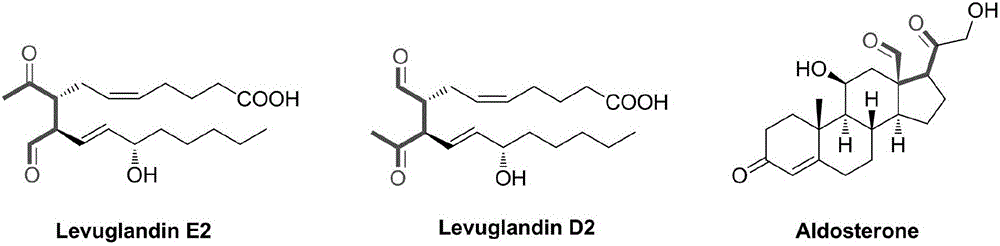
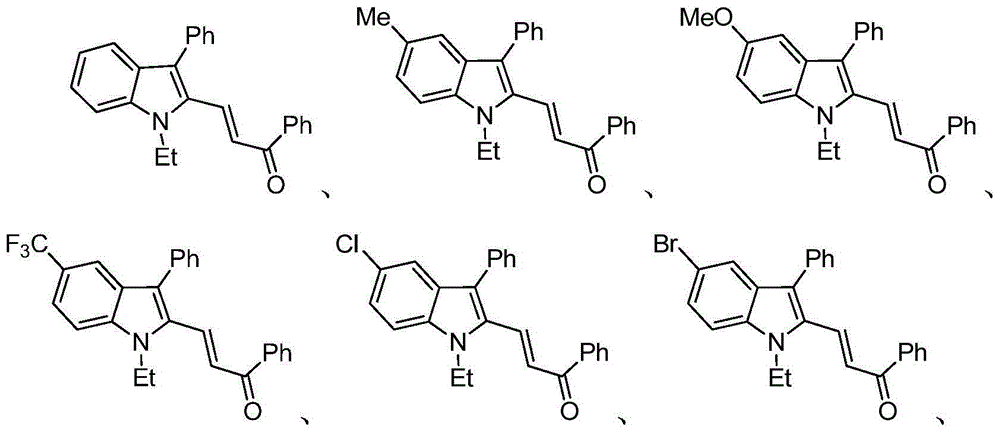
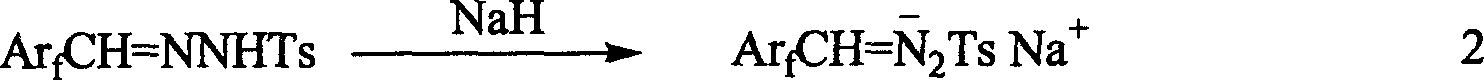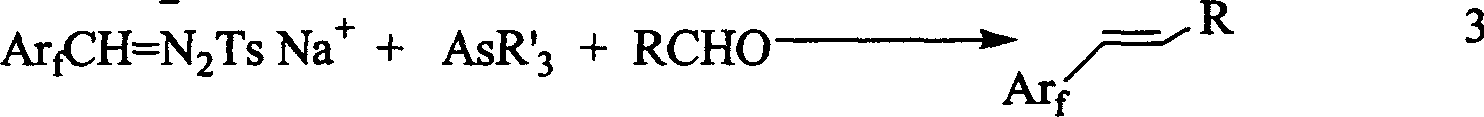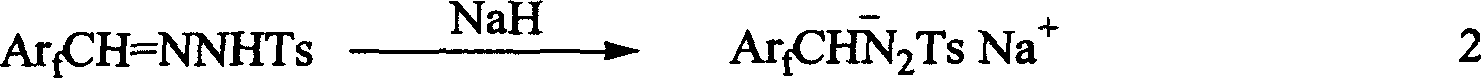Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "P-toluenesulfonyl hydrazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Modified foaming agent and preparation method thereof
ActiveCN1919901AImprove liquidityGood dispersionOther chemical processesMolecular sieveSodium bicarbonate
The invention discloses a modification vesicant, which consists of modification vesicant and molecular sieve, wherein the dosage of the molecular sieve is 0.5-30 parts by weight, when the modification vesicant is 100 parts by weight; the modification vesicant is elected from azobisformamide, 4, 4'-oxo dianil sulfuryl, benzene sulfonyl hydrazide, Unifor, dinitroso pentamethine fouramine and sodium bicarbonate or their mixtures. The modification vesicant is prepared by mixing up with the kibbling modification vesicant and molecular sieve in mixing plant proportions by weight.
Owner:HANGZHOU HI TECH FINE CHEM
Synthesis method of 25-hydroxycholesteryl acetate-7-p-toluenesulfonylhydrazone
The invention relates to the technical field of medicine chemical engineering and particularly relates to a synthesis method of 25-hydroxycholesteryl acetate-7-p- toluenesulfonylhydrazone. The synthesis method includes following steps: (1) performing a reaction between 25-hydroxy-7-ketocholesteryl acetate and p-toluenesulfonylhydrazide under a mechanical grinding condition; and (2) performing recrystallization to a reaction product in the step (1) to obtain the 25-hydroxycholesteryl acetate-7-p-toluenesulfonylhydrazone. The method is free of an acidic catalyst, is greatly reduced in usage of organic solvent, is simple in operation, is high in yield, is less in waste gas, waste water and solid waste and is simple in post-treatment. The product is easy to separate and purify.
Owner:ZHEJIANG GARDEN BIOCHEM HIGH TECH +2
1-aryl-2 perluoro or polyfluoro phenyl ethylene and its derirative, its synthesis and application
InactiveCN1475471ACarboxylic acid nitrile preparationOrganic compound preparationTosylhydrazoneCarbene
A 1-aryl-2 perfluoro (or polyfluoro) phenylethene and its derivative used as fluoric organic luminescent material or electrically conductive polymer is prepared from perfluoro (or polyfluoro) phenylformaldehyde through condensating with toluenesulfonyl diamine, dehydrogenating by sodium hydride, reacting on phase-transfer catalyst to generate perfluoro (or polyfluoro) phenyldiazo, catalytic decomposing by catalyst to generate metal carbene, capturing it by triphenyl (or trialkyl) arsenic, and condensating reaction on aldehide.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Formula of modified styrene-butadiene rubber (SBR)
The invention discloses a formula of modified styrene-butadiene rubber (SBR). The modified SBR comprises SBR and auxiliary additives, wherein the auxiliary additives comprise p-toluenesulfonhydrazide serving as a foaming agent, ethylene-vinyl acetate copolymer serving as a toughening agent, acrylate copolymer serving as an impact modifier, vinyltriethoxysilane serving as a coupling agent and a carbon fiber serving as a filling agent. After various modification aids are added into the SBR, the modified SBR has high flexibility and elasticity, and breaking elongation and tensile strength are improved; and in addition, an aid for improving reaction speed is added in the modification process, so that reaction time is greatly shortened, and processing cost is reduced.
Owner:SUZHOU XINGWU ENG PLASTIC
Chenodeoxycholic acid and preparation method of intermediate of chenodeoxycholic acid
The invention discloses chenodeoxycholic acid and a preparation method of an intermediate of the chenodeoxycholic acid. In the invention, commercial hyodeoxycholic acid is used as a raw material, andthe preparation method comprises the following steps: performing side chain carboxylmethylation,recrystallization,6 alpha-hydroxyl selective oxidation, and introducing into beta-bromine at the position 7 to obtain an intermediate III with the total yield of 16%; and reducing and hydrolyzing the intermediate III to obtain the chenodeoxycholic acid. The method is short in reaction route, is free ofreagents, such astrimethylchlorosilane and highly-toxic p-toluenesulfonylhydrazide, and is relatively low in environmental pollution; and the synthesized intermediate III plays an important role in the synthesis of cholic acid drugs.
Owner:SHANDONG RUIYING PIONEER PHARMA +1
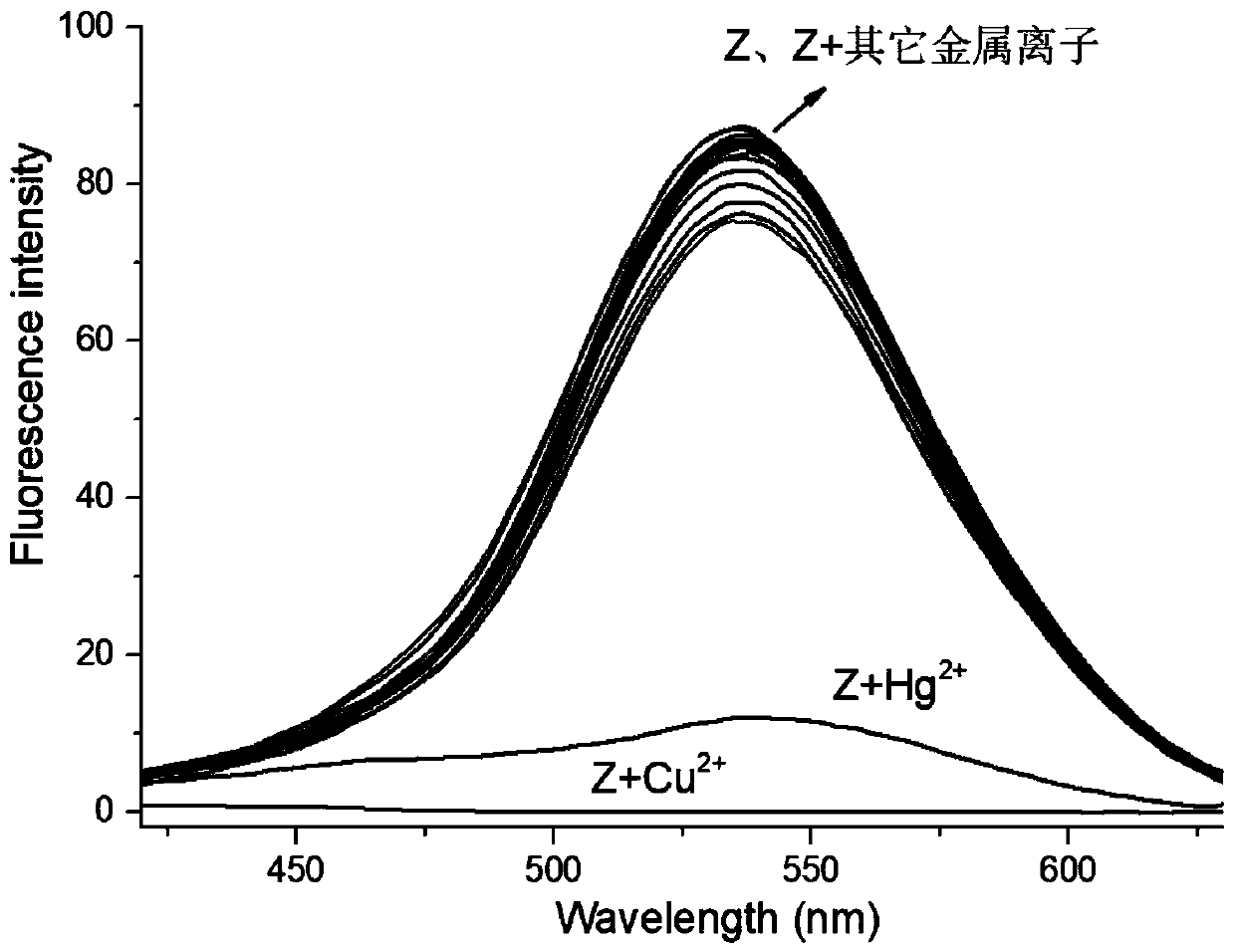
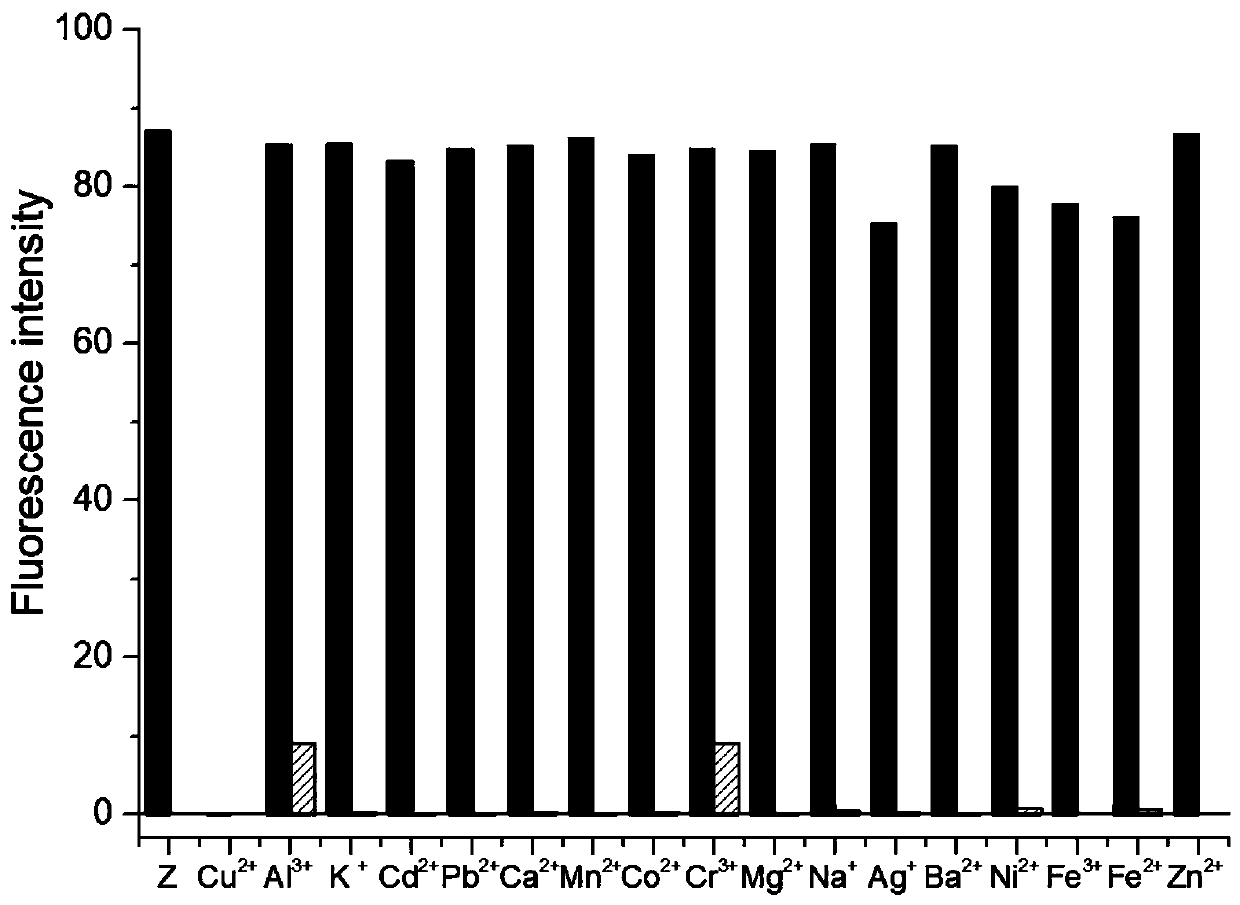
Bifunctional fluorescent probe for identifying copper ions and mercury ions as well as preparation method and application thereof
ActiveCN109722241ARaw material economySimple post-processingOrganic chemistryMaterial analysis by observing effect on chemical indicatorHexamethylenetetramineHydrazine compound
The invention discloses a bifunctional fluorescent probe for identifying copper ions and mercury ions as well as a preparation method and application thereof relates to a bifunctional fluorescent probe as well as a preparation method and application thereof, and aims at solving the problems that the existing probe for independently identifying the copper ions and the mercury ions is complex in synthesis steps, and low in selectivity and sensitivity. The bifunctional fluorescent probe is a covalent combination of 2-(2-hydroxy phenyl) benzothiazole and p-toluene sulfonyl hydrazine. The method comprises the following steps: 1, reacting 5-methylsalicylaldehyde with o-aminobenzenethiol to obtain a compound 1; 2, reacting the compound 1 obtained in the step 1 with urotropine to obtain a compound2; 3, reacting the compound 2 obtained in the step 2 with the p-toluene sulfonyl hydrazine to prepare a target compound z. The synthesis of the bifunctional fluorescent probe can be completed by onlythree steps, and the post-treatment process is relatively simple; the probe can be used for identifying both the copper ions and the mercury ions, the selectivity is good, the anti-interference capability is high, and the detection limit is low. The bifunctional fluorescent probe is used for detecting heavy metal ions.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
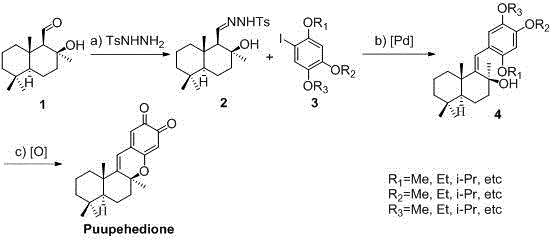
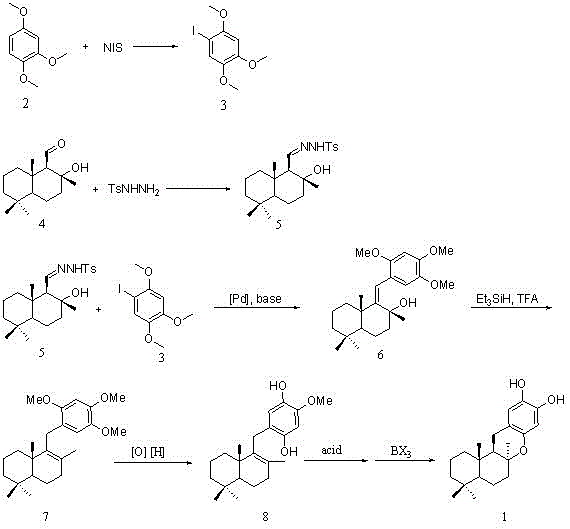
Synthesis method of marine natural product Puupehedione
ActiveCN106083803AFew reaction stepsSuitable for industrial productionOrganic chemistryChemical synthesisIsomerization
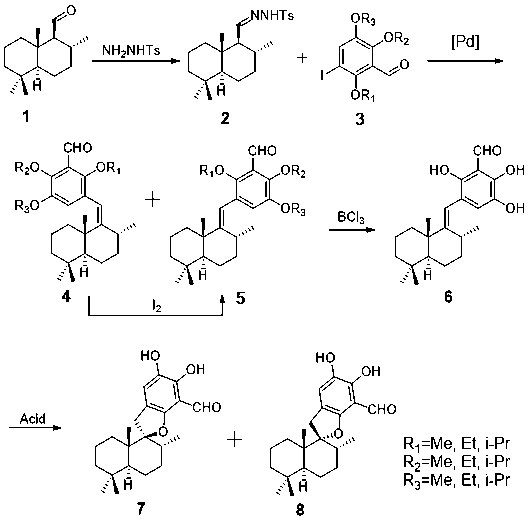
The invention relates to a synthesis method of a marine natural product Puupehedione, and belongs to the field of chemical synthesis. (-)salvia sclarea aldehyde and 1-iodine-2,4,5-trialkoxy benzene are used as starting materials, (-)salvia sclarea aldehyde and p-toluenesulfonhydrazide generate salvia sclarea hydrazone 2, salvia sclarea hydrazone 2 and 1-iodine-2,4,5-trialkoxy benzene form a framework compound 4 of Puupehedione under the catalysis of palladium in a coupling manner, the framework compound 4 is oxidized into an intermediate quinine under the action of an oxidizing agent, and quinine and C-8 site hydroxyl are subjected to reaction, so as to realize cyclization, isomerization and dealcoholization reactions to obtain natural product Puupehedione in one step. The synthesis method has the characteristics of being few in reaction steps, simple and convenient to operate, good in product selectivity and suitable for industrial production.
Owner:威海惠安康生物科技有限公司
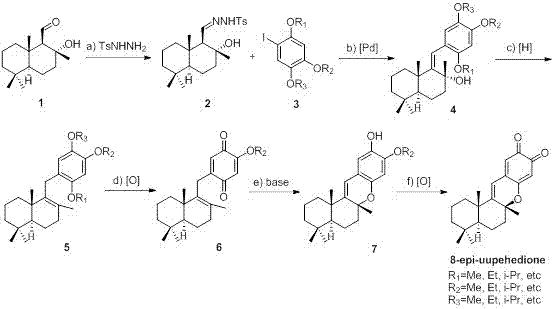
Synthetic method of 8-epi-puupehedione
The invention relates to a synthetic method of a sea natural product 8-epi-puupehedione, which belongs to the field of chemical synthesis. According to the invention, salvia sclarea aldehyde and 1-iodine-2,4,5-trialkoxy benzene are used as starting materials, salvia sclarea aldehyde and p-toluenesulfonhydrazide generate salvia sclarea hydrazone 2, the salvia sclarea hydrazone 2 and 1-iodine-2,4,5-trialkoxy benzene form a framework compound 4 of puupehedione under the catalysis of palladium in a coupling manner, the framework compound 4 is subjected to one-step hydroxyl reduction and double-bond isomerization under the action of a reducing agent to obtain an intermediate 5, the intermediate 5 is oxidized into an intermediate quinone compound 6 under the action of an oxidizing agent, and isomerization and Diels-Alder ring closure are realized for the quinone compound 6 under alkaline condition, then an intermediate 7 is obtained, and the intermediate 7 is further oxidized to obtain the final product 8-epi-puupehedione. The method has the characteristics of less reaction step, simple operation, and good products selectivity, and is suitable for industrial production.
Owner:威海创惠环保科技有限公司
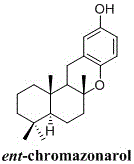
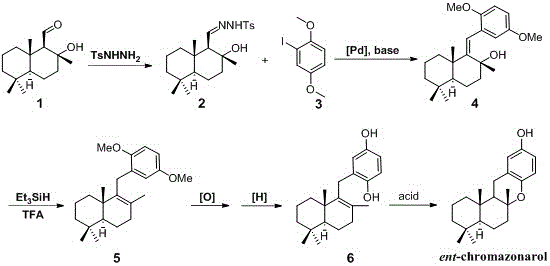
Synthetic method of marine terpenoid natural product namely ent-chromazonarol
ActiveCN105837549AFew reaction stepsSuitable for industrial productionOrganic chemistryChemical synthesisIsomerization
The invention relates to a synthetic method of a marine terpenoid natural product namely ent-chromazonarol, and belongs to the field of chemical synthesis. The synthetic method provided by the invention uses salvia sclarea aldehyde and 2-iodo-1,4-dimethoxybenzene as starting materials. Salvia sclarea sulfonyl hydrazone is generated by a condensation reaction between salvia sclarea aldehyde and p-toluenesulfonyl hydrazide, an olefinic alcohol compound is obtained by a coupling reaction between salvia sclarea sulfonyl hydrazone and 2-iodo-1,4-dimethoxybenzene, an olefinic terpene compound is obtained by an intra-molecular reductive reaction and a double-bond isomerization reaction of the olefin alcohol compound, an olefinic phenol compound is obtained by an oxidation reaction and a reduction reaction of the olefinic terpene compound, and a target marine terpenoid natural product namely ent-chromazonarol is obtained by a cyclization reaction of the olefinic phenol compound. The synthetic method provided by the invention has the characteristics of few reaction steps, simple and convenient operation, good product selectivity, suitability of industrial production, and the like.
Owner:威海创惠环保科技有限公司
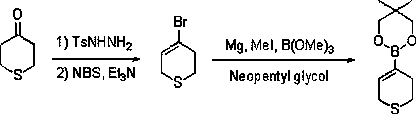
Method for synthesizing 3, 6-dihydro-2H-pyrazine (thiazine) furan-4-boric acid ester
ActiveCN105503927AAvoid ultra low temperatureRaw materials are easy to getGroup 3/13 element organic compoundsFuranSulfohydrazide
The invention discloses a method for synthesizing 3, 6-dihydro-2H-pyrazine (thiazine) furan-4-boric acid ester. According to the method, tetrahydropyrazole (thiazine) furan-4-ketone serves as the raw material, generates hydrazone with p-toluenesulfonhydrazide, then reacts with NBS / organic alkali to generate alkenyl bromide and next forms Grignard reagent with magnesium metal, after the Grignard reagent is formed, boron reagent is added for a reaction, and the 3, 6-dihydro-2H-pyrazine (thiazine) furan-4-boric acid ester is obtained. By means of the method, ultralow temperature, column chromatography and palladium catalyzed coupling in a literature method are avoided, raw materials are easy to obtain, cost is low, conditions are mild, and the method has potential industrial application and amplification prospect.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Synthetic method of marine natural product Puupehenol
InactiveCN106588854AFew reaction stepsSuitable for industrial productionOrganic chemistryBulk chemical productionChemical synthesisPerillaldehyde
The invention relates to a synthetic method of a marine natural product Puupehenol, and belongs to the field of chemical synthesis. The synthetic method comprises the steps: taking (+) aromatic perillaldehyde and hydroxyquinol as starting raw materials, firstly, utilizing (+) aromatic perillaldehyde and p-toluenesulfonhydrazide to form (+) aromatic perillasulfonyl hydrazone, then carrying out palladium-catalyzed cascade Carving migration insertion and intramolecular cyclization reaction of iodine substituted 1,2,4-trimethoxybenzene and (+) aromatic perillasulfonyl hydrazone in an alkali environment to obtain a marine natural product Puupehenol skeleton; and then carrying out hydroxy reduction and double-bond isomerization of a bicyclic sesquiterpanes part of the Puupehenol skeleton, and carrying out oxidation, reduction, cyclization and protection group removal to obtain the final target product Puupehenol. The synthetic method has the advantages of fewer reaction steps, simple operation, and good product selectivity, and is suitable for industrialized production.
Owner:HARBIN INST OF TECH AT WEIHAI
Synthetic method for 2-methyl-4-carbonyl-2,4-diphenyl butyraldehyde
ActiveCN107522606AImprove toleranceEasy to synthesizeOrganic compound preparationOrganic chemistry methodsKetoneTosylhydrazone
The invention relates to a synthetic method for 2-methyl-4-carbonyl-2,4-diphenyl butyraldehyde. The synthetic method comprises the following steps: firstly adding 1,1-dimethoxy-N,N-dimethyl methylamine into a methylbenzene solution of acetophenone, after stirring and performing a heating reaction, quenching a product with water, and then carrying out extraction, drying and purification to obtain (E)-3-(dimethyl amino)-1-phenylprop-2-ene-1-ketone; slowly dropwise adding acetophenone into a methanol solution of p-toluenesulfonhydrazide to obtain p-toluenesulfonylhydrazone; and carrying out a reaction on (E)-3-(dimethyl amino)-1-phenylprop-2-ene-1-ketone, p-toluenesulfonylhydrazone, copper hydroxide and potassium carbonate in argon shield, and purifying a reaction product to obtain 2-methyl-4-carbonyl-2,4-diphenyl butyraldehyde. The method is wide in substrate application range, the tolerance for various functional groups is high, cheap copper is taken as a catalyst, and the reaction condition is mild; and a synthetic 1,4-ketoaldehyde compound can be easily converted into various useful synthetic structures, and carries an alpha-quaternary three-dimensional center.
Owner:NANJING UNIV OF TECH
Super-hydrophobic-lubricating composite anti-icing coating with porous structure and preparation method thereof
ActiveCN111303738AReduce adhesionReduce hydrophobicityPolyurea/polyurethane coatingsPolymer scienceSulfohydrazide
The invention belongs to the field of new materials, and particularly relates to a super-hydrophobic-lubricating composite anti-icing coating with a porous structure and a preparation method thereof.The coating is composed of a foamed porous super-hydrophobic coating and a bionic joint lubricating liquid, and the super-hydrophobic-lubricating composite anti-icing coating of the super-hydrophobicfoamed porous structure is prepared by compounding low-molecular polybutadiene, polyacrylamide, waterborne polyurethane and alkyl ketene dimer and then heating and foaming p-toluenesulfonyl hydrazine.Blended components of the bionic joint lubricating fluid of the obtained coating and low-molecular-weight polybutadiene can significantly reduce the adhesive force of an ice layer, so that the adhesive force of the ice layer is reduced to 5 kPa or below, and an excellent ice-resistant effect is achieved.
Owner:西安华特节能技术有限公司
Pearl wool packing material
The invention discloses a pearl wool packing material. The pearl wool packing material is prepared from following raw materials, by weight, 80 to 100 parts of low density polyethylene, 10 to 20 parts of a foaming agent, 5 to 10 parts of a dispersant, 3 to 5 parts of an anti-oxidant, 1 to 5 parts of an activator, and 10 to 15 parts of a luminous powder. The foaming agent is one or a combination of a plurality of ingredients selected from 3,3-disulphohydrazide diphenyl sulfone, OBSH, p-Toluenesulfonhydrazide, 2,4-methylbenzene disulphohydrazide, p-(N-methoxyl formamido) benzenesulfonyl hydrazide, and preferably 3,3-disulphohydrazide diphenyl sulfone. The dispersant is one selected from N,N-ethylene bis-stearamide, glyceryl tristearate, and oleic acid acyl. The anti-oxidant is prepared by mixing butyl hydroxy anisd, butylated hydroxytoluene, and tert-Butylhydroquinone at a weight ratio of 1-1.5:1.2-2.5:1. The pearl wool packing material is capable of shining automatically; no extra illumination equipment is needed; strength is high; the pearl wool packing material is light, and is high in toughness.
Owner:GUANGXI JIN HONG GREEN PACKAGING TECH
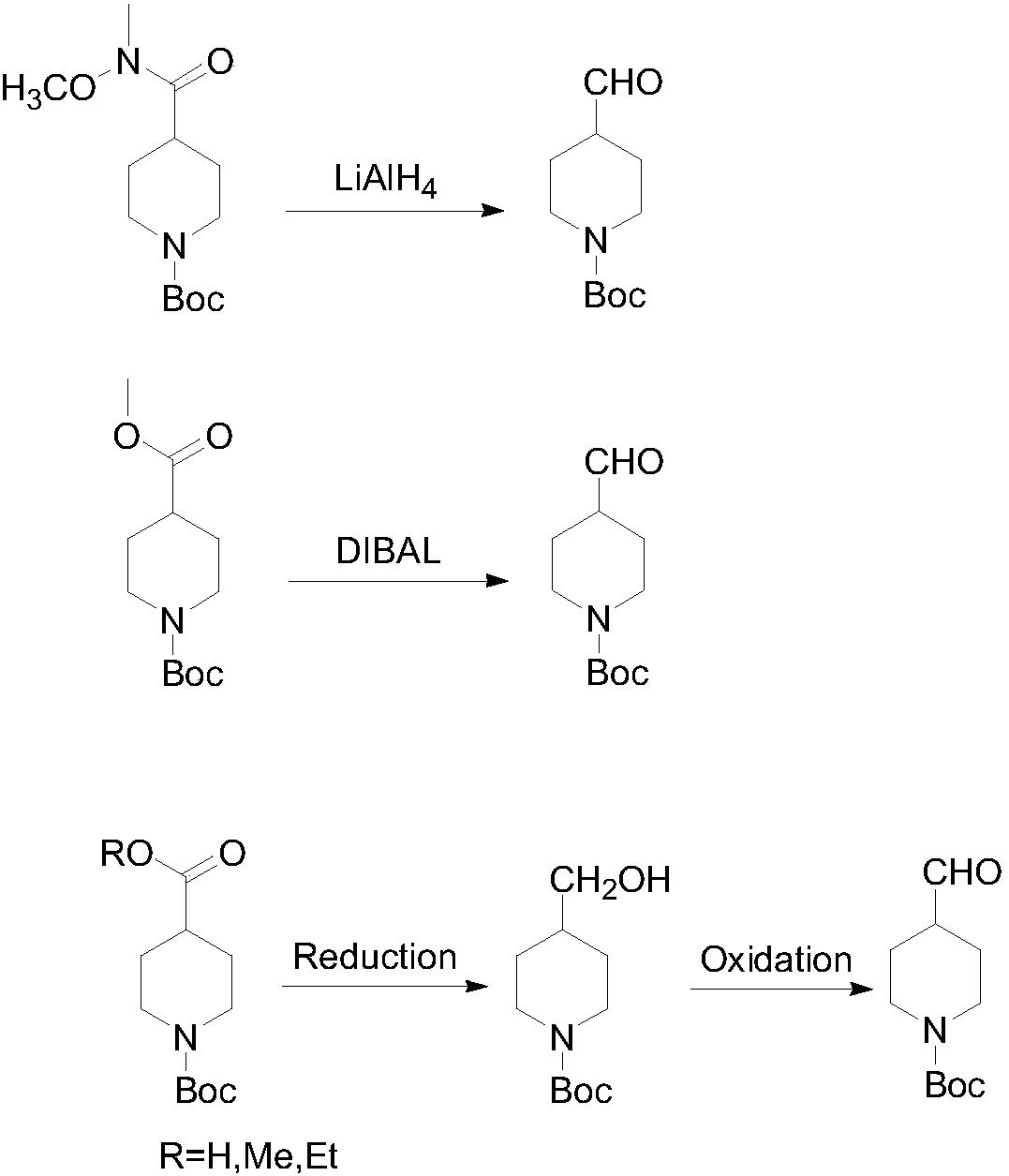
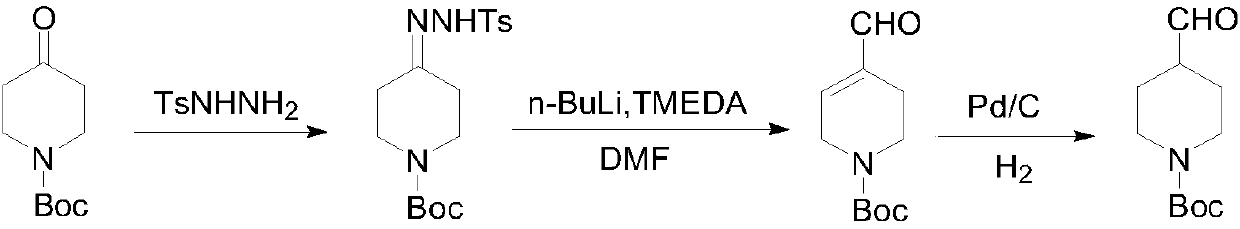
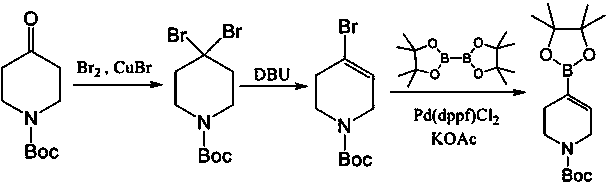
Preparing method of N-Boc-4-piperidine arboxyaldehyde
ActiveCN107652226AMild reaction conditionsThe reaction is easy to operateOrganic chemistryPalladium on carbonHydrazone
The invention discloses a preparing method of N-Boc-4-piperidine arboxyaldehyde, and belongs to the technical field of organic chemistry. Firstly, N-Boc-4-piperidine is reacted with p-Toluenesulfonylhydrazide to generate ketone hydrazone, then butyl lithium / tetramethylethylenediamine / formylation reagent is added to be subjected to a reaction to obtain 1-Boc-4-formyl group-3,6-dihydro-2H-pyridine, and then palladium on carbon is catalyzed and hydrogenated to obtain N-Boc-4-piperidine arboxyaldehyde. The preparing method has the advantages of being easy to operate and high in yield, utilized original raw materials are cheap and easy to obtain, and the preparing method is a proper method for preparing N-Boc-4-piperidine arboxyaldehyde.
Owner:SHANGHAI HOBOR CHEM CO LTD
Synthesis method of Corallidictyal D
ActiveCN107915699AFew reaction stepsSuitable for industrial productionOrganic chemistry methodsBulk chemical productionChemical synthesisNatural product
The invention relates to a synthesis method of marine natural product Corallidictyal D, and belongs to the field of chemical synthesis. The synthesis method comprises the following steps: taking sesquiterpene aldehyde 1 and 1-iodo-2,4,5-tri-alkoxybenzaldehydes 3 as starting materials, generating sesquiterpene hydrazine 2 from the sesquiterpene aldehyde 1 and p-toluenesulfonhydrazide, and couplingthe sesquiterpene hydrazine 2 with the 1-iodo-2,4,5-tri-alkoxybenzaldehydes under the catalysis of palladium to construct a skeleton 4 and a skeleton 5 of Siphonodictyal B; converting the skeleton compound 4 into the skeleton compound 5 under the effect of iodine, and removing a protecting group from the skeleton compound 5 under the effect of boron trichloride to generate Siphonodictyal B (6); and then generating Corallidictyal C (7) and Corallidictyal D (8) under the effect of acid. The synthesis method has the characteristics of less reaction steps, simplicity and convenience in operation,good selectivity of products, suitability for industrial production and the like.
Owner:威海创惠环保科技有限公司
Chemical synthesis method of piperine
The invention relates to a chemical synthesis method of piperine, which comprises the following steps: (1) preparation of bromopiperidine; (2) preparation of alpha-p-tolylsulfonylfuran hydrazone; (3) preparation of piperidinyl-alpha-diolefine aldehyde; (4) preparation of piperidinyl-alpha-diolefine acid; and (5) preparation of piperine.In the invention, the piperidine and bromine react to generate the bromopiperidine, so the bromation selectivity is high; and the bromine mainly goes to the beta position of the piperidine, the yield can reach more than 78%, and the byproducts are fewer. The raw materials, including piperidine, bromine, furfural, p-toluenesulfonhydrazide, piperidine and the like are common raw materials in the market, and are easy to purchase and low in price, so that the raw material cost for producing the piperine is much lower than that of other methods.
Owner:天津市利发隆化工科技有限公司
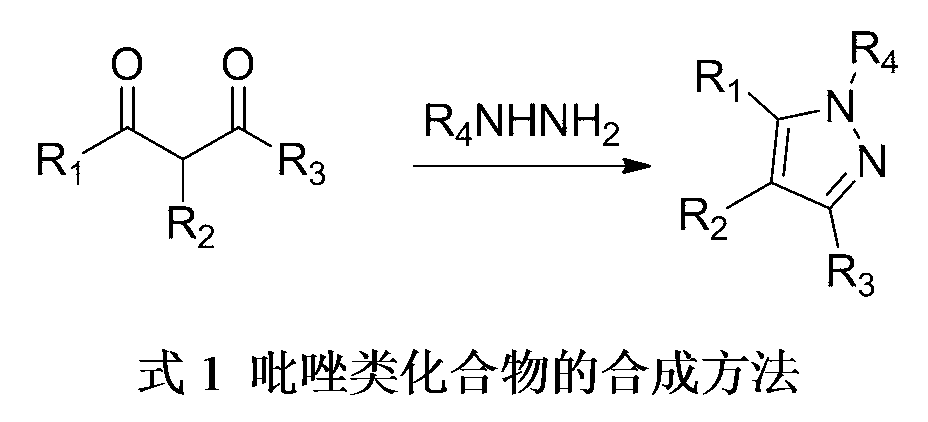
3,4,5-trisubstituted pyrazole compound and preparation method thereof
InactiveCN103275008ASingle structureHigh yieldOrganic chemistryAntineoplastic agentsFiltrationSolvent
The invention discloses a 3,4,5-trisubstituted pyrazole compound. The 3,4,5-trisubstituted pyrazole compound is characterized in that the structural general formula of the compound is shown in the specification; and each of R1, R2 and R3 in the specification is a mono-substituted aromatic ring, a mono-substituted heteroaromatic ring, a poly-substituted aromatic ring, or a poly-substituted heteroaromatic ring. The invention also discloses a preparation method of the 3,4,5-trisubstituted pyrazole compound. The method sequentially comprises the following steps: 1, carrying out a one-pot reaction of an olefin nitrine compound, aldehyde and p-toluenesulfonohydrazide in the presence of a solvent and an alkali at 60-80DEG C for 1-2h; and 2, carrying out pumping filtration or column chromatography of a material obtained in step 1 to obtain the 3,4,5-trisubstituted pyrazole compound. The 3,4,5-trisubstituted pyrazole compound is an ideal antitumor lead compound, and antitumor medicines having novel structures are expected to be found through modifying the structure of the 3,4,5-trisubstituted pyrazole compound.
Owner:ZHEJIANG UNIV
New preparation method of 5,7-pregnadiene-3,20-dione-diethyl ketal
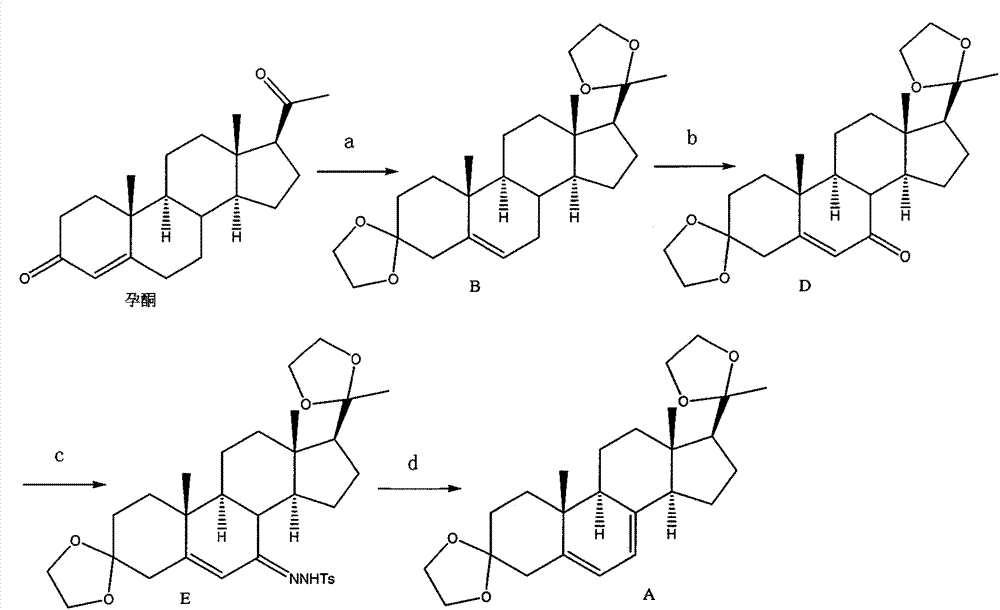
Disclosed is a preparation method of 5,7-pregnadiene-3,20-dione-diethyl ketal. The preparation method comprises: performing dehydration condensation on progesterone by using glycol to protect two carbonyls, so as to obtain a compound B; performing allylic selective oxidization on the compound B to obtain a compound D; performing condensation on the compound D and p-toluenesulfonhydrazide, so as to obtain a compound E; and performing a Shapiro reaction to remove hydrazone from the compound E, so as to obtain 5,7-pregnadiene-3,20-dione-diethyl ketal (a compound A).
Owner:TAIZHOU HISOUND PHARMA CO LTD
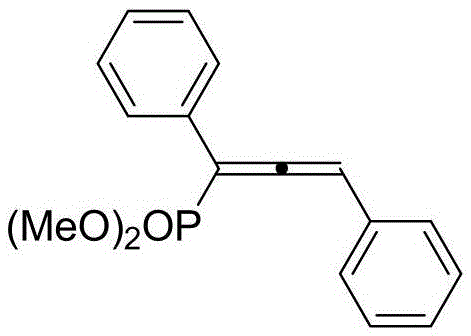
Preparation method of 1,1,3-tri-substituted divinyl dimethyl phosphate compound
InactiveCN105524108AImprove reaction efficiencyLower reaction costGroup 5/15 element organic compoundsArylHydrazone
The invention discloses a preparation method of a 1,1,3-tri-substituted divinyl dimethyl phosphate compound. The preparation method comprises the steps of enabling aryl diazonium phosphatide / alkyl tosylhydrazone phosphatide and terminal alkyne to react in an organic solvent under the action of a copper catalyst and alkali to obtain the 1,1,3-tri-substituted divinyl dimethyl phosphate compound. According to the method, coupling the aryl diazonium phosphatide / alkyl tosylhydrazone phosphatide and the terminal alkyne to obtain the 1,1,3-tri-substituted divinyl dimethyl phosphate compound is realized for the first time, the reaction efficiency is high, and the reaction cost is low. The aryl diazonium phosphatide is prepared through a known method; the alkyl tosylhydrazone phosphatide can be prepared by generating hydrazone through dimethyl benzoylphosphonate and p-toluenesulfonyl hydrazide, and then processing through potassium carbonate. The involved reaction operations are convenient and simple, and the preparation method has excellent tolerance and universality to functional groups, and can be widely used for preparing the 1,1,3-tri-substituted divinyl dimethyl phosphate compound.
Owner:PEKING UNIV
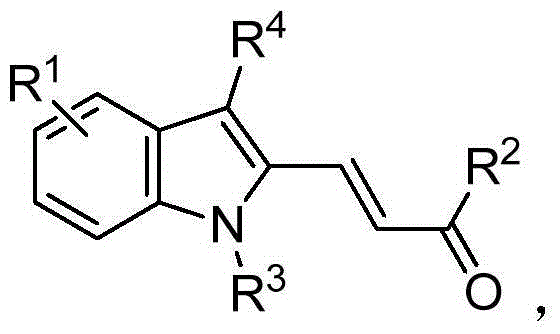
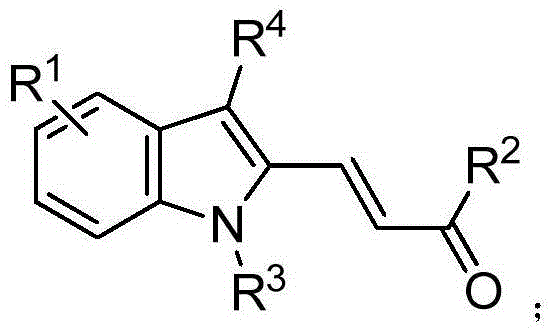
Polysubstituted indole compound and preparation method thereof
ActiveCN104341334ASimple and fast operationHas a resource advantageOrganic chemistrySolventP-toluenesulfonyl hydrazide
The invention belongs to the field of fine chemistry raw materials, and in particular relates to a polysubstituted indole compound and a preparation method thereof. The polysubstituted indole compound has a structural formula as shown in the specification. The preparation method of the polysubstituted indoles compound comprises the following steps: firstly adding a compound I as shown in the specificaiotion and p-toluenesulfonhydrazide in an organic solvent, refluxing to obtain a compound II as shown in the specification, and then adding a compound III as shown in the specification in the solvent, stirring and reacting to obtain the polysubstituted indole compound as shown in the specification under the effect of transition metal catalyst, ligand and alkali.
Owner:SOUTH CHINA UNIV OF TECH
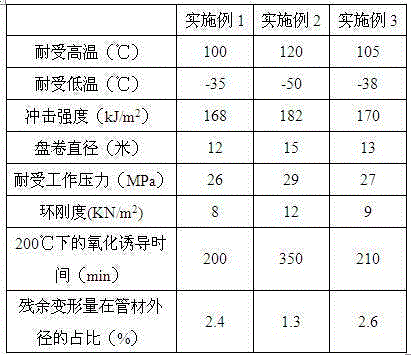
Ramie fiber salt elimination underground pipe used for saline-alkali soil and preparation method of ramie fiber salt elimination underground pipe
The invention provides a ramie fiber salt elimination underground pipe used for saline-alkali soil. The underground pipe comprises, by weight, 26-38 parts of ramie fiber, 45-53 parts of modified polypropylene, 4-9 parts of phenyl alkyl-sulfonate, 0.6-1.8 parts of p-toluenesulfonhydrazide, 1.4-1.9 parts of calcium stearate, 4-11 parts of devulcanized rubber powder elastomers, 1-3 parts of petroleum-based hydrocarbon oil, 6-12 parts of nanometer titanium dioxide, 7-14 parts of silicone rubber, 5-13 parts of chlorinated polypropylene resin and 1-5 parts of sulfosalicylic acid. The invention further provides a preparation method of the underground pipe. The underground pipe has the advantages that the tolerated high temperature can reach 100-120 DEG C; the coil diameter reaches 12-15 meters under the low temperature of -50DEG C to -35 DEG C; the impact strength is 168-182 kJ / m<2>; the tolerant working pressure reaches 26-29 MPa, and the ring stiffness is 8-12 KN / m<2>.
Owner:WEIFANG YOURONG IND
Power cable sheath material
InactiveCN105860359AEasy to processImprove mechanical propertiesPlastic/resin/waxes insulatorsInsulated cablesPolyethylene glycolAntimony trioxide
The invention discloses a power cable sheath material comprising the raw materials in parts by weight as follows: PVC resin, octyl-phenolic tackifying resin, fluororesin, polystyrene-butadiene copolymer, vinyl chloroacetate, ethyl acrylate, 2-mercaptobenzothiazole zinc salt, sorbitol anhydride trioleate, triethylene glycol monoisopropyl ether, p-toluenesulfonohydrazide, vinyl-tri(2-methoxyethoxy)silane, tetraethylene glycol monododecyl ether, diallyl amine, butyl stearate, 3-10 parts of titanium dioxide, nano-bauxite, carbon black, antimonous oxide, and polyethylene wax. The power cable sheath material provided by the invention has good processing performance and mechanical properties, and the prepared material has high strength, good flexibility, and long service life, and resists high temperature aging, oil permeation and chemical solvents.
Owner:ANHUI HUAXING CABLE GROUP
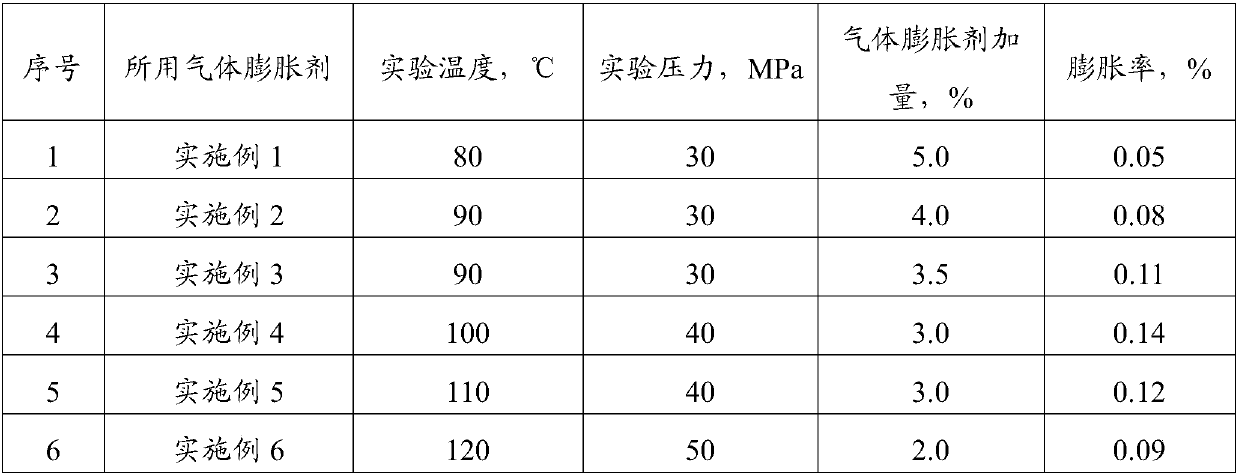
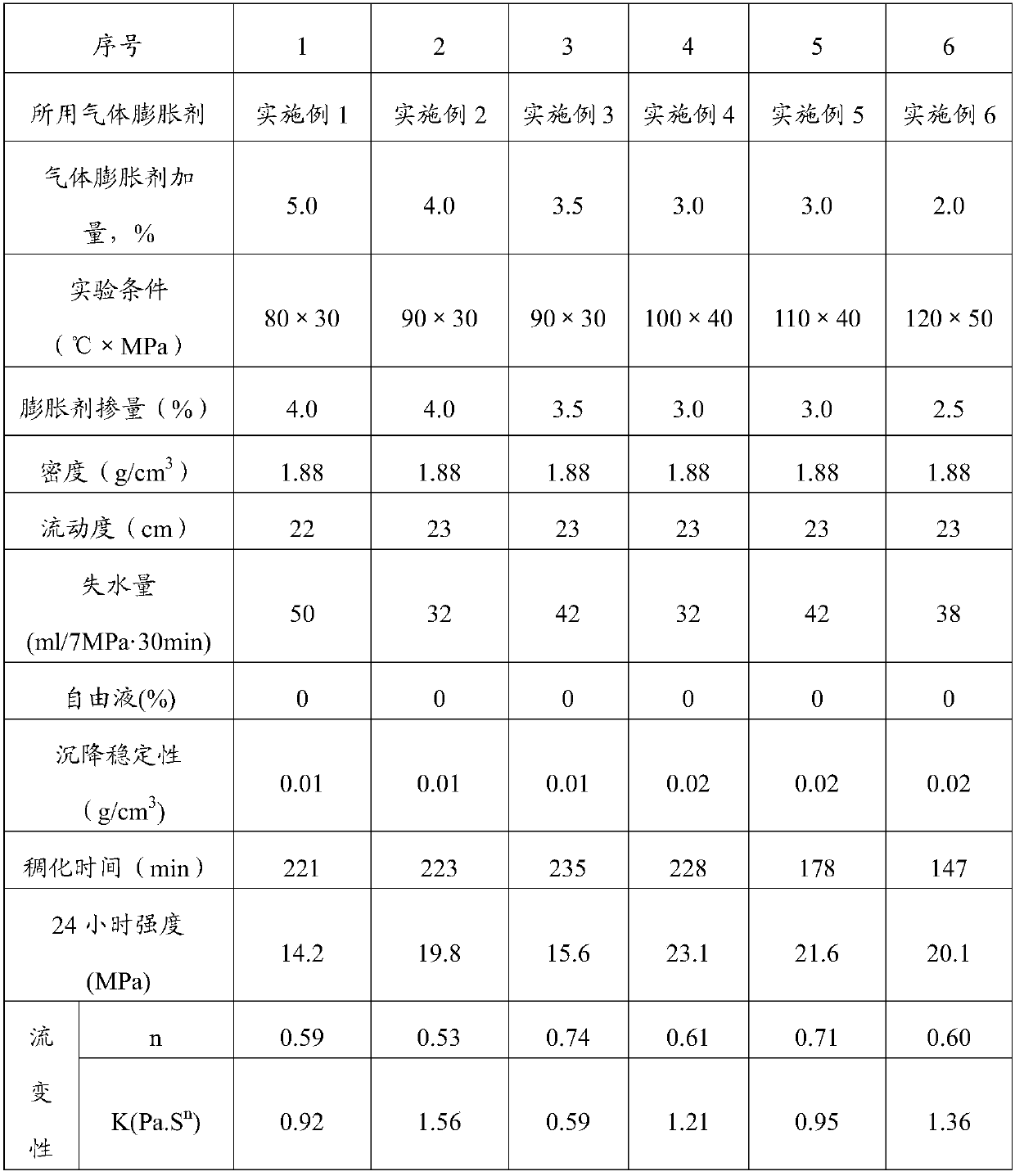
Gas expanding agent for cementing medium-temperature and high-temperature wells and application thereof
InactiveCN107760288AEvenly distributedExpansion is effectiveDrilling compositionAzodicarbonamideMaterials science
The invention discloses a gas expanding agent for cementing medium-temperature and high-temperature wells and application thereof, and belongs to the technical field of cement admixtures used in the field of cell cementing in an oilfield completion technology. The gas expanding agent comprises the following components in parts by weight: 20-30 parts of a gas generating agent, 5-10 parts of a gas generating aid, 0.5-1.0 part of a foam stabilizer and 40-90 parts of a filler, wherein the gas generating agent is at least one selected from N,N'-dinitrosopentamethyltetramine, azodicarbonamide, barium azodicarboxylate, 4,4'-oxybisbenzenesulfonyl hydrazide and p-toluenesulfonyl hydrazide; the gas generating aid is at least one selected from urea, sodium benzoate, zinc oxide, aluminum oxide and lead oxide. The gas expanding agent can meet requirements of on-site well cementing construction at the circulating temperature of 80-120 DEG C, effectively compensates the pressure loss and the volume shrinkage during cement stone solidification, improves the cement stone cementing quality and improves the anti-gas channeling capacity.
Owner:BC P INC CHINA NAT PETROLEUM CORP +2
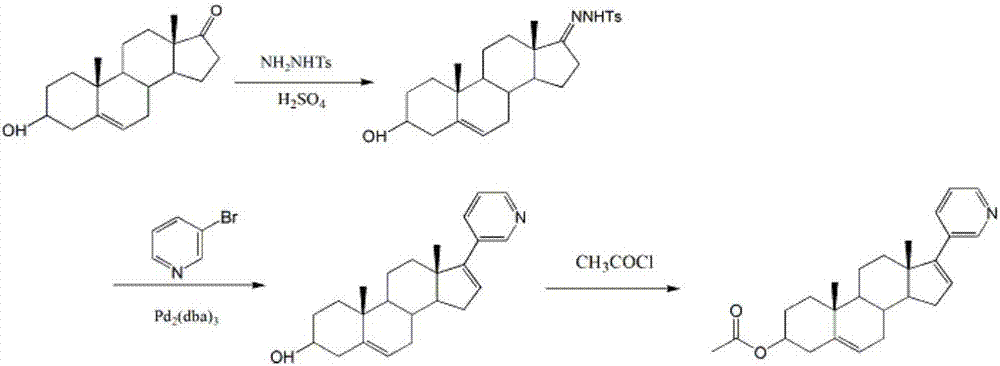
17-(3-pyridyl)-androstane-5,16-diene-3 beta-ol synthetic method
InactiveCN107325146AMild reaction conditionsRaw materials are cheap and easy to getSteroidsAbirateroneAndrostanes
The invention discloses a 17-(3-pyridyl)-androstane-5,16-diene-3 beta-ol synthetic method. According to the synthetic method, dehydroepiandrosterone is utilized as a raw material to be condensed with p-toluenesulfonhydrazide and generate coupling reaction with 3-bromopyridine to synthesize a target product of 17-(3-pyridyl)-androstane-5,16-diene-3 beta-ol (abiraterone). The synthetic method has the advantages of moderate reaction condition, low-price and easily available raw material and lower production cost.
Owner:李建恒
Synthesis method of abiraterone acetate
InactiveCN107056871AMild reaction conditionsRaw materials are cheap and easy to getSteroidsSynthesis methodsAcetylation
The invention discloses a synthesis method of abiraterone acetate. The method includes: taking dehydroepiandrosterone as the raw material, conducting condensation with p-toluenesulfonhydrazide, then carrying out coupling reaction with 3-bromopyridine, and conducting acetylation so as to synthesize the target product abiraterone acetate. According to the synthesis method provided by the invention, the route has mild reaction conditions, the raw materials are cheap and easily available, and the production cost is low.
Owner:李建恒
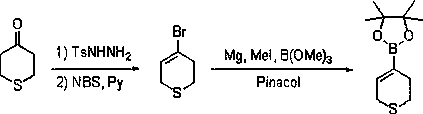
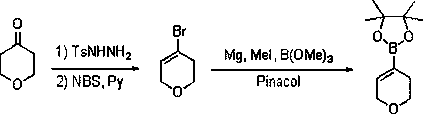
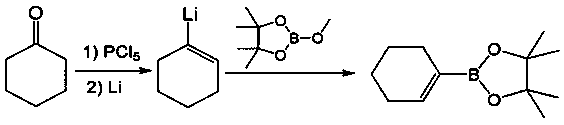
Preparation method of allylboronic acid pinacol ester
The invention provides a common preparation method of allylboronic acid pinacol ester. The common preparation method takes aliphatic ketone as a raw material and comprises the following steps: takingaliphatic ketone and p-toluenesulfonohydrazide as raw materials to react to generate p-toluenesulfonylhydrazone; taking the p-toluenesulfonylhydrazone and a Grignard reagent to react, so as to generate an intermediate allylmagnesium chloride; and taking the allylmagnesium chloride and methoxyboronic acid pinacol ester (isopropoxyboronic acid pinacol ester) to react, so as to prepare the allylboronic acid pinacol ester. A synthesis route has moderate reaction conditions, high yield and good commonality.
Owner:DALIAN RES & DESIGN INST OF CHEM IND
High-elastic anti-static sole and preparation method thereof
PendingCN114634681ALower surface resistivityGuaranteed antistatic effectSolesSodium bicarbonateFiber
The invention discloses a high-elastic anti-static sole and a preparation method thereof. The high-elastic anti-static sole is formed by compounding an outsole and an insole, the outsole is prepared from the following raw materials: polyvinyl chloride, mica powder, acetylene carbon black, carbon fibers, zinc oxide, a foaming agent, bis (tert-butylperoxy isopropyl) benzene, epoxidized soybean oil, an antioxidant, lauryl sodium sulfate and lauryl amine polyoxyethylene ether; the foaming agent is composed of p-toluenesulfonhydrazide, sodium bicarbonate and polyethylene glycol according to a certain proportion, the prepared shoe sole has low surface resistivity, the anti-static requirement can be met, after the mica powder is modified through limited lauryl amine polyoxyethylene ether, the mica powder is cooperatively used with the carbon fibers, acetylene carbon black and sodium dodecyl sulfate, and the anti-static performance of the shoe sole is improved. Therefore, the antistatic effect of the prepared sole is ensured.
Owner:达州市嘉源体育用品有限公司
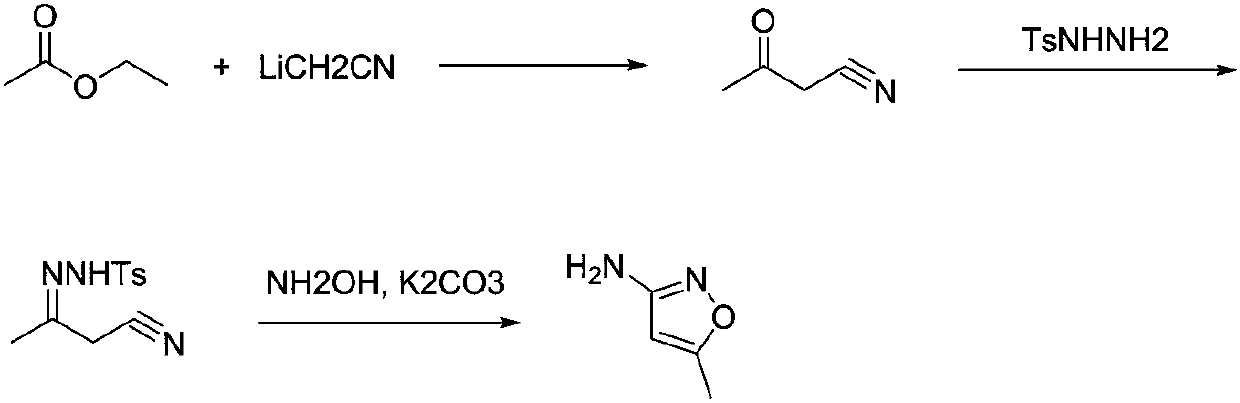
Preparation method for 3-amino-5-methylisoxazole
ActiveCN107721941ALow priceEasy to buy and transportOrganic chemistryHydroxylamine3-amino-5-methylisoxazole
The invention discloses a preparation method for 3-amino-5-methylisooxazole, belonging to the technical field of organic chemistry. The preparation method is completed through three steps: with an easily-available raw material namely ethyl acetate as a starting material, carrying out a reaction with acetonitrile in the presence of an alkali metal so as to form acetyl acetonitrile, then carrying out a reaction with p-toluenesulfonhydrazide so as to form hydrazone, and carrying out a ring closing reaction with hydroxylamine under an alkaline condition so as to obtain the 3-amino-5-methylisoxazole. According to the invention, raw materials in the reactions are common; chloroform or carbon tetrachloride in a traditional method are avoided; and analogous isomers of the 3-amino-5-methylisoxazoleare not detected in the reaction process.
Owner:浦拉司科技(上海)有限责任公司
Preparation method of alpha-alkenyl boronic acid pinacol esters
InactiveCN103012455AHigh yieldSimple and fast operationGroup 3/13 element organic compoundsSulfohydrazideBoronic acid
Owner:DALIAN NETCHEM CHIRAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com