Bacillus coagulans preparation and preparation method thereof
A technology of bacillus coagulans and preparations, applied in the field of bacillus coagulans preparations and its preparation, can solve the problems of high production cost not suitable for industrial application, lower actual utilization rate of probiotic preparations, complicated coating process, etc., and achieve recovery of microorganisms Effects of barrier function, inhibition of intestinal peristalsis, and shortening of disease course
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Isolation and screening of embodiment 1 bacterial strain
[0039] 1. Primary screening of Bacillus
[0040] Take 1g of fermented sour cabbage, smash it with a masher, soak it with 10ml of 0.85% physiological salt for 10 minutes, put it into a sterile sample bag, beat it with a homogenizer, and pour it into a test tube, set the water bath at 70°C Heated for 10 minutes, and the suspension was diluted gradually, and 10 -1 、10 -2 、10 -3 Three dilution gradients of 100ul were spread on the MRS medium, and cultured in a 37°C incubator under aerobic conditions for 48h. After a single colony grew on the plate, microscopic examination was performed respectively. According to the results of microscopic examination, the applicant screened out 10 bacillus strains, which were named NH-1, NH-2, ..., NH-10 respectively.
[0041] 2. Re-screening of Bacillus
[0042] Liquid fermentation medium: weigh 3.0g yeast powder, 5.0 peptone, 2.0g beef extract, K 2 HPO 4 3g, MnSO 4 0.005g, ...
Embodiment 2
[0051] Example 2 strain identification
[0052] 2.1 Identification of colony morphology
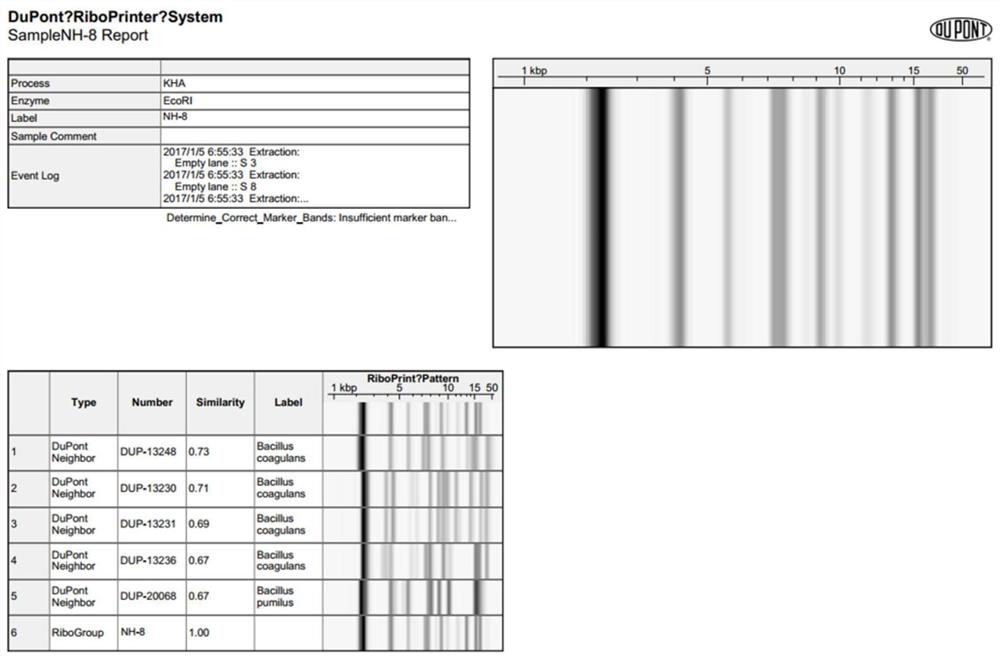
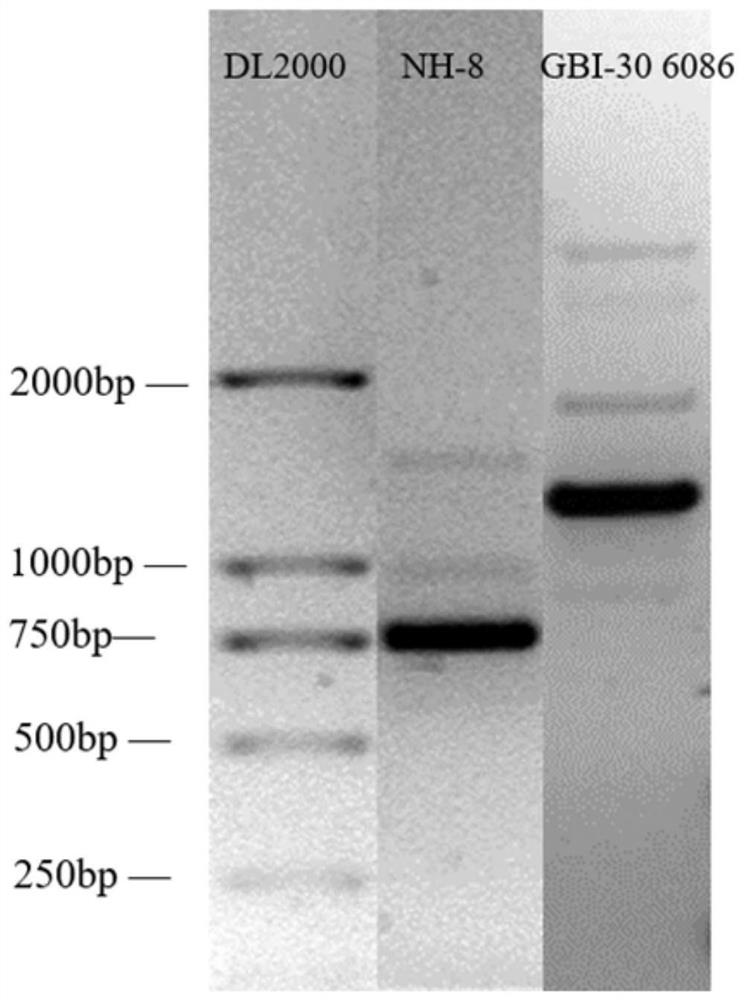
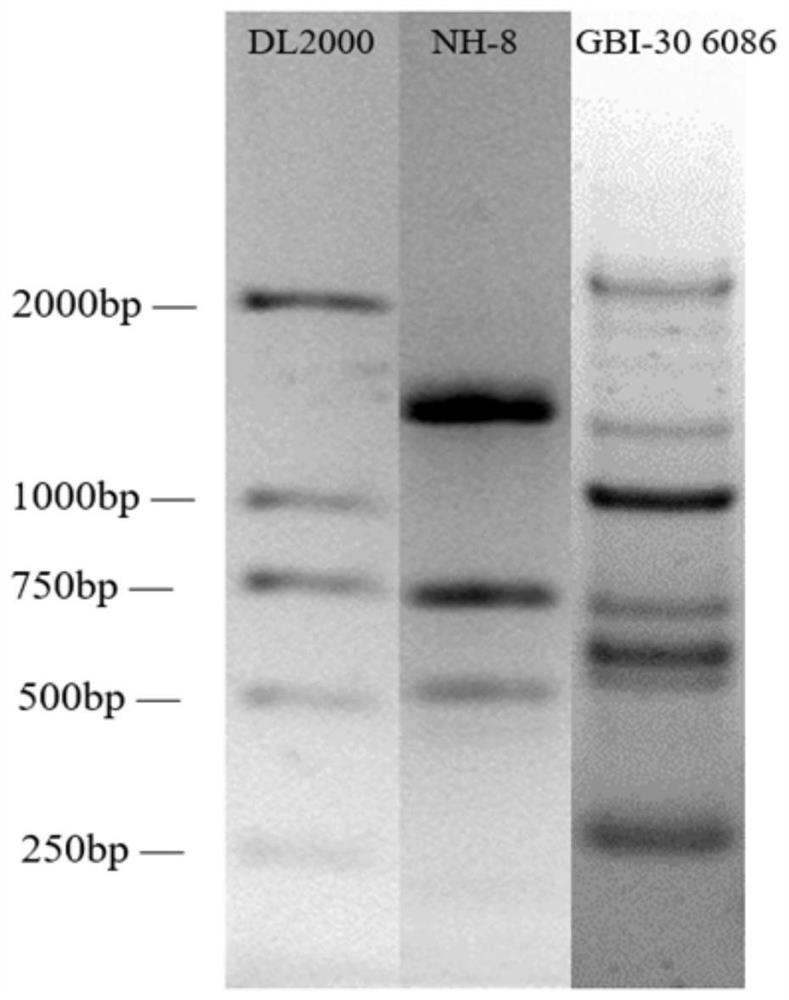
[0053] The single colony of the NH-8 strain is milky white and shiny, the surface of the colony is smooth and moist, the edges are neat, and the diameter of the colony is 2-3mm. Under the microscope, it is rod-shaped, arranged in single, pair or chain, and the spores are terminal.
[0054] 2.2 Identification of physiological and biochemical characteristics
[0055] The preparation of the inoculum in this embodiment is as follows: under sterile conditions, take an appropriate amount of fresh bacterial solution, centrifuge at 5000rpm for 5min, wash 2 times with PBS buffer, then resuspend with the same volume of PBS buffer and dilute 50 times as the inoculum. liquid.
[0056] 1. Temperature growth range experiment
[0057] Under sterile conditions, the inoculum was inoculated into 10 mL of MRS liquid medium at a 10% inoculation amount, and 10 mL of MRS liquid medium without inoculation w...
Embodiment 3
[0114] Example 3 Bacillus coagulans VHProbi C08 Salinity Tolerance Test
[0115] Under sterile conditions, inoculate 10% of the inoculum into 5 mL of MRS liquid medium with salt concentrations of 1%, 2%, 3%, 4%, 5%, 6%, 7%, and 8%. In this method, 5mL of MRS liquid medium without inoculation was used as a control, and placed in a constant temperature shaking culture at 37°C to observe whether the medium became turbid.
[0116] The results showed that Bacillus coagulans VHProbi C08 grew at a salt concentration of 1% to 7%, and did not grow at a salt concentration of 8%. The maximum salt concentration of Bacillus coagulans VHProbi C08 was 7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com