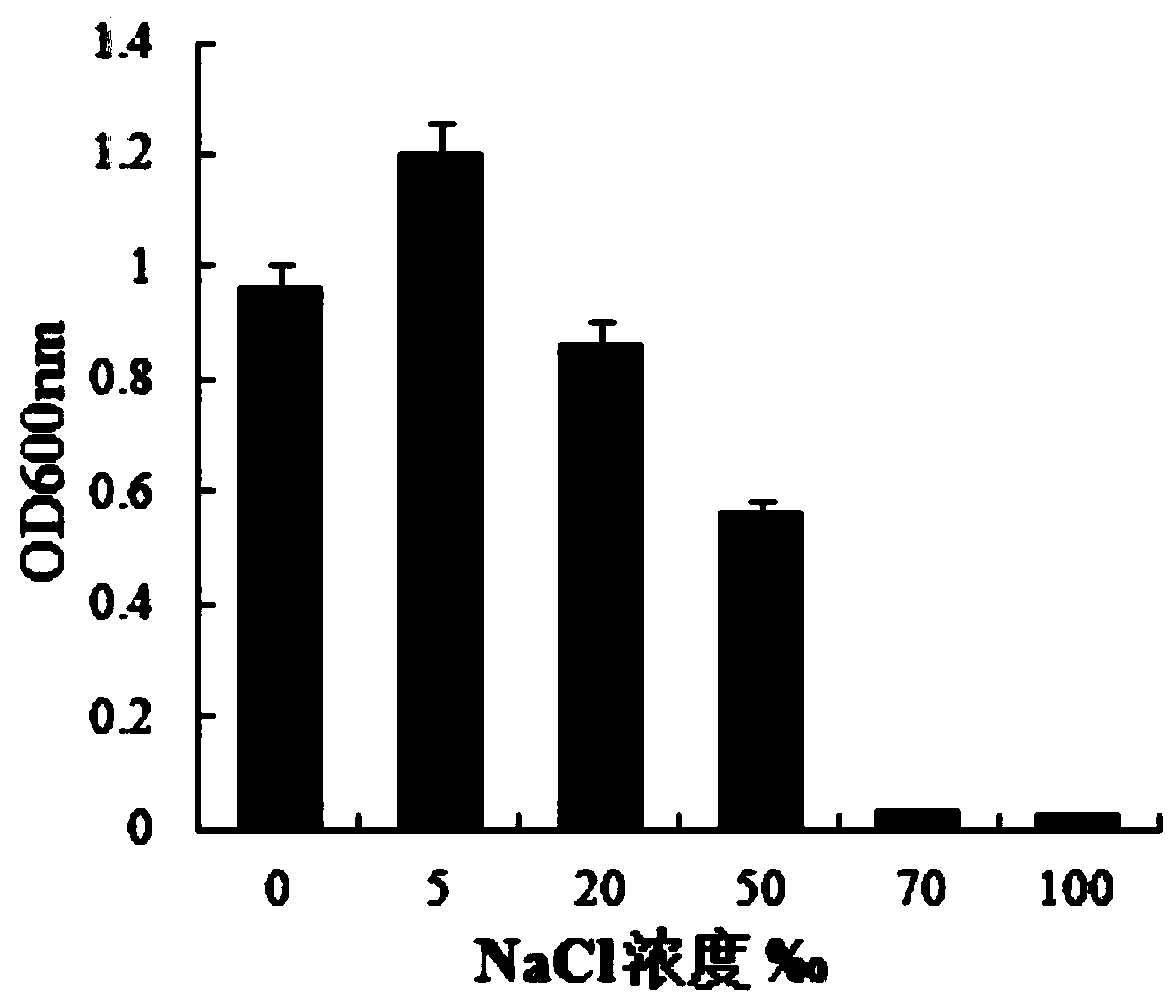
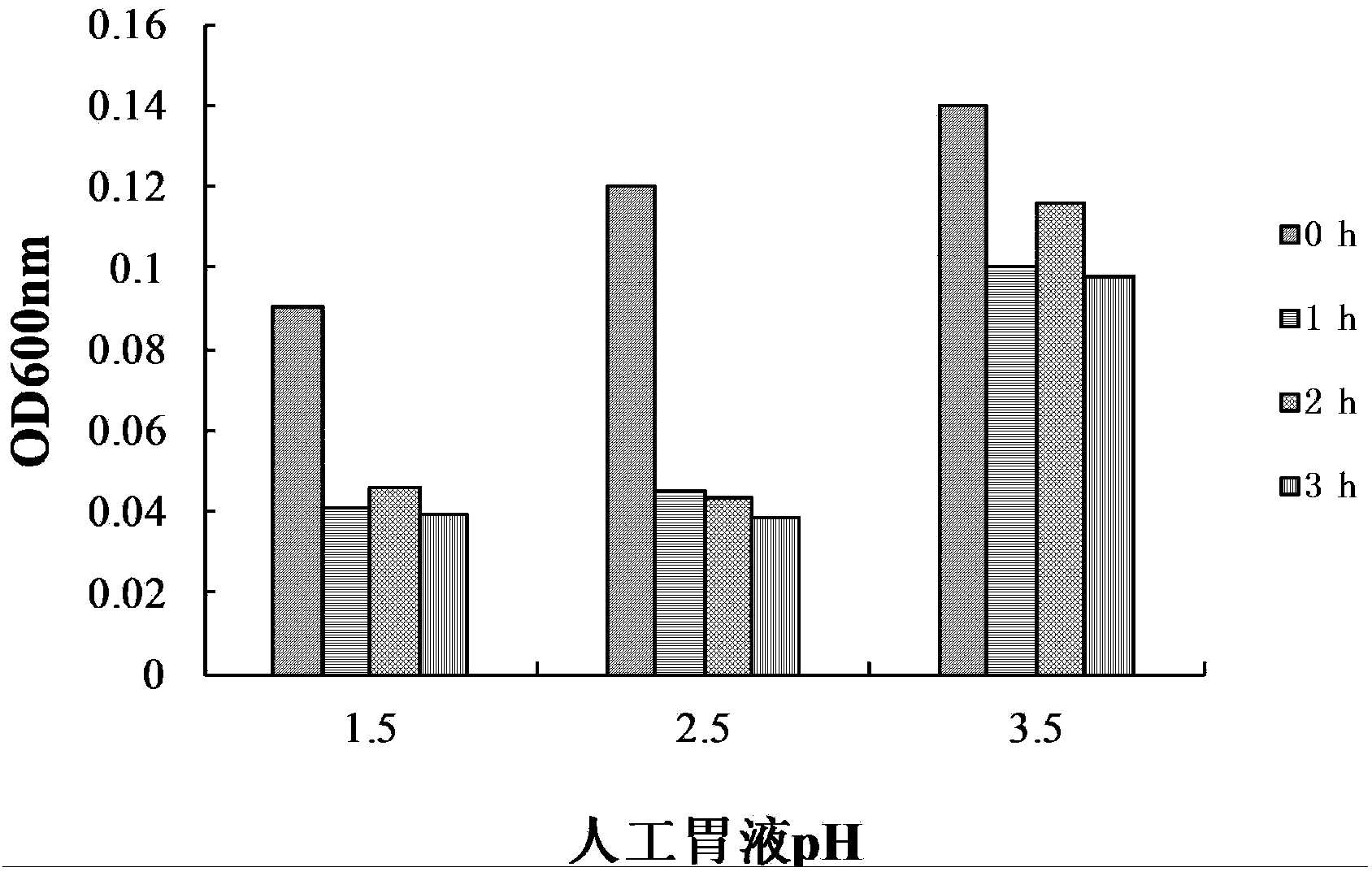
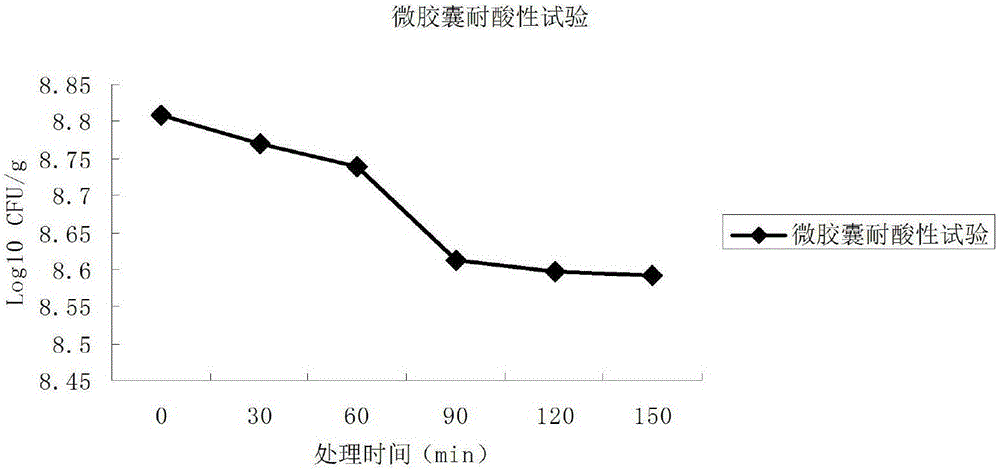
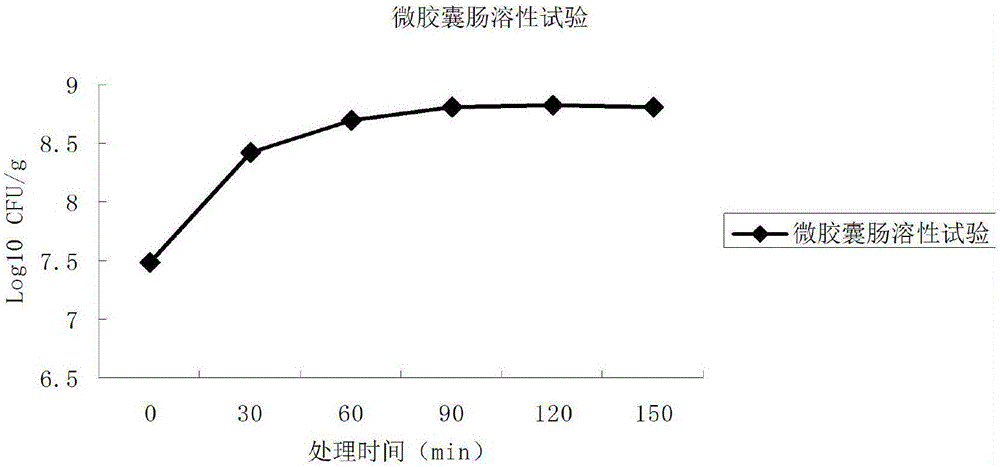
The invention relates to a probiotics microcapsule and a preparation method thereof. The preparation method comprises the steps of probiotic strain activation, fungus milk mixed solution preparation, microcapsule preparation, cooling drying and the like. The embedding rate of the probiotics microcapsule finished product prepared by the method disclosed by the invention is over 76 percent, and the number of living bacteria is larger than 10<8>CFU / g; the particles are uniform, and the average particle size is about 100 microns; the number of probiotics living bacteria is only reduced by 0.22 (Log10CFU / g) after heat preservation in artificially simulated gastric fluid with the temperature of 37 DEG C for 2.5 hours; probiotics in the microcapsule is basically released totally after heat preservation in artificially simulated succus entericus with the temperature of 37 DEG C for 90min, and the number of living bacteria can be up to 8.810+ / -0.548 (Log10CFU / g); after freeze drying, the survival rate of probiotics is up to 64.733+ / -5.221 percent; and after the microcapsule is stored within the temperature range of 4-37 DEG C for 28 days, the number of probiotics living bacteria is larger than 10<7>CFU / g.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
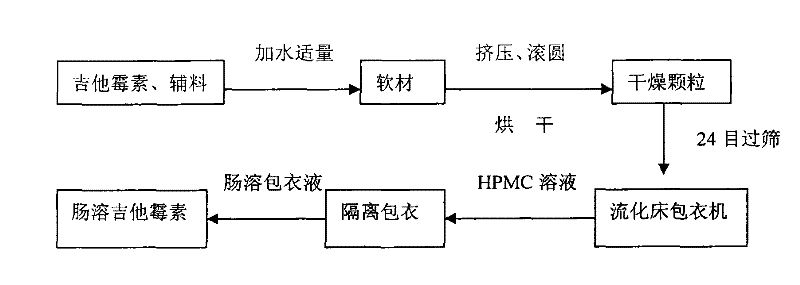
Production method of enteric-coated kitasamycin for feed
ActiveCN101611766AProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinMicroparticle
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
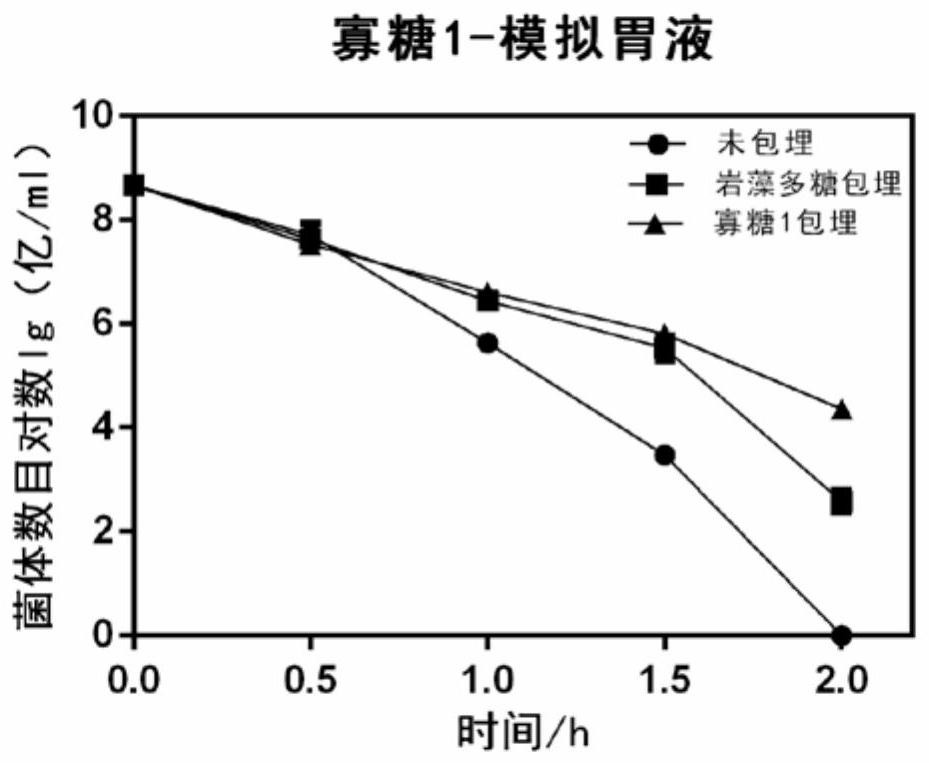
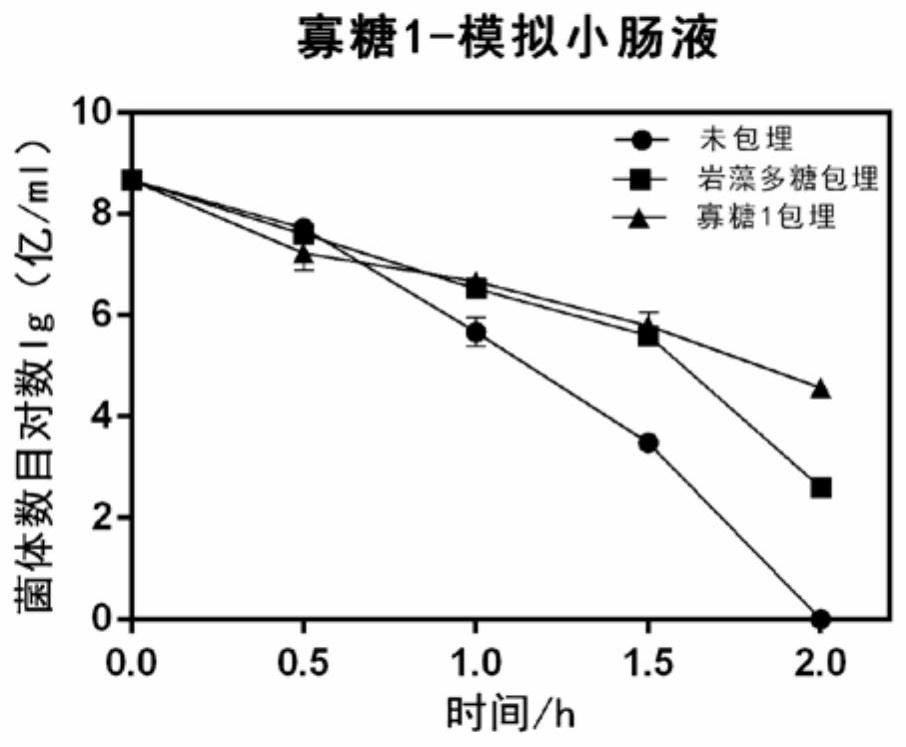
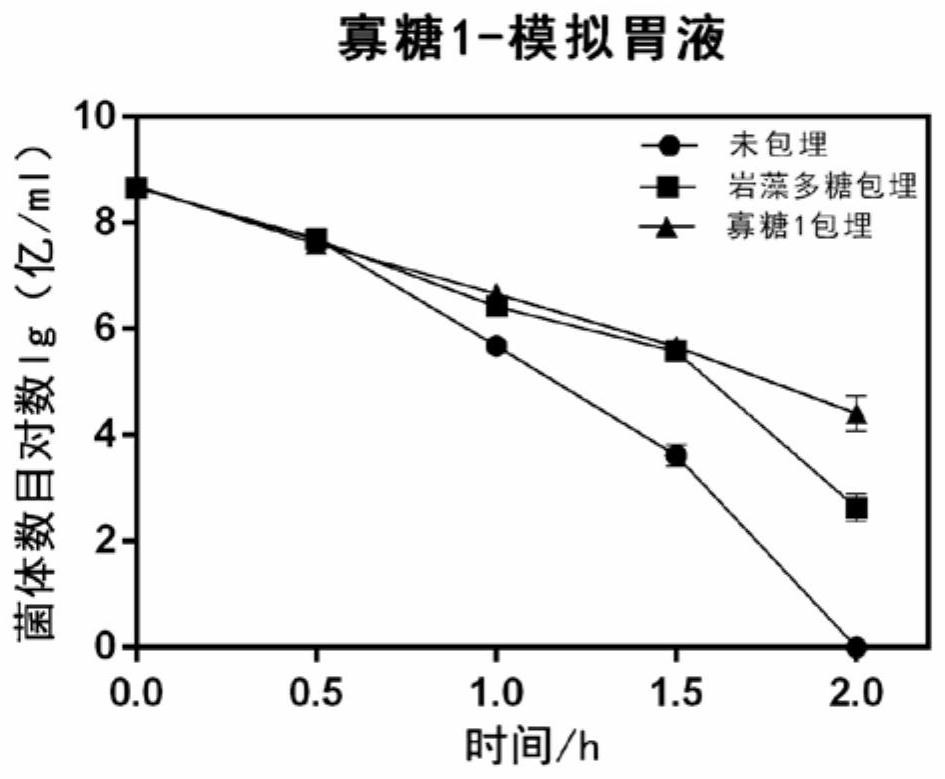
Application of fucoidin and hydrolyzed oligosaccharide thereof in preparing probiotic protection agent and method
ActiveCN110106163AStrong stress resistanceImprove survival rateOn/in organic carrierChemistryOligosaccharide
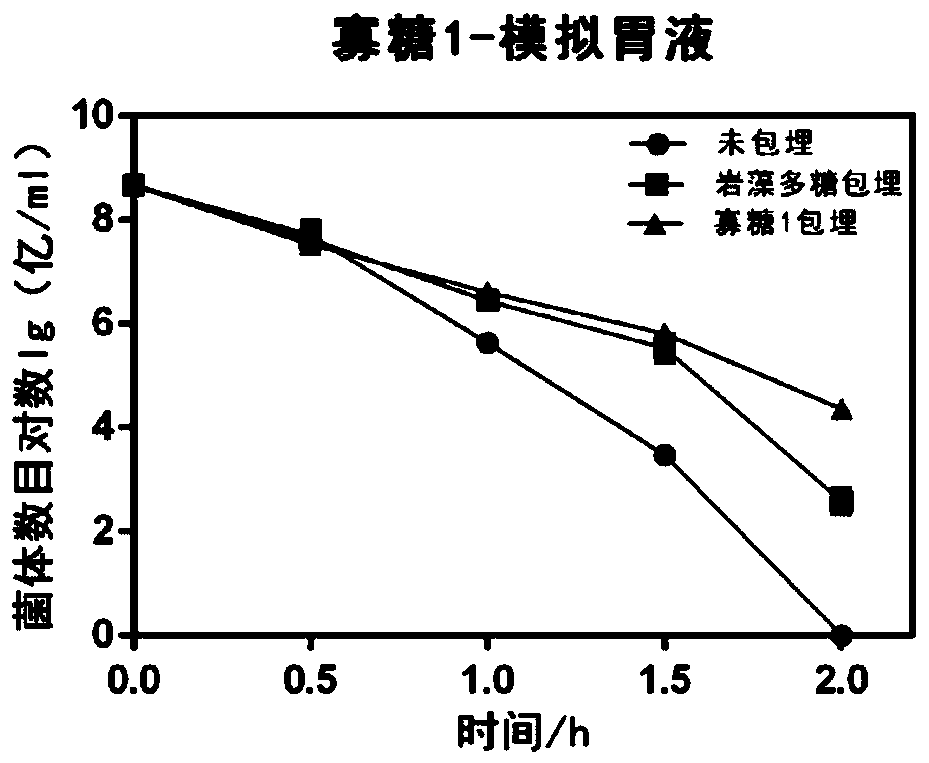
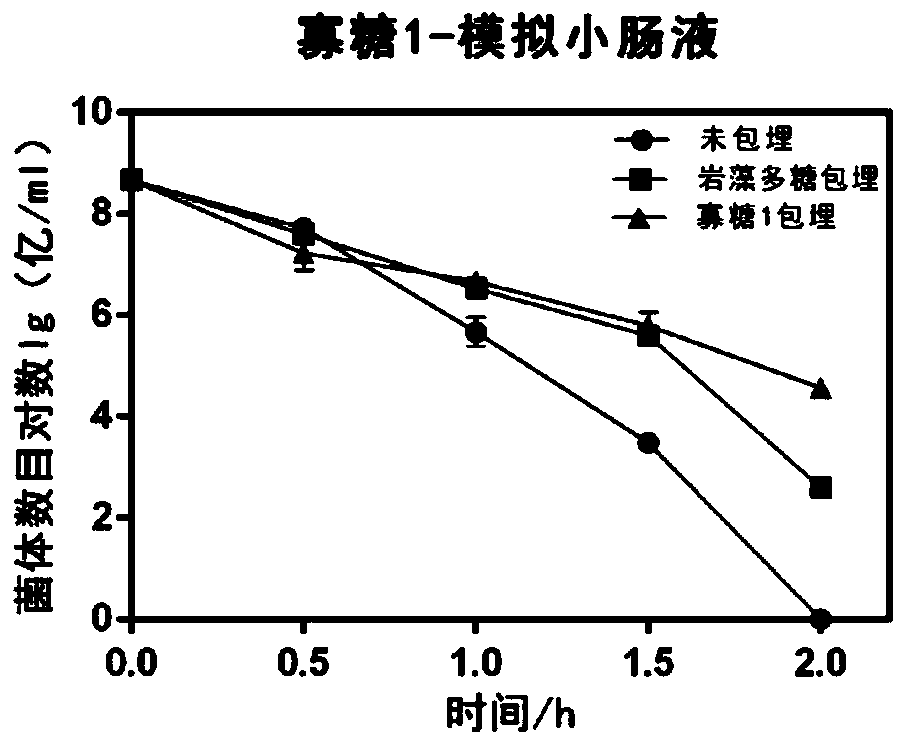
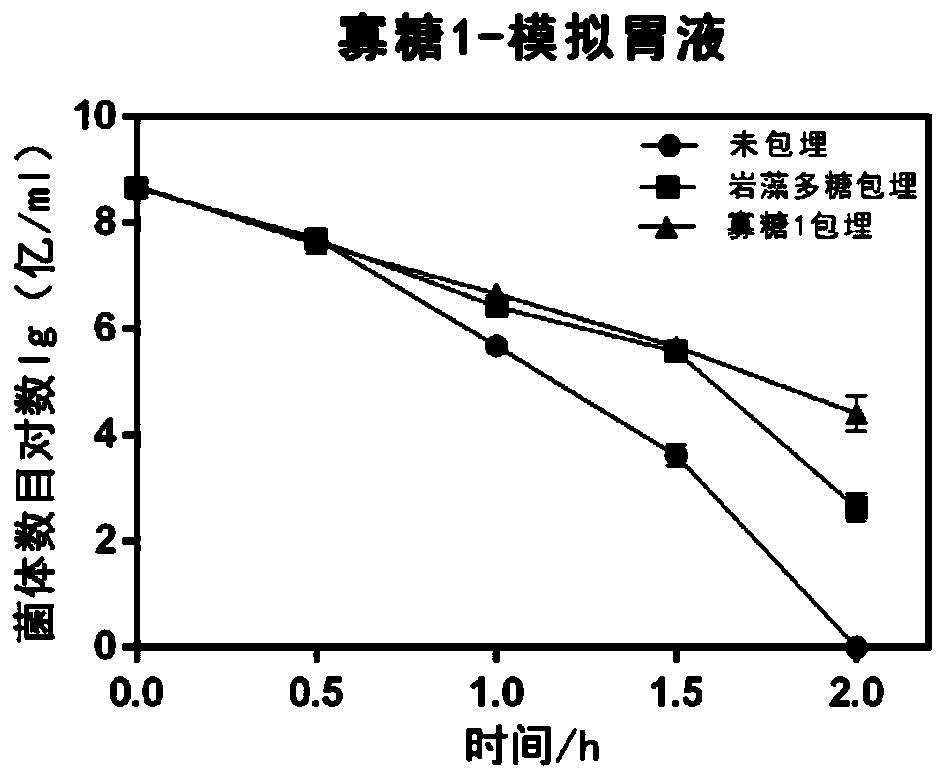
The invention discloses application of fucoidin and hydrolyzed oligosaccharide thereof in preparing a probiotic protection agent and a method. According to the application and the method, the fucoidinand the hydrolyzed oligosaccharide thereof embed probiotics for the first time, the survival conditions in gastric acid and small intestinal fluid are simulated, it is shown through a result that thesurvival time of the probiotics embedded by the fucoidin and the hydrolyzed oligosaccharide is prolonged, the embedding effect of the hydrolyzed oligosaccharide is better than that of the fucoidin, and a foundation is laid for prolonging the high activity of the probiotics in the gastrointestinal environment.
Owner:SOUTHWEST UNIVERSITY +1
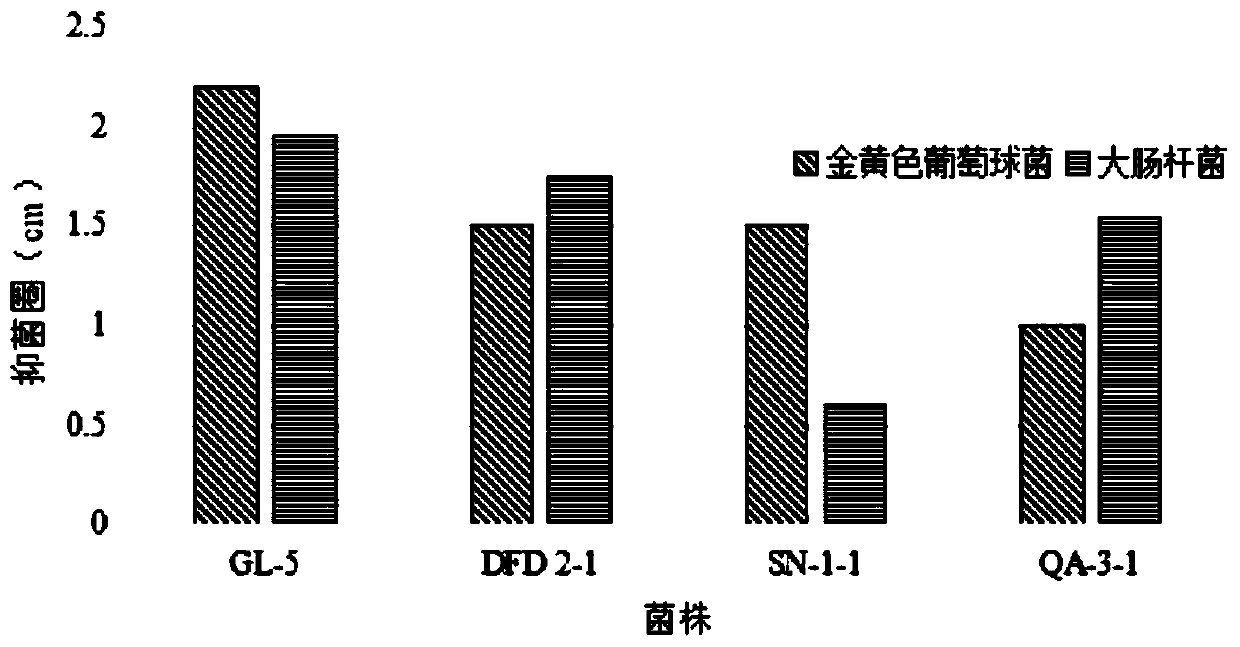
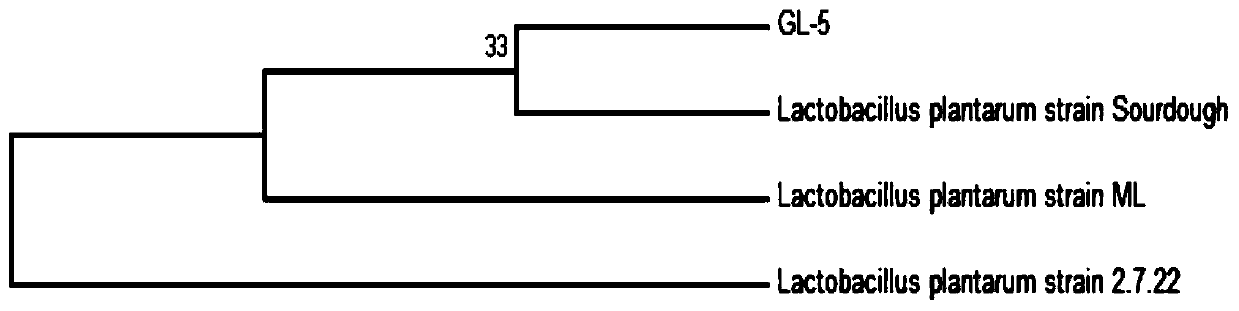
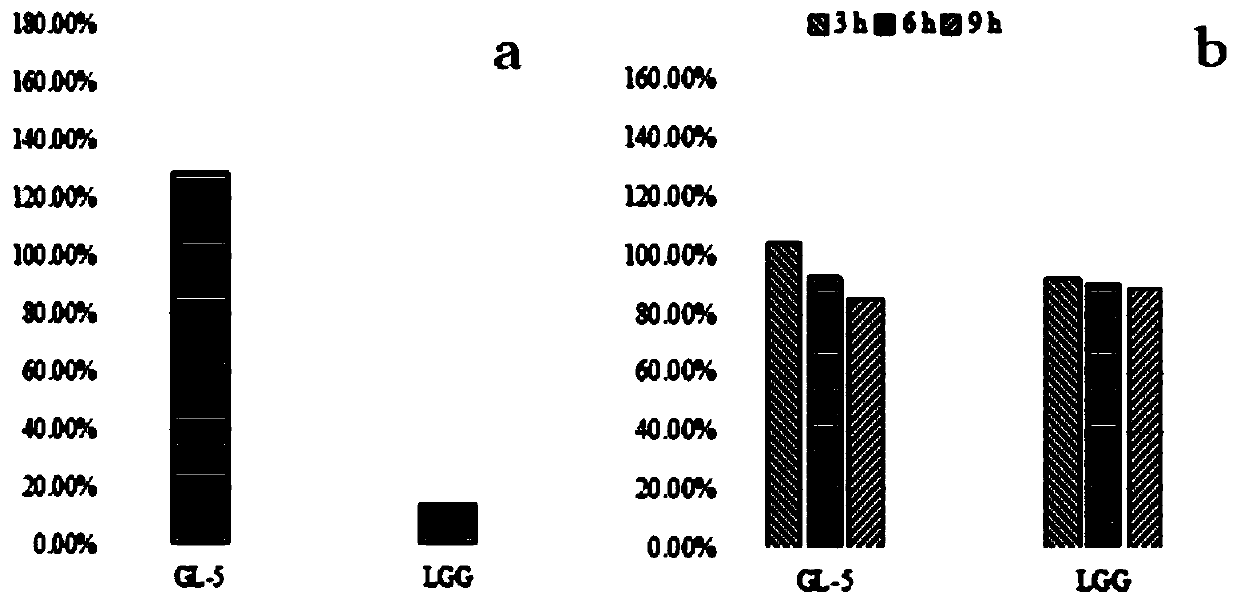
Lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof
ActiveCN111304117AHigh antibacterial activityImproves antioxidant activityAntibacterial agentsBacteriaBiotechnologyEscherichia coli
The invention belongs to the technical field of microbes and particularly relates to lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof. The lactobacillus plantarumGL-5 with oxidation resisting activity disclosed by the invention is screened out from a home-made fermented dairy product of a herdsman's house of a Qinghai Guoluo region with an altitude of 4,000mor more, is collected in China General Microbiological Culture Collection Center of China Committee for Culture Collection of Microorganisms on November, 21, 2019 and has an accession number of CGMCCNo. 18988. The lactobacillus plantarum GL-5 has a 16S rRNA sequence shown in SEQ ID NO: 1. The lactobacillus plantarum GL-5 provided by the invention has relatively good bacteriostatic activity, and activity of enteropathogenic bacteria, i.e., staphylococcus aureus and escherichia coli can be remarkably inhibited; the lactobacillus plantarum GL-5 provided by the invention has relatively high oxidation resisting activity and can remarkably tolerate catalase; and the lactobacillus plantarum GL-5 provided by the invention has relatively high gastrointestinal fluid tolerance and cholate tolerance,and the survival rate of the lactobacillus plantarum GL-5 after culture in simulated gastric fluid and intestinal fluid is remarkably higher than that of lactobacillus rhamnosus LGG.
Owner:LANZHOU UNIVERSITY
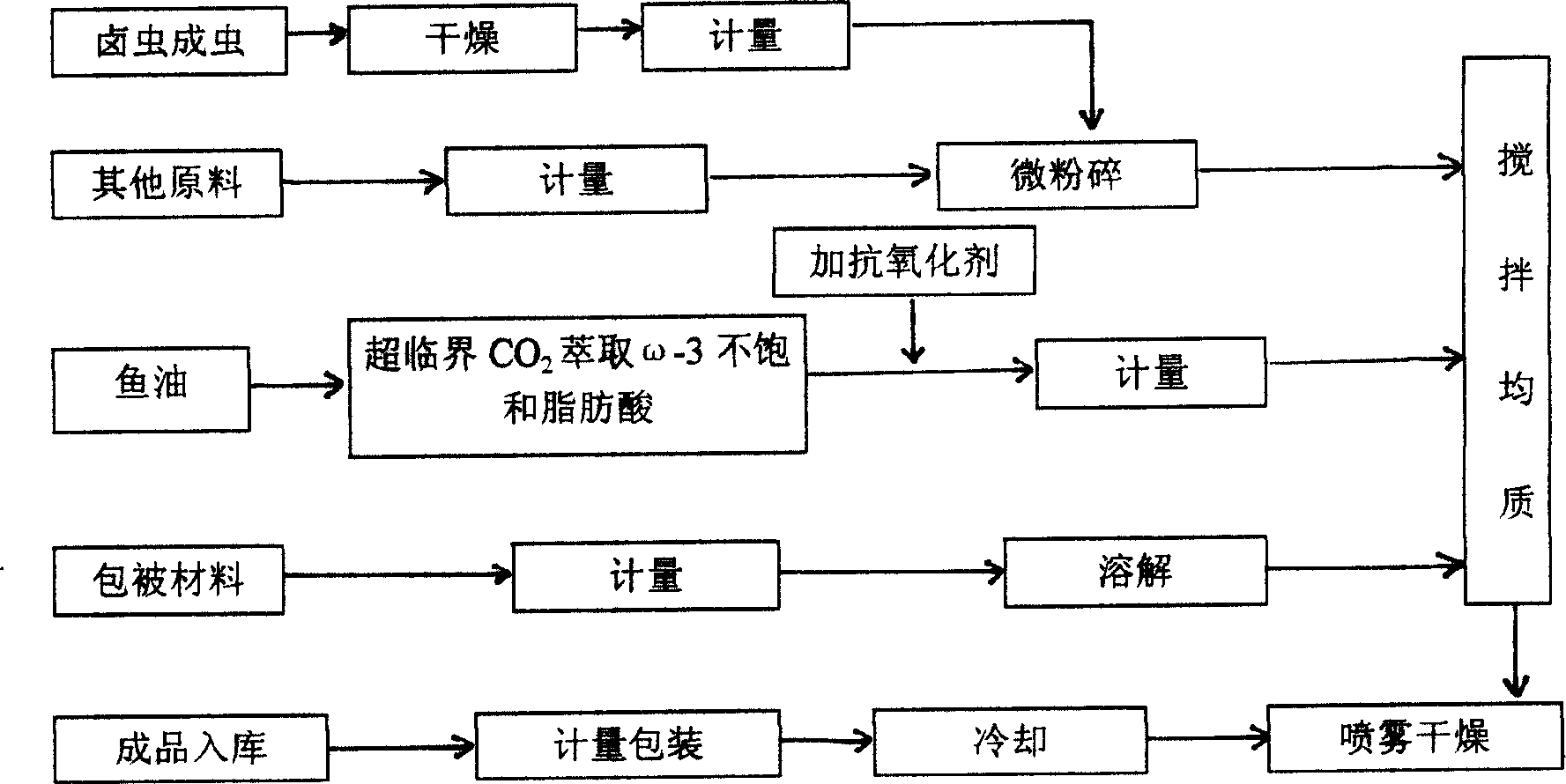
Aquatic seedling breeding microcapsule and its production method
InactiveCN1520740AHigh nutritional valueClimate change adaptationAnimal feeding stuffAquatic productWater soluble
The present invention discloses microcapsule for aquiculture and features that the microcapsule consists of Luchong insect powder 50-80 wt%, omega fatty acid 1-5 wt%, fish meal 8-25 wt%, premixed material 3-5 wt%, and glutin and arabic gum as enveloping material 10-25 wt%. The microcapsule contains balanced nutrients and no antibiotic and chitin, and is less water soluble and easy to be digested and absorbed in intestinal. It is one kind of green nutritive material for aquiculture.
Owner:浙江恒兴饲料有限公司
Docetaxel solid lipid nanoparticle lyophilized preparation and preparation method
InactiveCN104586817AImprove adhesionExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationDocetaxel
The invention discloses a docetaxel solid lipid nanoparticle lyophilized preparation and a preparation method. The quaternary ammonium salt chitosan-modified docetaxel solid lipid nanoparticle lyophilized preparation comprises the following components in parts by weight: 1 part of docetaxel, 20-50 parts of lipid materials, 10-25 parts of emulsifier, 3-7 parts of quaternary ammonium salt chitosan and 150-200 parts of lyophilization protecting agent. The preparation method comprises the following steps: 1) preparation of an oil phase; 2) preparation of diffusion liquid; 3) preparation of nanoparticle suspension; 4) modification of nanotparticles; and 5) lyophilized drying. The quaternary ammonium salt chitosan-modified docetaxel solid lipid nanoparticle has pH universality, the dissolution speed of the medicament can be effectively increased, the problem that the traditional chitosan modified docetaxel solid lipid nanoparticles are instable in succus entericus can be improved, the oral bioavailability can be improved, and the clinical application of the docetaxel solid lipid nanoparticle lyophilized preparation becomes possible.
Owner:SUZHOU UNIV
Bacillus coagulans BC99 and screening method thereof
InactiveCN111548968AStrong stress resistanceImprove toleranceBacteriaMicroorganism based processesBiotechnologyFeces
The invention discloses bacillus coagulans BC99 and a screening method thereof. The preservation number of the bacillus coagulans is CGMCC No.19487, the preservation time is March 18, 2020, the preservation unit is China General Microbiological Culture Collection Center (CGMCC), and the preservation address is No.3, Yard 1, West Beichen Road, Chaoyang District, Beijing. The bacillus coagulans BC99provided by the invention is derived from baby excrement, has relatively strong stress resistance, has relatively high tolerance to high temperature, artificial gastric juice, artificial bile salt and artificial intestinal juice, can preserve relatively high survival rate, and is suitable for being applied to food processing and animal husbandry.
Owner:武汉微康益生菌研究院有限公司
Hydrogel for assisting in controlling diet and preparation method and application thereof
ActiveCN112262984AIncrease satietyImprove solubilityAcidic food ingredientsFood ingredient as gelling agentCelluloseWater soluble polysaccharides
The invention discloses hydrogel for assisting in controlling diet and a preparation method and application thereof, and belongs to the technical field of medical assistance. The hydrogel with pH sensitivity is prepared by taking sodium carboxymethylcellulose, water-soluble polysaccharide macromolecules and polybasic carboxylic acid as raw materials and performing cross-linking reaction; the obtained hydrogel can swell in acidic gastric juice and neutral small intestine juice and can scatter and dissolve in alkaline colon juice; and by controlling the using amount of the polybasic carboxylic acid and adopting the non-ionic water-soluble polysaccharide macromolecules, the obtained hydrogel has a higher swelling ratio in a stomach acidic environment. The hydrogel disclosed by the invention can be used for preparing hydrogel capsules for assisting in controlling the diet, is safe and harmless to a human body, has very good satiety and does not influence the absorption of the human body tonutrients.
Owner:吴良平
Ipiaconitine enteric-coated preparation
InactiveCN110882228AImprove stabilityLong term storagePeptide/protein ingredientsAntipyreticBiotechnologyChemical synthesis
The invention discloses an ipiaconitine enteric-coated preparation. The raw materials of the preparation comprise, by weight, 15 to 20 parts of ipiaconitine raw material medicine, 1.5 to 2 parts of dextrin, 1 to 1.5 parts of calcium sulfate, 1.8 to 2 parts of starch, 0.1 to 0.5 part of talcum powder, 2 to 2.5 parts of starch slurry, 4 to 5.3 parts of croscarmellose sodium, 0.5 to 1 part of acrylicresin type I, 0.5 to 1 part of acrylic resin type II, 12 to 15 parts of an ethanol solution, 0.2 to 0.4 part of propylene glycol, 0.3 to 0.5 part of castor oil, 0.1 to 0.5 part of magnesium stearateand 0.03 to 0.05 part of Chinese wax. The ipiaconitine raw material medicine is produced through chemical synthesis and high performance liquid chromatography separation and refining processes. The ipiaconitine enteric-coated preparation is stable in biological activity, simple in pilot plant test process, low in cost, free of disintegration when being eroded by gastric juice, rapid in disintegration when being eroded by intestinal juice, convenient for absorption of effective substances, high in stability, capable of being stored for a long time, common in raw materials and suitable for large-scale production.
Owner:WUHAN YICHENG BIOTECH CO LTD
Lactobacillus capable of adsorbing or degrading enterotoxin and application thereof
ActiveCN104988104AGood acid and bile salt resistanceImprove the body's immunityBacteriaMicroorganism based processesEnterotoxinFunctional food
The invention discloses a lactobacillus capable of adsorbing or degrading enterotoxin and an application thereof. The preservation number of the lactobacillus is CCTCC No: M2015128. The lactobacillus can adsorb or degrade enterotoxin and has the good effects of resisting acid, cholate, artificial gastric juice and artificial intestinal juice. The lactobacillus is specifically characterized in that the enterotoxin can be adsorbed or degraded, and good enterotoxin removal capacity is achieved; growth of common pathogenic bacteria can be restrained; the lactobacillus can resist acid and cholate and can survive in the digestive tract. In specific application, a method includes the step that freeze-dried bacterial powder is dissolved in water at normal temperature to be taken orally or is directly added according to a certain proportion to make functional food or healthcare products. The lactobacillus is screened from multiple lactobacilli, has the high enterotoxin removal capacity and good performance, and can be used for human body healthcare.
Owner:山东凤凰生物科技股份有限公司
Compound lactic acid bacterium preparation and application thereof in preparing feed additive
ActiveCN110804571ARaise the level of fermentationHigh viable count fermentation brothBacteriaClimate change adaptationBiotechnologyStaphyloccocus aureus
The present invention relates to the technical field of microorganisms, specifically discloses a compound lactic acid bacterium preparation and an application thereof in preparing a feed additive, andrelates to a strain of lactobacillus casei F209. The strain has relatively strong tolerance on simulated gastric juice, bile salt and artificial intestinal fluid, besides produces acid rapidly, is high in yield of L-lactic acid, has inhibitory effects on escherichia coli and staphylococcus aureus and shows relatively strong probiotic characteristics. After co-fermentation of the strain and saccharomyces boulardii SH94, the number of viable lactic acid bacteria of the product is greater than or equal to 30x10<8> CFU / g and the number of viable yeasts is greater than or equal to 0.3x10<8> CFU / g,and after 3 months of storage at 4 DEG C, 20 DEG C and 30 DEG C, survival rates of the lactic acid bacteria are 92%, 76%, and 67%, respectively and the survival rates of the yeasts are 72%, 57%, and42%, respectively. The compound microorganism agent can significantly improve production performance when applied to procambarus clarkii and nursery pig breeding, and has better application prospects.
Owner:HUAZHONG AGRI UNIV
Novel salmonella bacteriophage microencapsulated microspheres and preparation method thereof
PendingCN113812518AAffinityKeep aliveAnimal feeding stuffAccessory food factorsMicrosphereOligosaccharide
The invention provides a preparation method of novel salmonella bacteriophage microencapsulated microspheres. The method comprises the following steps of mixing a cationic etherified starch solution and a bacteriophage suspension, and uniformly stirring to obtain a first mixed solution; uniformly stirring and mixing the first mixed solution, a sodium alginate solution, a xanthan gum solution and a nano TiO2 solution to obtain a second mixed solution; and dropwise adding the second mixed solution into a calcium chloride solution to form calcium alginate gel, filtering and collecting bacteriophage microspheres, putting the bacteriophage microspheres into a cationic chitosan oligosaccharide solution, coating for 30 minutes, and filtering and collecting the bacteriophage microencapsulated microspheres. In actual use, after the bacteriophage microspheres enter gastric juice, the bacteriophage surface coated material can effectively resist erosion of gastric acid and prolong the survival time of bacteriophage, and after the bacteriophage microspheres enter intestinal juice, the bacteriophage surface coated material can react with the intestinal juice and is cracked to release the bacteriophage in the material so as to achieve the sterilization effect. The method has the advantages that microencapsulation preparation is carried out at room temperature, the process is simple, the application range is wide, and the cost is low; and the raw materials are wide in source, cheap and easy to obtain, and the bacteriophage in the product is high in activity and encapsulation efficiency and can be directly applied to feed addition.
Owner:HEBEI UNIV OF ENG
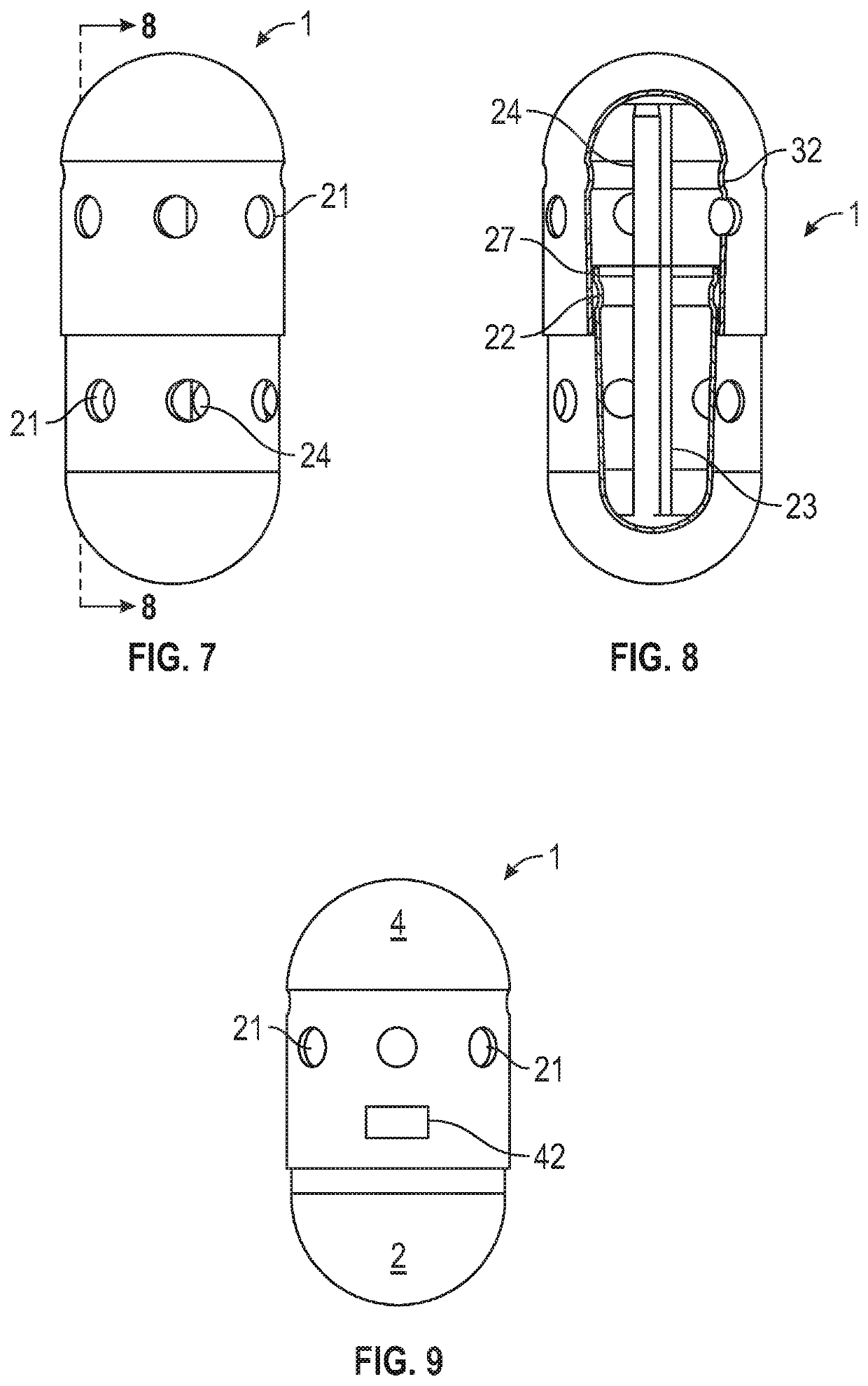
Sampling system capsule
A non-invasive system for sampling gastrointestinal microbiota with their associated environment for discovery, characterization, medical research, diagnostics, and treatment using an ingestible, non-digestible sampling capsule. The capsule device, with ports open for acquiring a sample of gastrointestinal microbiota and content, is placed inside an immediate or delayed release capsule. When the outer capsule dissolves according to its specifications, enteric fluid and content enters the sampling capsule through the ports triggering a hydrophilic stop to release a pulling force to close the ports by enveloping the capsule outer casing over the inner casing, capturing the sample, and holding it sealed until it completes passage through the digestive track, and is recovered from feces for analysis.
Owner:JONES JAMES PHILLIP
Lactococcus lactis subsp.hordniae having high antioxidant activity and application thereof
ActiveCN110885767AIncrease profitImprove conversion rateMilk preparationBacteriaBiotechnologyStaphylococcus lactis
The invention relates to a lactococcus lactis subsp.hordniae having the high antioxidant activity. The lactococcus lactis subsp.hordniae is lactococcus lactis subsp.hordniae JYZ4. According to the invention, the lactobacillus is preserved in China General Microbiological Culture Collection Center at a preservation site being the Institute of Microbiology, Chinese Academy of Sciences, No.3, Yard 1,West Beichen Road, Chaoyang District, Beijing and has a preservation number of CGMCC No.17892 on a preservation date of June 3, 2019. The lactobacillus is separated from soy sauce residues and the in-vitro probiotic characteristic research shows that the lactobacillus is resistant to acid, artificial gastric juice and intestinal juice and has high hydrophobicity and antioxidant activity. The lactococcus lactis subsp.hordniae not only can be applied to fermentation of soy sauce residues to make the fodder with high palatability but also can be combined with streptococcus thermophilus, lactobacillus bulgaricus to ferment yoghourt and the like and has broad application prospects and good commercial value.
Owner:NANCHANG UNIV
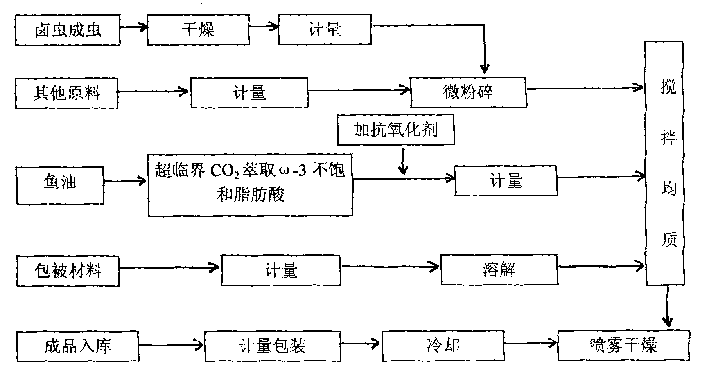
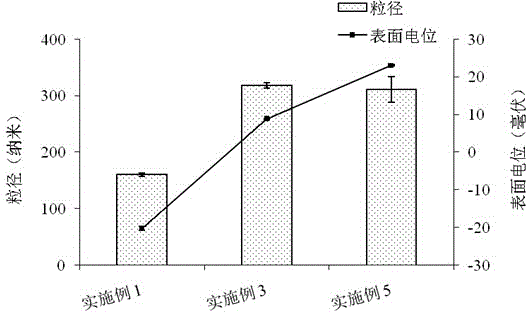
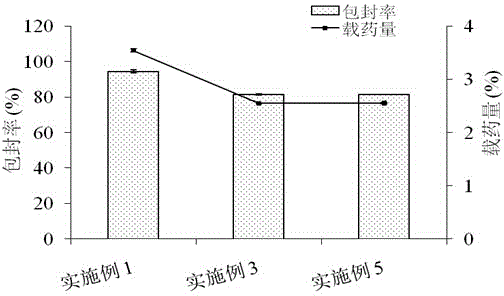
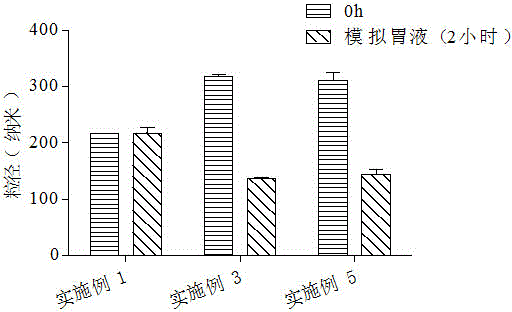
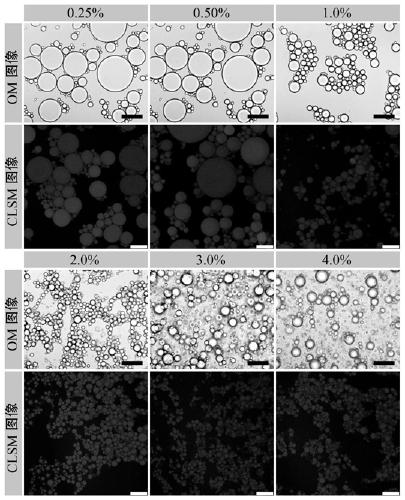
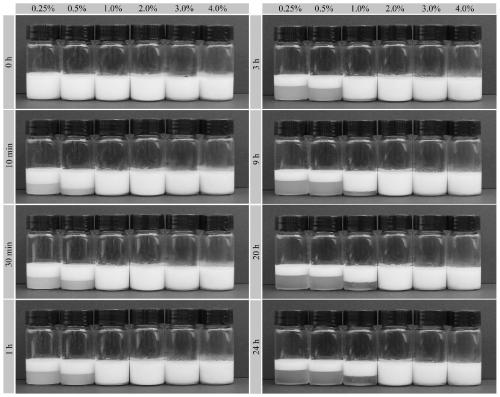
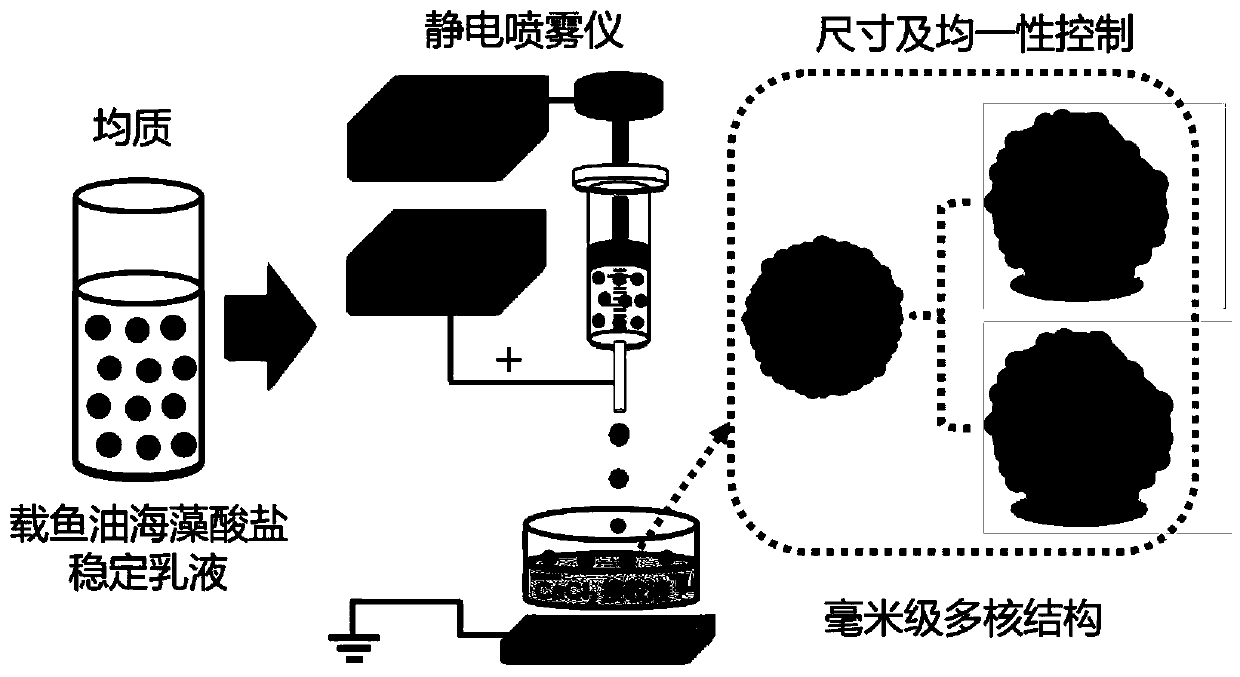
Millimeter-level polykaryon capsules for transporting nutrient substances/medicines to small intestines and realizing slow release and preparation method and application of millimeter-level polykaryon capsules
ActiveCN111450077AWith particle size uniformityEvenly distributedAntipyreticMetabolism disorderFormularyActive agent
The invention provides millimeter-level polykaryon capsules for transporting nutrient substances / medicines to small intestines and realizing slow release and a preparation method and application of the millimeter-level polykaryon capsules. The millimeter-level polykaryon capsules comprise a wall material and oil substances, wherein the oil substances are evenly or unevenly distributed on the innerwall of the wall material in the manner of uniform or ununiform oil droplets, so that the millimeter-level polykaryon capsules are obtained. Bulges are formed on the surfaces of the millimeter-levelpolykaryon capsules, and the oil droplets are placed in the ridges. The wall material is calcium ion cross-linking alginate or calcium cross-linking alginate and a surfactant. The entire preparation method is simple and easy to operate, complex polymer modification and formulas are not added, and the prepared millimeter-level polykaryon capsules are mainly released during simulation of small intestinal juice and are not released in simulation of gastric juice. The millimeter-level polykaryon capsules are particularly suitable for medication through mouths to specially transport fish oil to thesmall intestines and realize slow release of the fish oil / medicines in the small intestines, and can be used for transporting nutrient substances and medicines as specific carriers.
Owner:SHANGHAI OCEAN UNIV
Production method of enteric-coated kitasamycin for feed
ActiveCN101611766BProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinGastric fluid
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
Development of Lipid Matrix Granules with Incorporation of Polysaccharides for Effective Delivery of an Antimicrobial Essential Oil
PendingUS20210236427A1Antibacterial agentsHydroxy compound active ingredientsBiotechnologyAntibiotic resistance
Antibiotics have long been used at sub-therapeutic levels to control incidences of post-weaning diarrhea and to improve growth performance in pigs. However, the current trend world-wide is to eliminate the use of in-feed antibiotics due to increased public concerns over the spread of antibiotic resistance in bacterial pathogens, which poses a threat to public health. Alternatives to in-feed antibiotics are needed. Thymol essential oil exhibits strong in vitro antibacterial activity; however; direct inclusion of essential oils to pig feeds has limited efficacy due to their high volatility, low stability during feed processing, interactions with other feed components and poor availability in lower gut. To solve these problems, we developed lipid matrix beads using thymol and a fatty acid with incorporation of 2% polysaccharides via a melt-granulation technique. Laurie acid was identified as a suitable carrier for thymol. In vitro release of thymol from lipid matrix granules was determined using simulated salivary fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF), respectively. The lipid matrix granules with 2% polysaccharides exhibited a slow release rate (%) of essential oil and fatty acid in SSF (21.2±2.3; 36±1.1), SGF (73.7±6.9; 54.8±1.7) and SIF (99.1±1.2; 99.1±0.6), respectively. However, the lipid matrix granules without polysaccharides had quick release (%) of essential oil and fatty acid from the SSF (79.9±11.8; 84.9±9.4), SGF (92.5±3.5; 75.8±5.9) and SIF (93.3±9.4; 93.3±4.6), respectively.
Owner:UNIVERSITY OF MANITOBA
Preparing method of radix scutellariae-gypsum oral preparation capable of relieving sore throat
InactiveCN105012967AGuaranteed stabilityGuaranteed uniformityPharmaceutical delivery mechanismUnknown materialsSodium cyclamateGastric juices
The invention belongs to the technical field of traditional Chinese medicine compound preparation and particularly relates to a preparing method of radix scutellariae-gypsum oral preparation capable of relieving sore throat. The method includes the steps of firstly, extracting volatile oil of gypsum, fructus forsythiae, mint and perilla leaf in a microwave manner; secondly, adding buffalo horn grinding fluid into artificial gastric juice extract and artificial intestinal juice extract; thirdly, jointly decocting fructus forsythiae, mint, perilla leaf and gypsum residues with radix scutellariae, rhizoma anemarrhenae, radix rehmanniae, red peony root, spina gleditsiae, dandelion and liquorice; fourthly, welling mixing the volatile oil clathrate compound and the extract obtained in the first step and the third step, adding the buffalo horn extract in the second step, adding 0.1% of agar and 0.2% of sorbic acid, well mixing, immediately filtering, cooling, adding the volatile oil clathrate compound obtained in the first step, 1% of apple essence and 1.5% of sodium cyclamate, adding distilled water to reach 1000ml, split charging into glass ampoules of 10ml, and sterilizing. The method is simple and easy to operate and stable in experiment results.
Owner:TONGHUA GOLDEN-HORSE PHARM IND CO LTD
Bacillus coagulans preparation and preparation method thereof
ActiveCN113528368AWon't dissolveStrong heat resistanceBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention relates to the technical field of functional microorganisms, and particularly provides a bacillus coagulans preparation and a preparation method thereof. The bacillus coagulans preparation is prepared by carrying out spray drying on a mixed solution of bacillus coagulans and a coating material, is high in viable count, good in tolerance to artificial gastric acid and intestinal juice and very high in heat resistance, and can be widely applied to the fields of foods, health-care products and the like.
Owner:QINGDAO VLAND BIOTECH INC +1
Lactobacillus paracasei with the ability to decompose oil and its application
ActiveCN110591952BSignificant lipase activityExcellent acid and bile salt resistanceBacteriaMicroorganism based processesBiotechnologyFood additive
Owner:中科利君股份有限公司
Portable enteral feeding apparatus
An enteral feeding apparatus includes a pod having an expansile pouch which defines a reservoir for enteral fluid and a gas impermeable barrier surrounding the pouch. The pod has an inlet port for delivery of enteral fluid into the pouch and an outlet port having a seal which is pierceable to release enteral fluid from the pouch for delivery to a PEG via a feeding line. The expansile pouch provides the sole force by which enteral fluid is delivered from the pouch through a regulator. The system can accommodate a range of enteral fluids with a wide range of viscosities.
Owner:ROCKFIELD MEDICAL DEVICES LTD
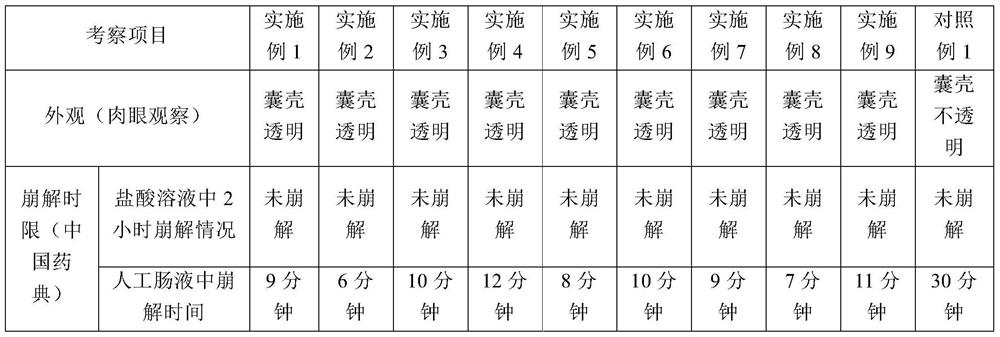
A kind of plant-type enteric-coated soft capsule
ActiveCN105534945BHigh transparencyDisintegrates quicklyOrganic active ingredientsMulti-step food processesBiotechnologySoftgel
The invention discloses a plant type enteric soft capsule. The capsule comprises dispensing and content, and is prepared by the steps of preparing dispensing, preparing filler, pressing soft capsules and drying. The shell of the plant type enteric soft capsule has good transparency, has a disintegration time in accordance with the specification of domestic and international pharmacopeias, namely having the characteristics that the shell is not disintegrated in a hydrochloric acid solution (artificial gastric juice) within two hours and quickly disintegrated in artificial intestinal juice within 15 minutes. Compared with enteric coated soft capsules, the plant type enteric soft capsule has the advantages that the coating process is reduced, the process is simplified, an organic solvent is not used, safe production and environmental protection are benefitted, efficiency can be improved, and cost can be reduced.
Owner:SIRIO PHARMA CO LTD
Lactobacillus reuteri SXDT-32 and application thereof
ActiveCN114836358AEnhanced inhibitory effectPromote growthAntibacterial agentsBacteriaBiotechnologyEscherichia coli
The invention relates to the field of probiotics, in particular to lactobacillus reuteri SXDT-32 and application thereof. The preservation number of the strain is CGMCC (China General Microbiological Culture Collection The strain has a good inhibition effect on escherichia coli and salmonella; the strain can be colonized in an animal body, and has strong tolerance to artificial cholate, artificial gastric acid and artificial intestinal juice; the feed additive can be firmly adhered and colonized in animal intestinal tracts, inhibits growth of pathogenic microorganisms in the intestinal tracts, improves animal intestinal tract immunity, relieves animal colitis, reduces animal diarrhea and death rate, improves animal intestinal tract health, and also can improve animal growth performance.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
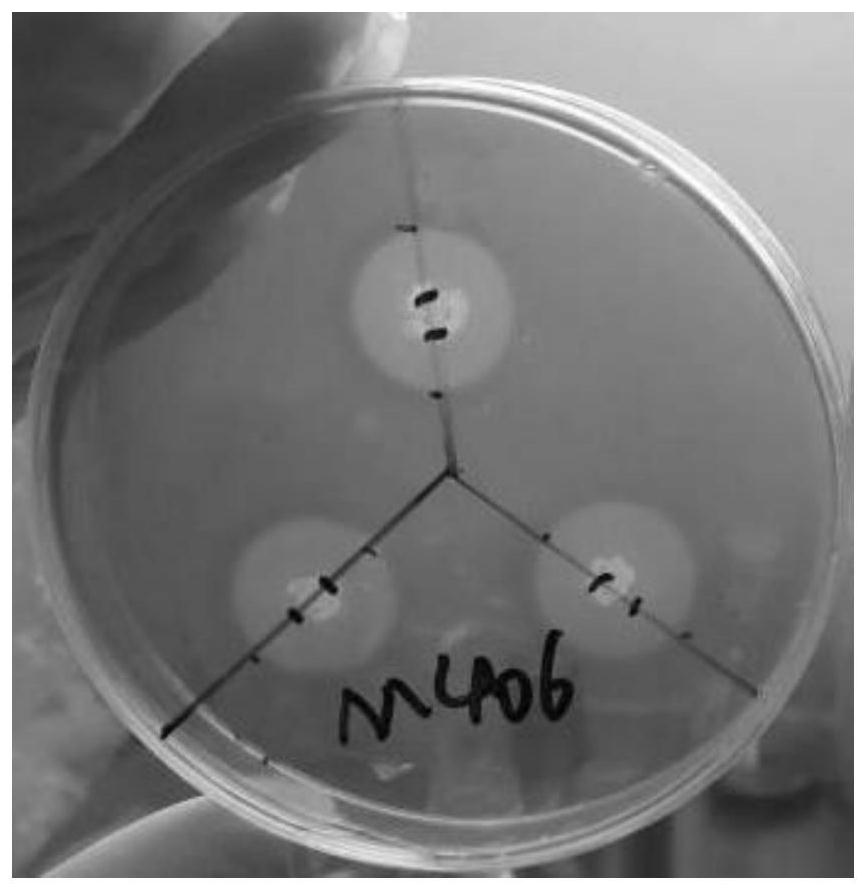
Bacillus subtilis m406 and its application in the preparation of bacteriocin and cellulase
ActiveCN109161498BImprove use valueHigh nutritional valueAntibacterial agentsBacteriaBiotechnologyAntibacterial activity
The invention discloses a bacillus subtilis M406 and its application in preparing bacteriocin and cellulase. Bacillus subtilis (Bacillus subtilis) M406 strain, its preservation number is CCTCC NO: M 2018234. The present invention focuses on one trait and supplements multiple traits to screen out a strain of probiotics, which is Bacillus subtilis M406, which improves the use value of the probiotics. The antibacterial active substance produced by the bacillus subtilis M406 can inhibit various harmful bacteria including S.typhimurium, Salmonella enteritidis, S.aureus, E.coli and Listeria monocytogenes in the intestinal tract of animals. The probiotics can ferment various sugars to produce acid and lower the pH value of intestinal juice. The bacillus subtilis M406 produces cellulase, which can effectively degrade structural carbohydrates in roughage, convert part of NSP into soluble sugar, fat, protein and amino acid components, and improve the nutritional value of roughage.
Owner:HUAZHONG AGRI UNIV
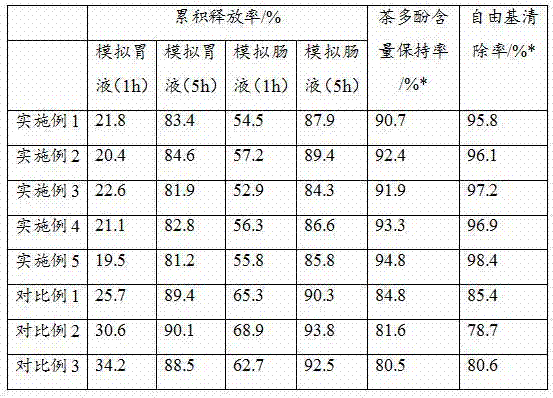
Multifunctional tea polyphenol healthcare product and preparation process thereof
ActiveCN107982514AImprove bioavailabilityImprove stabilityOrganic active ingredientsNervous disorderPhenolic content in teaSodium Caseinate
The invention discloses a multifunctional tea polyphenol healthcare product and a preparation process thereof. The healthcare product is prepared from the following raw materials in parts by weight: tea polyphenol, pollen typhae polysaccharide, yeast powder, sodium caseinate, a potato extract, alpha-cyclodextrin, soy isolate protein, sucrose octanoate and sodium octadecyl sulfate. The multifunctional tea polyphenol healthcare product disclosed by the invention is good in stability and can be slowly released in a simulated gastric fluid and intestinal fluid, the content of tea polyphenol is still 90% or greater after the healthcare product is placed for one month in a room temperature environment, meanwhile the free radical clearing rate of the healthcare product is as high as 95% or greater, the bioavailability of the tea polyphenol is greatly increased, and the healthcare product has relatively rich healthcare effects.
Owner:SUZHOU HEALTH COLLEGE
Application and method of fucoidan and its hydrolyzed oligosaccharides in the preparation of probiotic protective agent
ActiveCN110106163BStrong stress resistanceImprove survival rateOn/in organic carrierBiotechnologyFucoidan
The invention discloses the application and method of fucoidan and its hydrolyzed oligosaccharides in the preparation of probiotic protective agents. In the present invention, for the first time, fucoidan and its hydrolyzed oligosaccharides are embedded in probiotics, and then simulated in gastric acid and small intestinal fluid. Survival, the results show that the survival time of probiotics embedded with fucoidan and its hydrolyzed oligosaccharides is prolonged, and the effect of hydrolyzed oligosaccharides embedded is better than that of fucoidan, which lays the foundation for prolonging the high activity of probiotics in the gastrointestinal environment Base.
Owner:SOUTHWEST UNIV +1
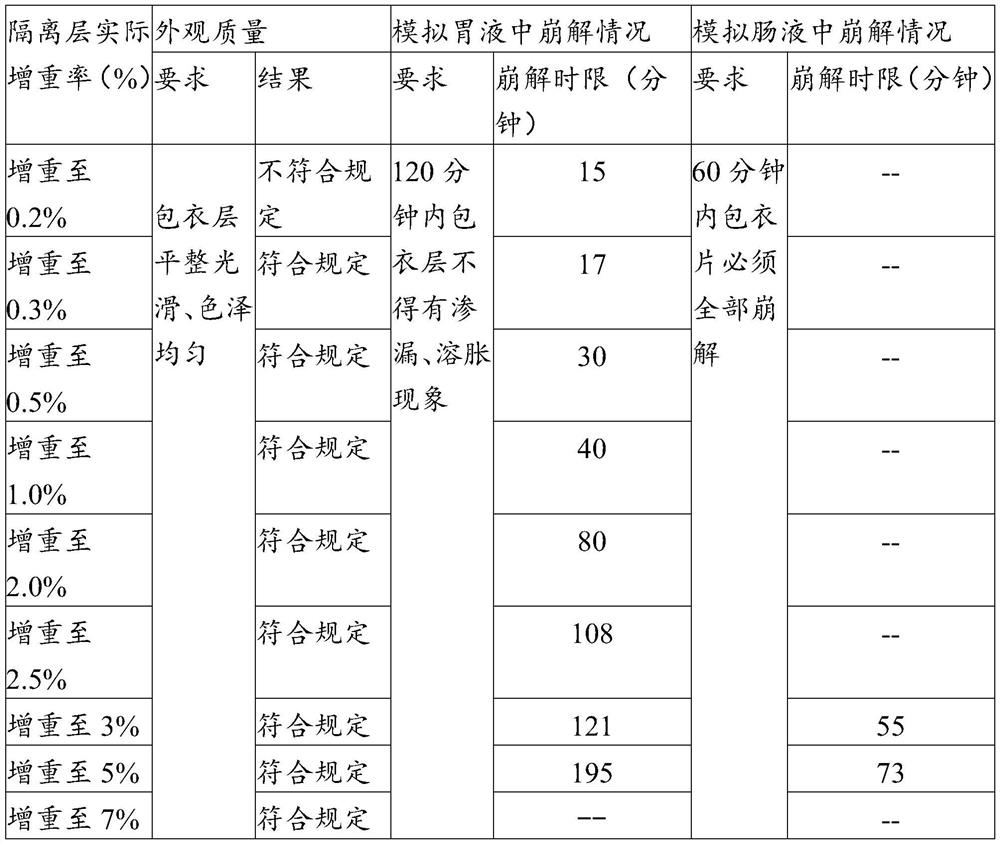
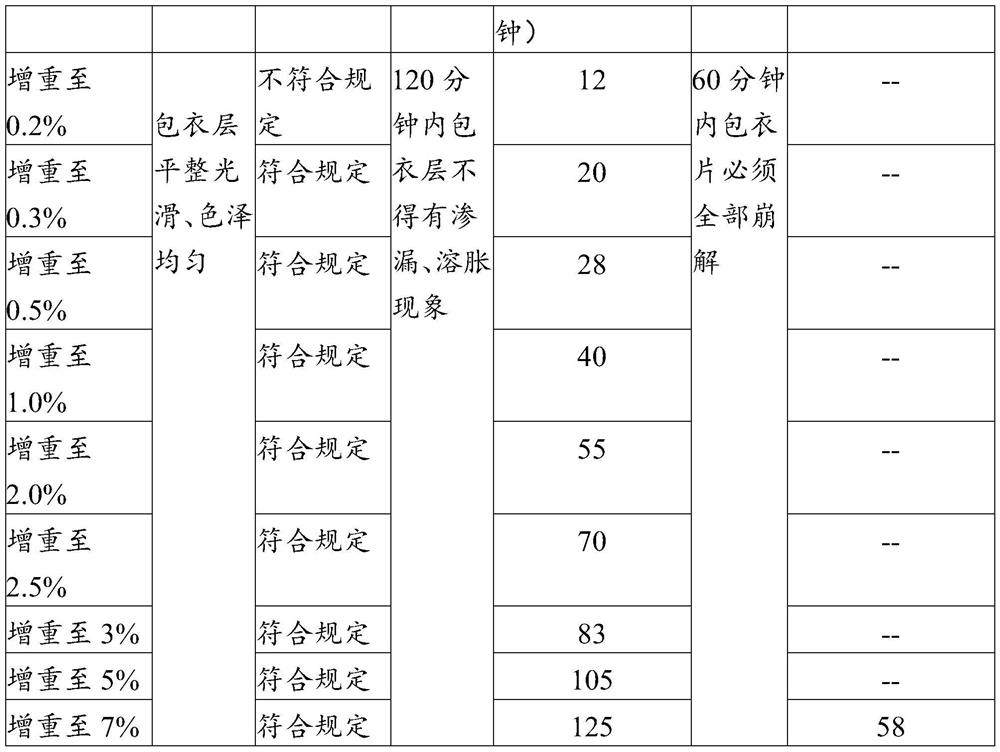
Enteric coating material and its preparation method and enteric products
The invention relates to an enteric coating material, a preparation method thereof and an enteric product. The enteric coating material of the present invention includes an isolation layer coating material and an enteric outer layer coating material for wrapping the material to be coated in sequence, wherein, in parts by weight, the isolation layer coating material includes: 2-8 parts of glue; 0.1-2 parts of hydroxypropyl methylcellulose or sodium carboxymethyl cellulose; 0.1-2 parts of magnesium stearate; glycerin, propylene glycol, polyethylene glycol, sorbitol, triethyl citrate 1-10 parts of one or more of esters, mannitol and isomalt; 40-180 parts of 75v / v%-85v / v% ethanol. The raw material cost of the coating material of the invention is low, the preparation method is simple, and the coated product can meet the use requirements of not disintegrating in simulated gastric juice for 2 hours and disintegrating in intestinal juice within 1 hour.
Owner:QINGDAO SHENGBANG HEALTH FOOD CO LTD
A strain of Lactobacillus plantarum capable of regulating ampicillin-induced intestinal flora disturbance
ActiveCN107988123BImprove toleranceGood probiotic propertiesMilk preparationBacteriaBiotechnologyAmpicillin
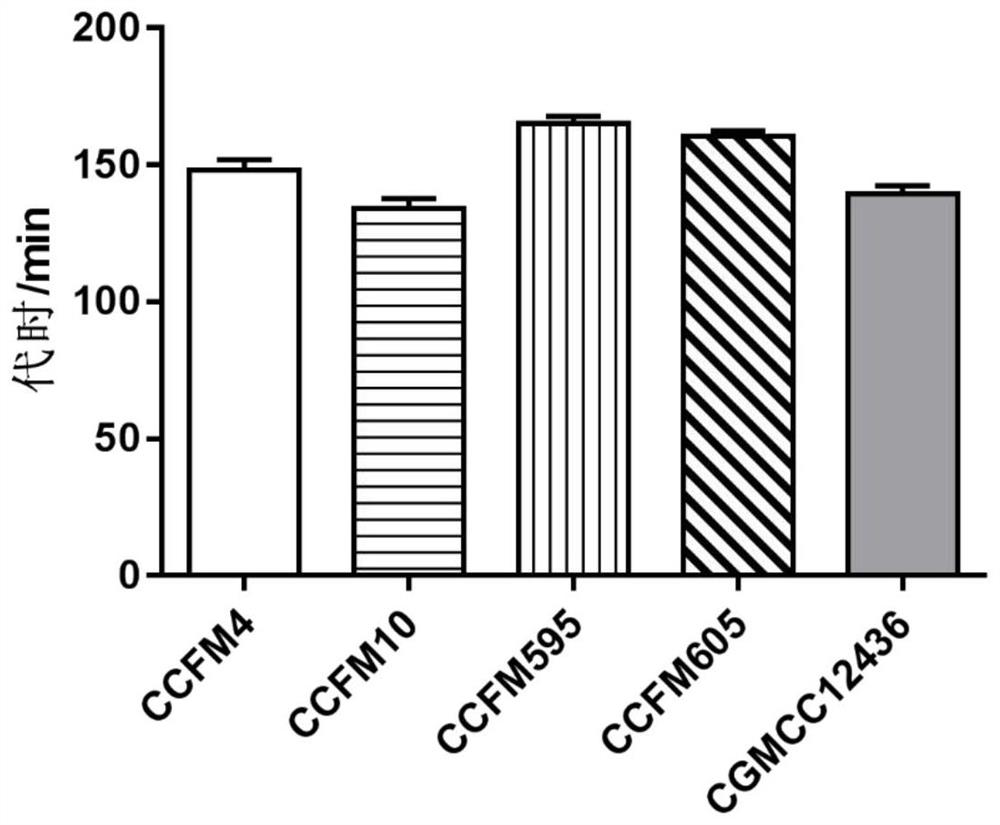
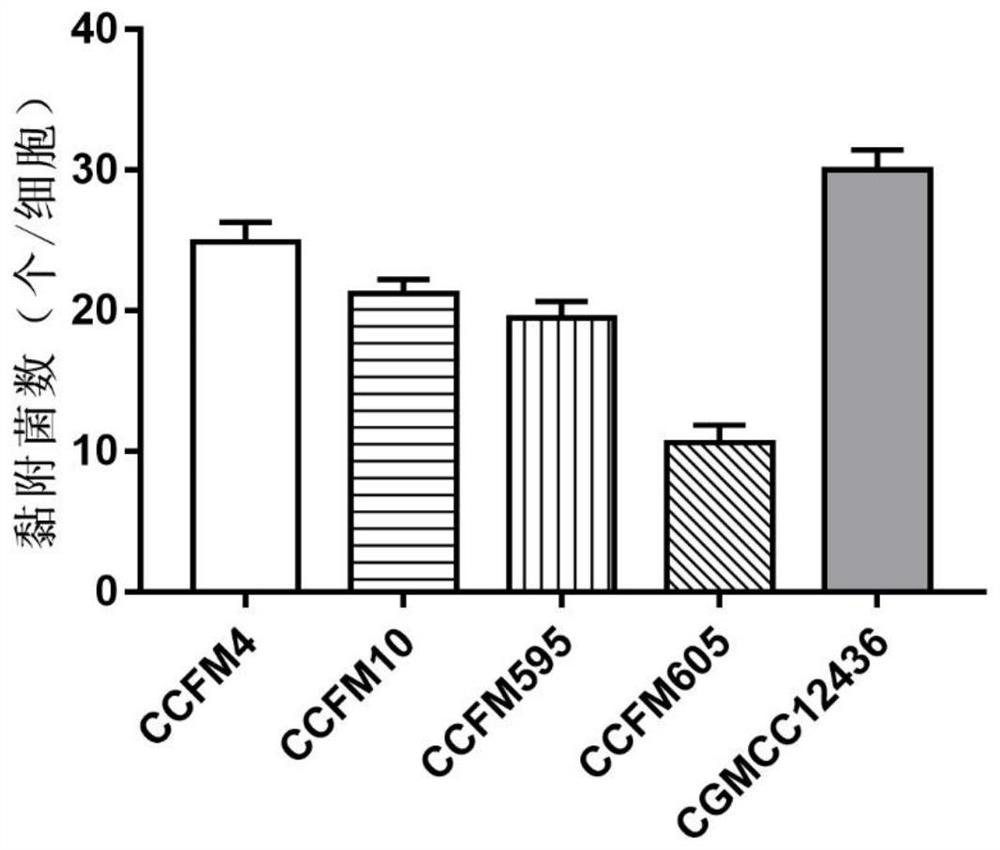
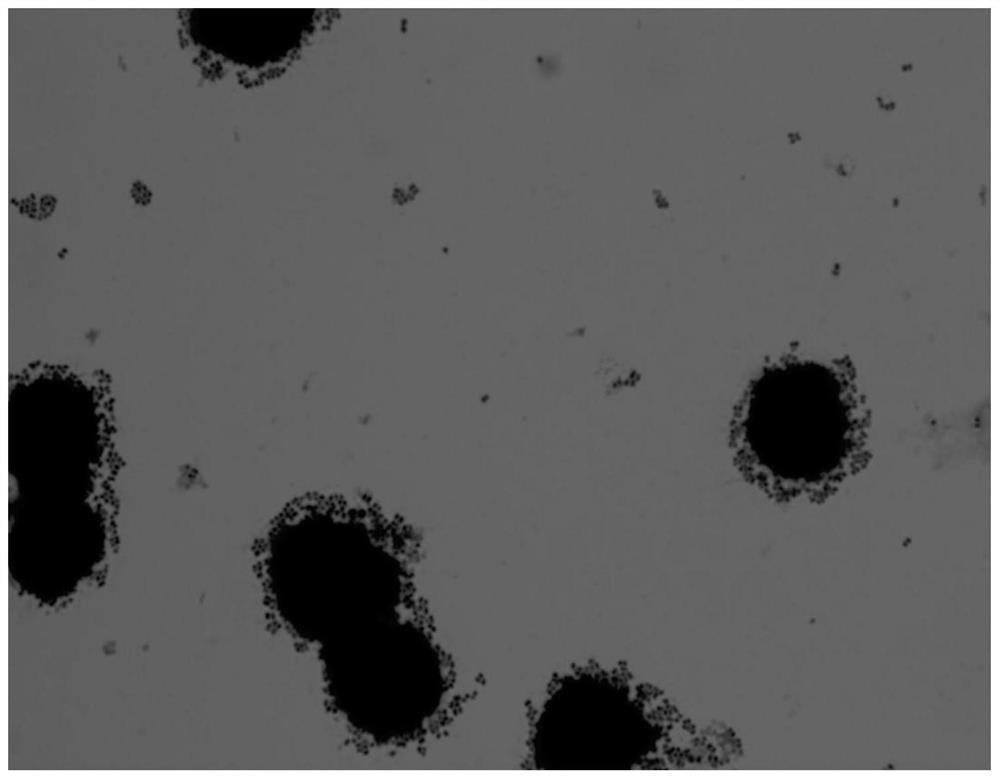
The invention discloses a strain of Lactobacillus plantarum capable of regulating ampicillin-induced intestinal flora disturbance, which belongs to the technical field of food. The present invention is to expand the cultivation of Lactobacillus plantarum (Lactobacillus plantarum) CGMCC12436, supplemented with skim milk, trehalose and sucrose, and prepare a biological preparation after freeze-drying. The live bacteria content of Lactobacillus plantarum CGMCC12436 in this preparation is greater than 10 9 CFU / g. The Lactobacillus plantarum CGMCC12436 provided by the present invention has a relatively low growth generation time, a high survival rate in simulated gastric juice and small intestinal juice in vitro, good adhesion to human HT-29 cells, and relatively low drug resistance in the drug sensitivity test. lower. The biological preparation made by the Lactobacillus plantarum can significantly restore the intestinal flora imbalance caused by ampicillin in mice, improve intestinal function, and has a good market application prospect.
Owner:JIANGNAN UNIV
Fish feed additive containing traditional Chinese medicine ingredients
InactiveCN113016963AImprove antioxidant capacityImprove meat qualityMetabolism disorderClimate change adaptationBiotechnologyNutrition
The invention discloses a fish feed additive containing traditional Chinese medicine ingredients, and relates to the field of fish culture, and the fish feed additive comprises the following components in parts by weight: 10-16 parts of tartaric acid, 8-10 parts of betaine, 7-9 parts of a phagostimulant, 7-10 parts of Toxicodendron vernicifluum leaves, 12-15 parts of zanthoxylum ailanthus, 5-10 parts of licorice, 2.5-4 parts of purslane, 1.5-2 parts of medicated leaven, 3-5 parts of polygonum multiflorum, 2-4 parts of gynostemma pentaphyllum and 20-30 parts of bile acid. The tartaric acid, the phagostimulant, the medicated leaven and the purslane are added into the additive disclosed by the invention, so that the feeding frequency of fishes in sultry weather can be increased, the effect of increasing appetite can be achieved, secretion of gastric juice and intestinal juice is promoted, nutrient absorption of organisms is promoted, nutrient substances are promoted to enter the organisms more quickly, and the food intake of the fishes is increased; the fish feed additive solves the problem that fishes are unwilling to ingest or have a slow ingestion speed in sultry weather, uses Chinese herbal medicines for compatibility, is derived from natural components of plants, is green and environment-friendly, has no toxic effects, and has high social and economic benefits.
Owner:深圳市沙利文生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com