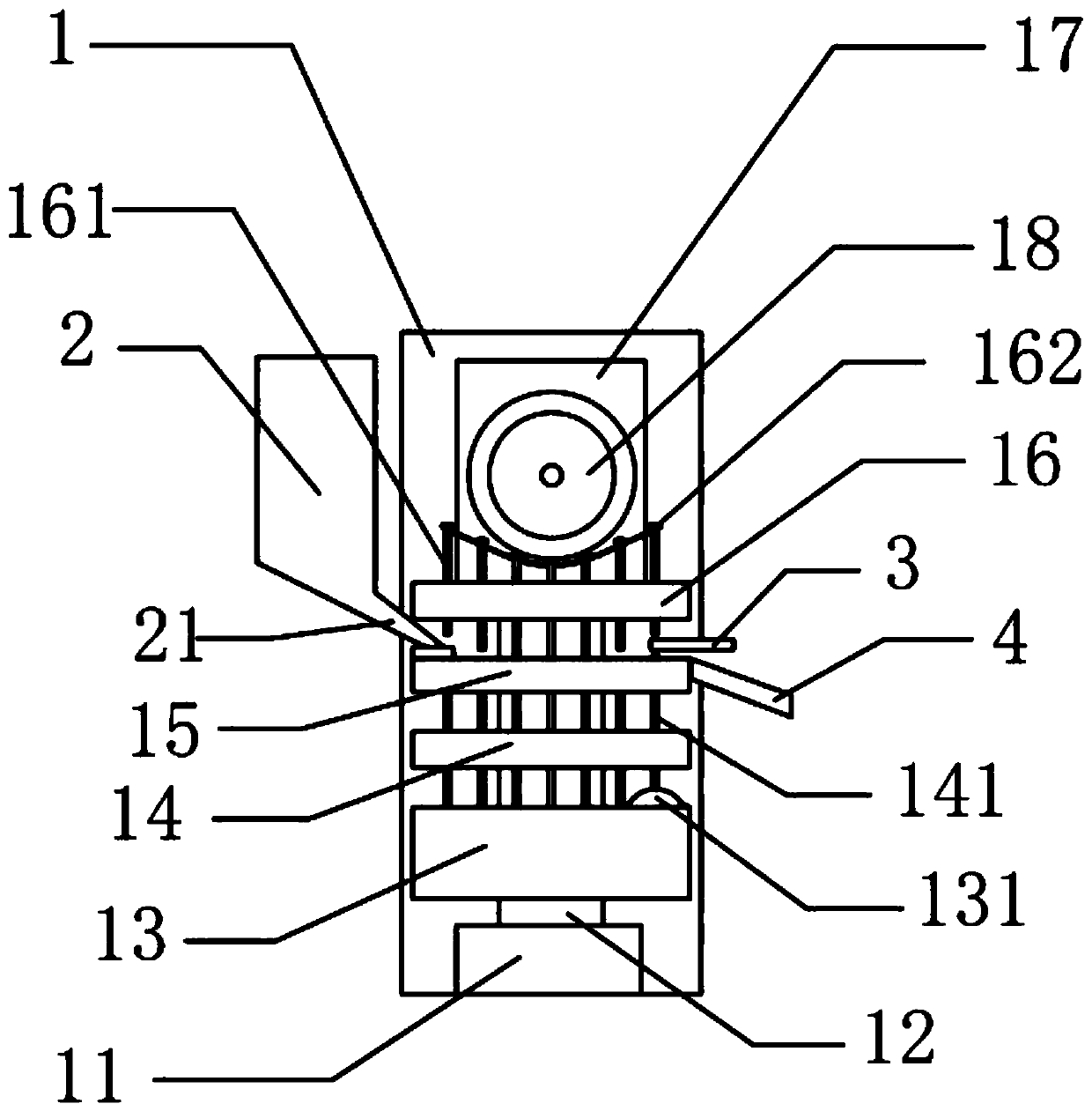
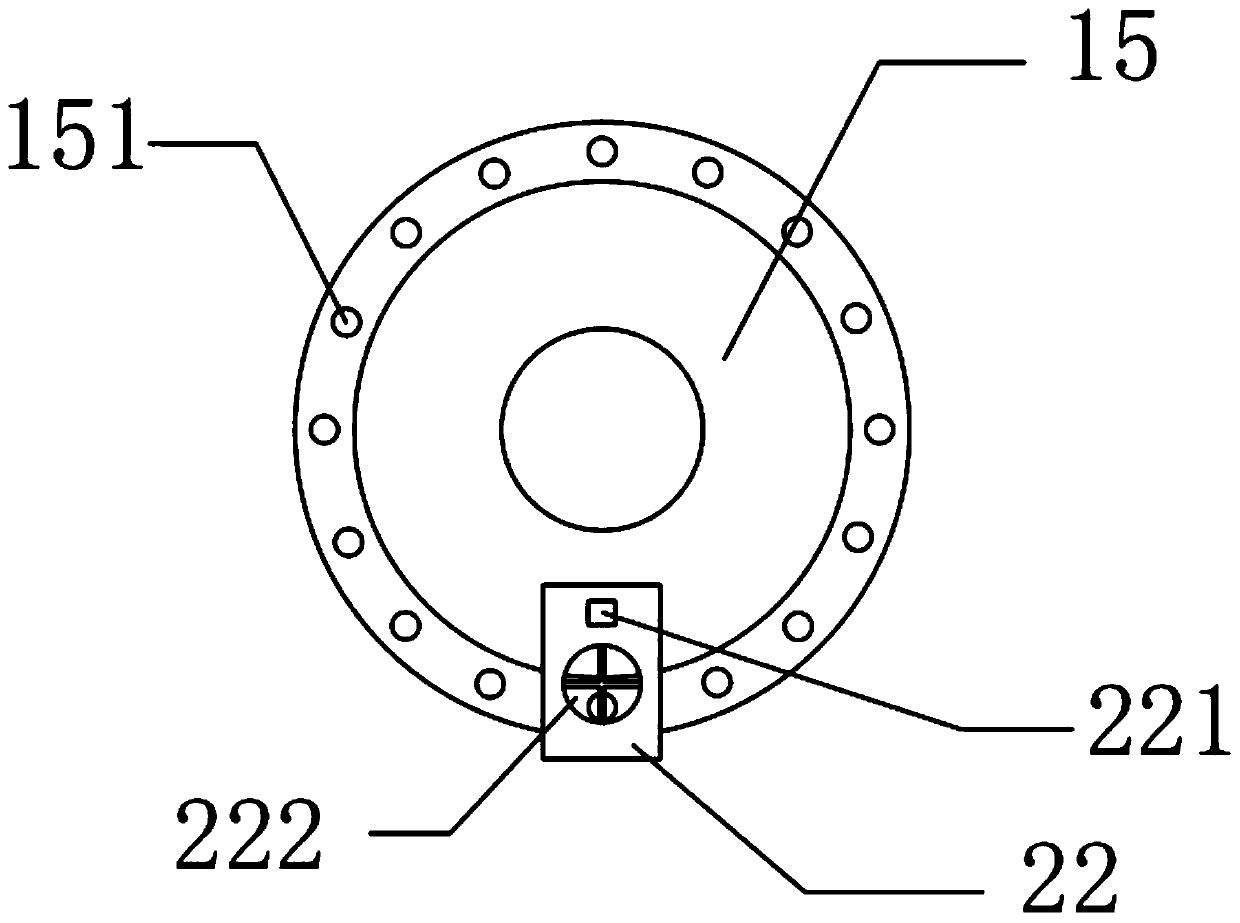
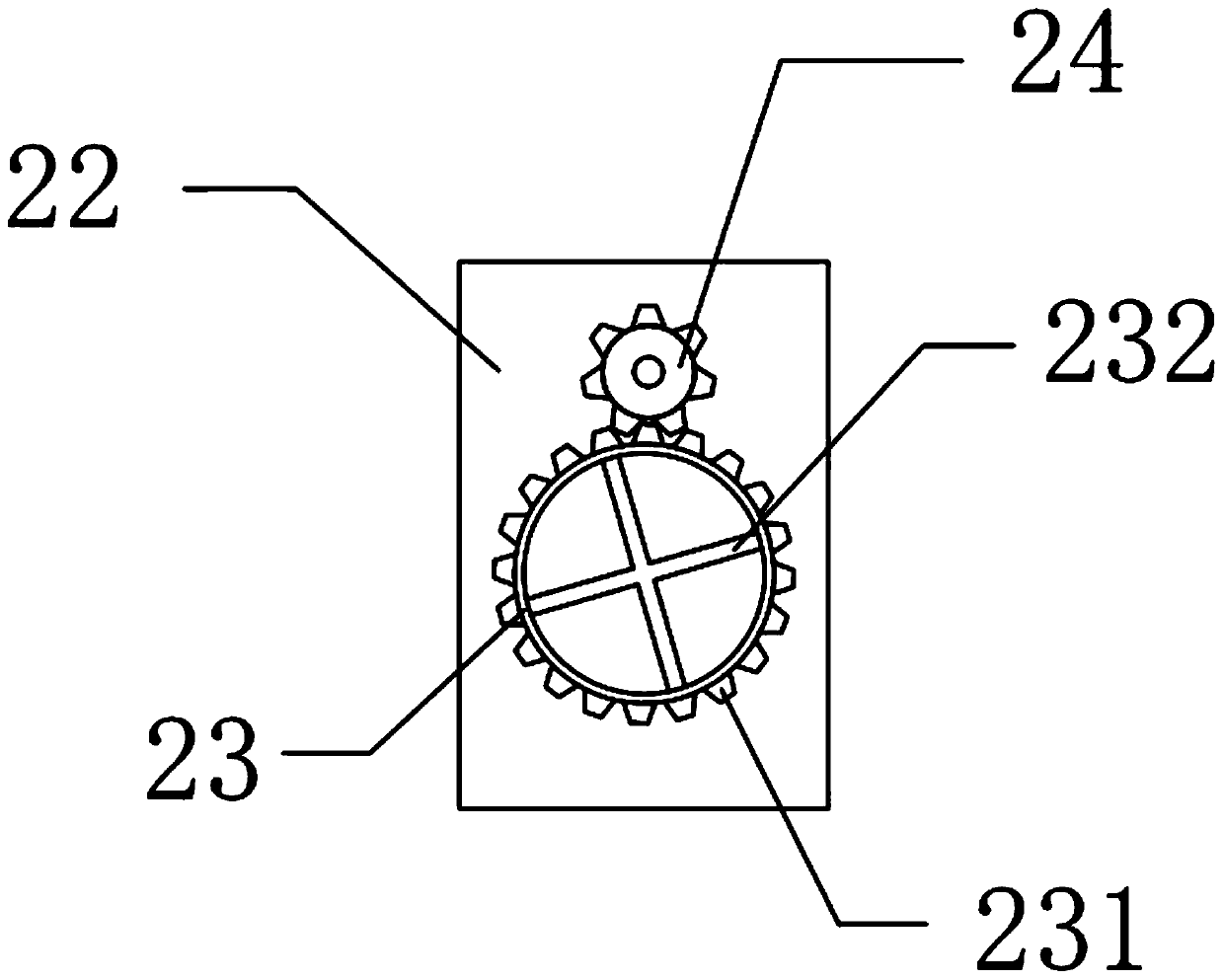
Ipiaconitine enteric-coated preparation
A technology of Ipiwutide and enteric-coated preparations, which is applied in anti-inflammatory agents, pill delivery, peptide/protein components, etc., to achieve the effect of convenient absorption, simple pilot test process, and promotion of cell differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] An enteric-coated preparation of ipiwutide, made of the following raw materials in parts by weight: 15 parts of ipiwutide bulk drug, 1.5 parts of dextrin, 1 part of calcium sulfate, 1.8 parts of starch, 0.1 part of talcum powder, and 2 parts of starch slurry , 4 parts of croscarmellose sodium, 0.5 parts of acrylic resin type I, 0.5 parts of acrylic resin type II, 12 parts of ethanol solution, 0.2 parts of propylene glycol, 0.3 parts of castor oil, 0.1 part of magnesium stearate, Sichuan wax 0.03 copies;
[0043] The ipiwutide enteric-coated preparation is made by the following steps:
[0044] Step S1: Add the ipiwutide raw material drug, dextrin, calcium sulfate, starch, and talcum powder into a pulverizer, and pulverize it. Calcium and starch are added to the mixer and mixed for 5-10 minutes to prepare the first mixture. The first mixture and starch slurry are added to the stirring tank, and the stirring speed is 500-800r / min, and stirring is carried out for 20-30 min...
Embodiment 2
[0054] An enteric-coated preparation of ipiwutide, made of the following raw materials in parts by weight: 18 parts of ipiwutide bulk drug, 1.7 parts of dextrin, 1.3 parts of calcium sulfate, 1.9 parts of starch, 0.3 parts of talcum powder, and 2.3 parts of starch slurry , 4.5 parts of croscarmellose sodium, 0.7 parts of acrylic resin type I, 0.3 parts of acrylic resin type II, 13.5 parts of ethanol solution, 0.3 parts of propylene glycol, 0.4 parts of castor oil, 0.3 parts of magnesium stearate, Sichuan wax 0.04 copies;
[0055] The ipiwutide enteric-coated preparation is made by the following steps:
[0056] Step S1: Add the ipiwutide raw material drug, dextrin, calcium sulfate, starch, and talcum powder into a pulverizer, and pulverize it. Calcium and starch are added to the mixer and mixed for 5-10 minutes to prepare the first mixture. The first mixture and starch slurry are added to the stirring tank, and the stirring speed is 500-800r / min, and stirring is carried out fo...
Embodiment 3
[0061] An enteric-coated preparation of ipiwutide, made of the following raw materials in parts by weight: 20 parts of ipiwutide bulk drug, 2 parts of dextrin, 1.5 parts of calcium sulfate, 2 parts of starch, 0.5 parts of talcum powder, and 2.5 parts of starch slurry , 5.3 parts of croscarmellose sodium, 1 part of acrylic resin type I, 1 part of acrylic resin type II, 15 parts of ethanol solution, 0.4 parts of propylene glycol, 0.5 parts of castor oil, 0.5 parts of magnesium stearate, Sichuan wax 0.05 copies;
[0062] The ipiwutide enteric-coated preparation is made by the following steps:
[0063] Step S1: Add the ipiwutide raw material drug, dextrin, calcium sulfate, starch, and talcum powder into a pulverizer, and pulverize it. Calcium and starch are added to the mixer and mixed for 5-10 minutes to prepare the first mixture. The first mixture and starch slurry are added to the stirring tank, and the stirring speed is 500-800r / min, and stirring is carried out for 20-30 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com