Docetaxel solid lipid nanoparticle lyophilized preparation and preparation method
A technology of solid lipid nano and docetaxel, which is applied in the direction of microcapsules, nanocapsules, and capsule delivery, can solve the problems of unfavorable drug absorption, electrostatic repulsion, etc., reduce the first-pass effect, increase adhesion, The effect of extending the contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
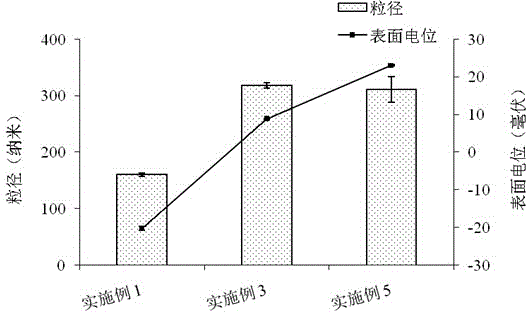
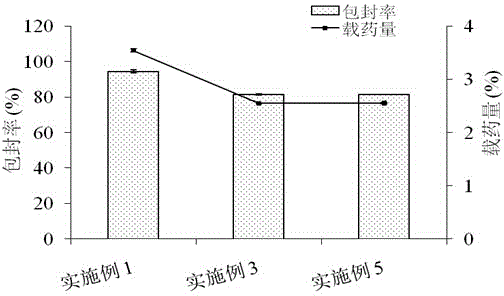
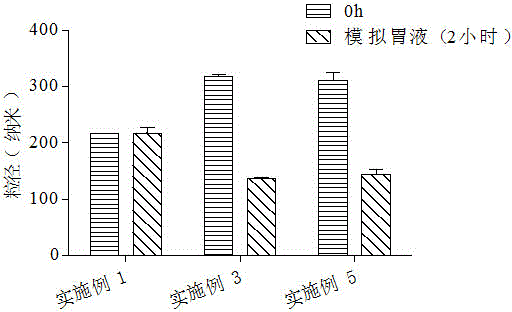
[0043] Example 1: Preparation of unmodified docetaxel solid lipid nanoparticles.
[0044] First, weigh 3 mg of docetaxel and 20 mg of lecithin and place them in a vial, add 2 ml of dichloromethane to dissolve to obtain a drug solution; secondly, weigh 60 mg of glyceryl monostearate, melt it in a water bath at 70°C, and mix with The above drug solution was mixed to obtain a uniform oil phase; then, the above oil phase was added to 4ml Tween 80 aqueous solution (the mass concentration of Tween 80 was 1.0g / 100ml), and 200w ultrasonic dispersion was performed for 60s to obtain a dispersion liquid. The above dispersion was stirred at room temperature for 3 hours, and the organic solvent was evaporated to obtain 4ml of docetaxel solid lipid nanoparticle suspension, which contained 0.123g of docetaxel solid lipid nanoparticles, and the particle diameter was 160.5nm. The potential is -20.2mv. According to the weight-to-volume ratio of lyoprotectant:suspension=2.5g:100ml, add 0.1g suc...
Embodiment 2
[0045] Example 2: Preparation of chitosan-modified docetaxel solid lipid nanoparticles.
[0046] First, weigh 3 mg of docetaxel and 20 mg of lecithin and place them in a vial, add 2 ml of dichloromethane to dissolve to obtain a drug solution; secondly, weigh 60 mg of glyceryl monostearate, melt it in a water bath at 70°C, and mix with The above drug solution was mixed to obtain a uniform oil phase; then, the above oil phase was added to 4ml Tween 80 aqueous solution (the mass concentration of Tween 80 was 1.0g / 100ml), and 200w ultrasonic dispersion was performed for 60s to obtain a dispersion liquid. The above-mentioned dispersion liquid was stirred at room temperature for 3h, and the organic solvent was evaporated to obtain 4ml docetaxel solid lipid nanoparticle suspension; then, according to the docetaxel solid lipid nanoparticle suspension: chitosan aqueous solution =1:2 volume ratio, slowly drop 8ml chitosan aqueous solution (the mass concentration of chitosan is 0.1g / 100m...
Embodiment 3
[0047] Example 3: Preparation of chitosan-modified docetaxel solid lipid nanoparticles.
[0048] First, weigh 3 mg of docetaxel and 20 mg of lecithin and place them in a vial, add 2 ml of dichloromethane to dissolve to obtain a drug solution; secondly, weigh 60 mg of glyceryl monostearate, melt it in a water bath at 70°C, and mix with The above drug solution was mixed to obtain a uniform oil phase; then, the above oil phase was added to 4ml Tween 80 aqueous solution (the mass concentration of Tween 80 was 1.0g / 100ml), and 200w ultrasonic dispersion was performed for 60s to obtain a dispersion liquid. The above-mentioned dispersion liquid was stirred at room temperature for 3h, and the organic solvent was evaporated to obtain 4ml docetaxel solid lipid nanoparticle suspension; then, according to the docetaxel solid lipid nanoparticle suspension: chitosan aqueous solution =1:4 volume ratio, slowly drop 16ml chitosan aqueous solution (the mass concentration of chitosan is 0.1g / 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com