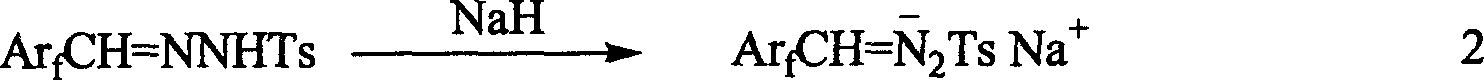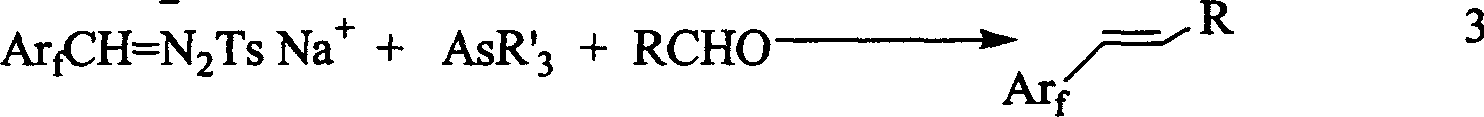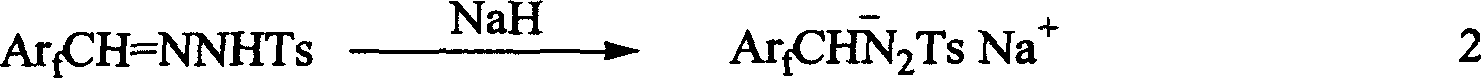1-aryl-2 perluoro or polyfluoro phenyl ethylene and its derirative, its synthesis and application
A technology of polyfluorophenylethylene and its derivatives, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problem of poor cis-trans selectivity of product olefins, poor cis-trans selectivity of products, and preparation problems. The conditions are high, and it is beneficial to industrial production, the steps are short, and the operation is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0029] Example 11. Preparation of perfluorobenzaldehyde p-toluenesulfonyl hydrazone by condensation reaction
[0030] Add 9.9 g (50 mmol) of perfluorobenzaldehyde, 9.3 g (50 mmol) of p-toluenesulfonyl hydrazide and 90 ml of absolute ethanol to a 150 ml bottle, and react at room temperature under nitrogen protection for 24 hours. TLC detects that the raw material has reacted. The solvent was removed under pressure and dried under vacuum to obtain 18.2 g, with a yield of 99%. 2. Preparation of perfluorobenzaldehyde p-toluenesulfonylhydrazone sodium salt by hydrogen abstraction reaction of sodium hydride
[0031] Add 3.9 g (10.7 mmol) of perfluorobenzaldehyde p-methylbenzenesulfonyl hydrazide and 120 ml of anhydrous ethyl ether dried with sodium to a 250 ml bottle under ice bath conditions, and slowly add 0.43 g (content 60 % In mineral oil), after 24 hours of reaction at room temperature, anhydrous and oxygen-free filter, washed 3 times with 60 ml of sodium-d...
Embodiment 21
[0033] Example 21. The condensation reaction is the same as that of Example 12. The hydrogen abstraction reaction of sodium hydride is the same as that of Example 13. The preparation of the Wittig reaction 1-p-nitrophenyl-2-perfluorophenylethylene
[0034] Add 230 mg (0.75 mmol) of triphenylarsenic, 3 mg (0.007 mmol) of rhodium acetate dimer, 7 mL of 1,4-dioxane, and 18-crown-6 10 to a 15 mL bottle dried with nitrogen. Mg (0.0375 mmol), 76 mg (0.5 mmol) of p-nitrobenzaldehyde and 290 mg (0.75 mmol) of sodium salt of perfluorobenzaldehyde p-toluenesulfonyl hydrazone. After the addition of the materials, the reaction solution was vigorously stirred at room temperature for ten minutes, and then the reaction solution was immersed in an oil bath at 35°C for 5 hours. After the reaction was quenched by adding 2 ml of water, the reaction was then separated, and the water phase was used 2 ml Extract 2 times with hexyl acetate. Combine the organic phases, dry with ...
Embodiment 31
[0035] Example 31. The combined reaction is the same as in Example 12. The hydrogen abstraction reaction of sodium hydride is the same as in Example 13. The preparation of 1-p-bromophenyl-2-perfluorophenylethylene is catalyzed by the Wittig reaction
[0036] Add 230 mg (0.75 mmol) of triphenylarsenic, 3 mg (0.007 mmol) of rhodium acetate dimer, 7 mL of 1,4-dioxane, and 18-crown-6 10 to a 15 mL bottle dried with nitrogen. Mg (0.0375 mmol), 92 mg (0.5 mmol) of p-bromobenzaldehyde and 290 mg (0.75 mmol) of sodium salt of perfluorobenzaldehyde p-toluenesulfonyl hydrazone. After the addition of the materials, the reaction solution was vigorously stirred at room temperature for ten minutes, and then the reaction solution was immersed in an oil bath at 35°C for 5 hours. After the reaction was quenched by adding 2 ml of water, the reaction was then separated, and the water phase was used 2 ml The ethyl acetate was extracted twice. Combine the organic phases, dry w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com