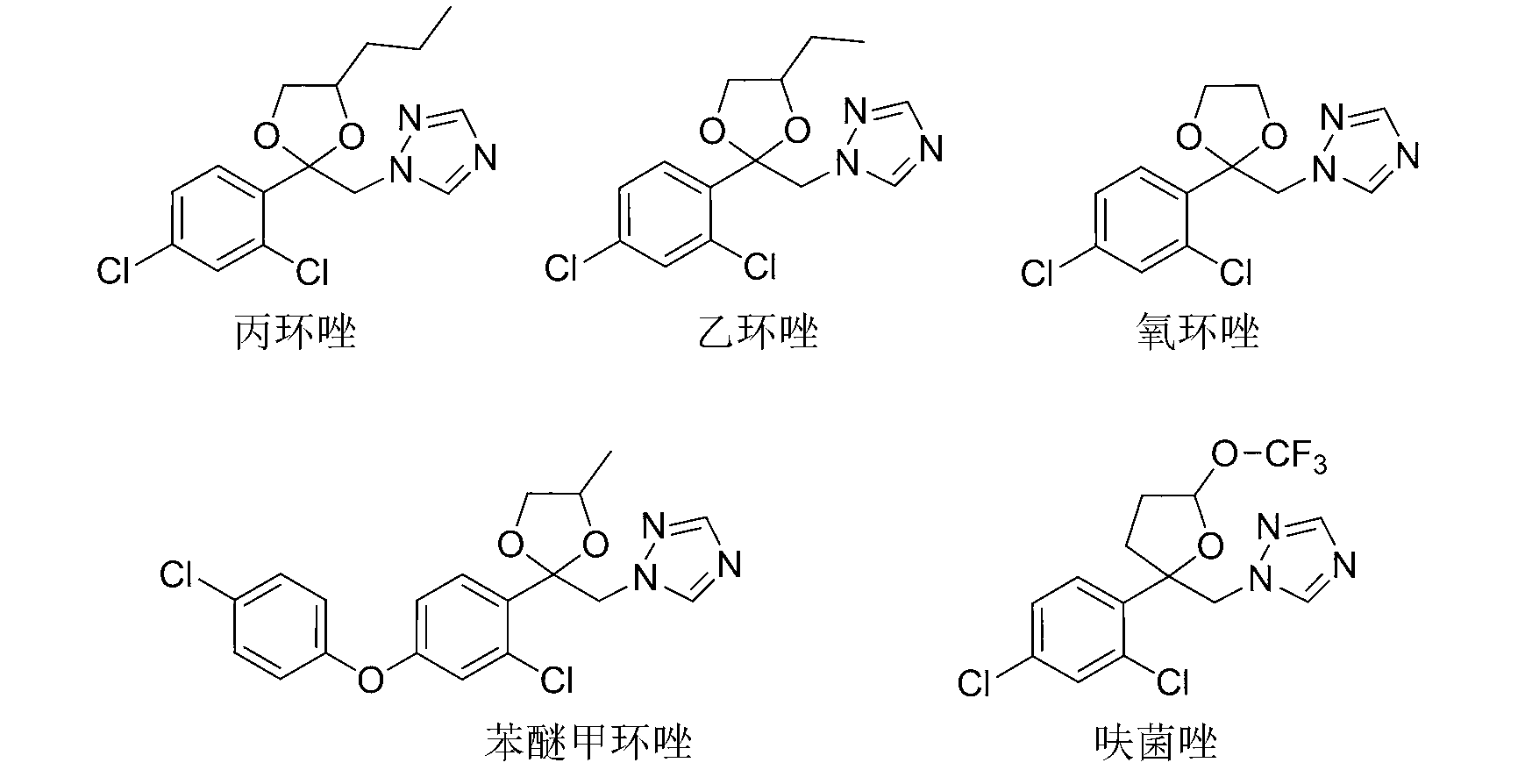
2-(1,2,4-triazole-1-methyl)-2-(coumarone-5-radical)-1,3-dioxolane and application thereof
A technology of dioxolane and methyl, which is applied in the application field of fungicides and can solve the problems of high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
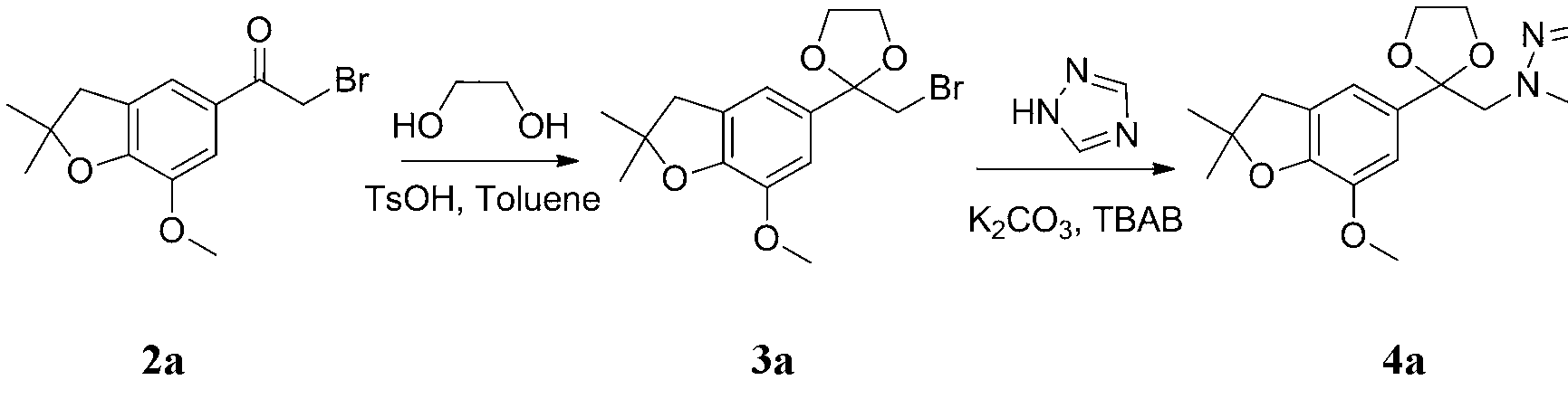
[0030] 2-(1,2,4-triazole-1-methyl)-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)- Preparation of 1,3-dioxolane (4a)
[0031]
[0032] 1.50 g (5 mmol) 7-methoxy-5-bromoacetyl-2,2-dimethyl-2,3-dihydrobenzofuran (2a), 0.47 g (7.5 mmol) ethylene glycol, 20 ml toluene and 0.10 g p-toluenesulfonic acid, stirred and refluxed for 2.5 h. After the reaction solution was cooled to room temperature, it was washed with water, and the organic layer was dried with anhydrous sodium sulfate, solvent removal, and column chromatography to obtain a pale yellow liquid 2-bromomethyl-2-(7-methoxy-2,2-dimethyl -2,3-dihydrobenzofuran-5-yl)-1,3-dioxolane (3a), yield 79.3%.
[0033] 0.28 g (4 mmol) of 1,2,4-triazole, 15 ml of DMF, 0.75 g of potassium carbonate, and 0.10 g of tetrabutylammonium bromide were stirred and refluxed for 30 min, and 1.03 g (3 mmol) of 2- Bromomethyl-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-1,3-dioxolane(3a) in DMF, After dropping, continue to react ...
Embodiment 2
[0035] 4-Methyl-2-(1,2,4-triazole-1-methyl)-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran- Preparation of 5-yl)-1,3-dioxolane (4b)
[0036]
[0037] The experimental operation was the same as in Example 1, and the condensation reaction was 4 hours to obtain light yellow liquid 4-methyl-2-bromomethyl-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzene Furan-5-yl)-1,3-dioxolane 3b, yield 81.3%; substitution reaction solvent is DMSO, reaction 10h, beige solid 4-methyl-2-(1,2,4- Triazole-1-methyl)-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-1,3-dioxolane 4b , m.p.107.5~108.5℃, yield 71.1%; 1 H NMR (CDCl 3 , 400 MHz), δ: 1.08 (d, J = 6.4 Hz, 3H, CH 3 ), 1.52(s, 6H, 2×CH 3 ), 3.03(s, 2H, furan ring CH 2 ), 3.09 (dd, J = 6.8 Hz, J = 7.6 Hz, 1H, OCH 2 ), 3.91 (dd, J = 6.8 Hz, J = 7.6 Hz, 1H, OCH 2 ), 3.87(s, 3H, OCH 3 ), 4.09~4.14(m, 1H, OCH), 4.47(s, 2H, NCH 2 ), 6.88 (s, 1H, benzene ring 4-H), 6.92 (s, 1H, benzene ring 6-H), 7.95 (s, 1H, triazole ring 5-H), 8.21 (s...
Embodiment 3
[0039] 4-Propyl-2-(1,2,4-triazole-1-methyl)-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran- Preparation of 5-yl)-1,3-dioxolane (4c)
[0040]
[0041] The experimental operation is the same as in Example 1, and the condensation reaction takes 4.5 hours to obtain light yellow liquid 4-propyl-2-bromomethyl-2-(7-methoxy-2,2-dimethyl-2,3-dihydro Benzofuran-5-yl)-1,3-dioxolane 3c, yield 80.4%; Substitution reaction 12h, 4-propyl-2-(1,2,4-triazole-1 -methyl)-2-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-1,3-dioxolane (4c), yield rate 38.8%, 1 H NMR (DMSO, 400 MHz), δ: 0.82~1.28 (m, 7H, CH 2 CH 2 CH 3 ), 1.40(s, 6H, 2×CH 3 ), 3.00 (s, 2H, furan ring CH 2 ), 3.08(t, J=7.2 Hz, 1H, OCH 2 ), 3.82(t, J = 7.2Hz, 1H, OCH 2 ), 3.75(s, 3H, OCH 3 ), 3.88~3.91(m, 1H, OCH), 4.51(s, 2H, NCH 2 ), 6.81 (s, 1H, benzene ring 4-H), 6.89 (s, 1H, benzene ring 6-H), 7.92 (s, 1H, triazole ring 5-H), 8.35 (s, 1H, triazole ring Ring 3-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com