Polysubstituted indole compound and preparation method thereof
A technology for indoles and compounds, which is applied in the field of polysubstituted indoles and their preparation, can solve the problems of harsh reaction conditions, difficult to meet industrial production requirements, difficult to obtain reaction raw materials, etc., and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
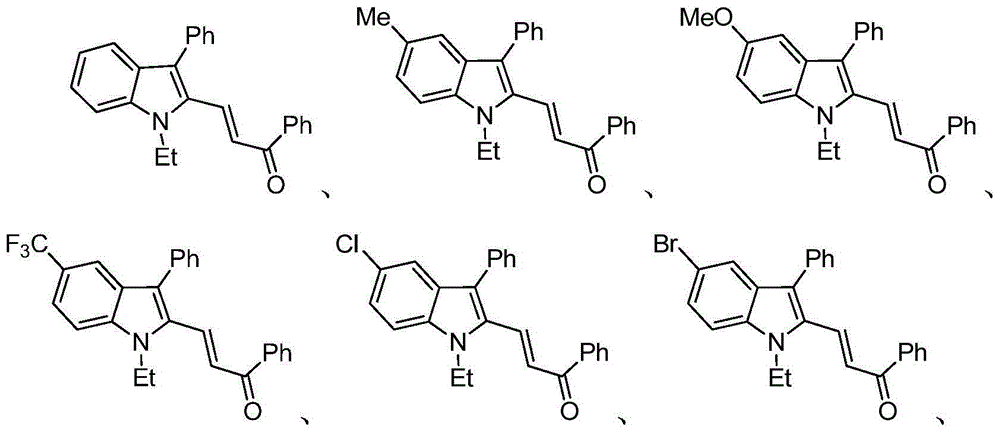
Examples
Embodiment 1
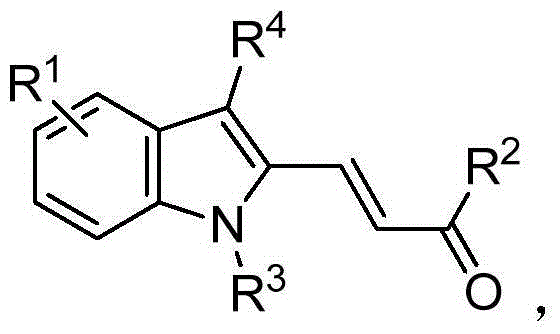
[0047] A multi-substituted indole compound, the preparation method is as follows:
[0048] (1) Add 70mL of absolute ethanol to a 250mL single-necked flask, and then add 10mmol of And 10mmol of p-toluenesulfonyl hydrazide, reflux reaction at 70°C for 12 hours to carry out dehydration condensation, recrystallization after cooling to room temperature to obtain
[0049] Among them, the R in 2 for hydrogen, R 4 is phenyl;
[0050] (2) Under nitrogen protection, add 5mmol of step (1) gained in 100mL three-necked flask , 0.25 mmol of Pd 2 (dba) 3 , 0.5mmol of dppf, 20mmol of potassium carbonate, 25mL of tetrahydrofuran and 10mmol of After stirring and reacting at 90°C for 6 hours, cool to room temperature, remove the solvent, and separate by column chromatography to obtain the multi-substituted indole compound, whose structural formula is Yield 76%;
[0051] Among them, the Middle R 1 for hydrogen, R 3 Ethyl (-CH 2 -CH 3 ).
[0052] For the polysubstituted indo...
Embodiment 2
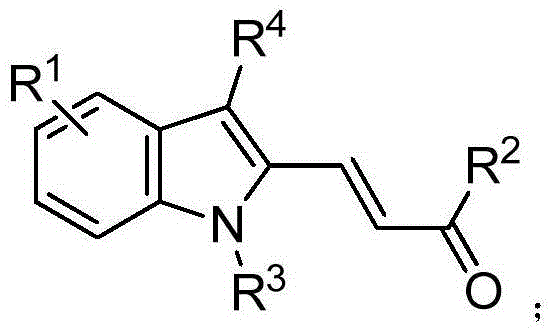
[0057] Add 60mL of absolute ethanol to a 250mL single-necked flask, and then add 10mmol of And 10mmol of p-toluenesulfonyl hydrazide, reflux reaction at 70°C for 12 hours to carry out dehydration condensation, recrystallization after cooling to room temperature to obtain
[0058] (2) Under nitrogen protection, add 5mmol of step (1) gained in 100mL three-necked flask 0.25 mmol of Pd 2 (dba) 3 , 0.5mmol of dppf, 20mmol of potassium carbonate, 25mL of tetrahydrofuran and 10mmol of After stirring and reacting at 90°C for 6 hours, cool to room temperature, remove the solvent, and separate by column chromatography to obtain the multi-substituted indole compound, whose structural formula is
[0059] Among them, the Middle R 1 for hydrogen, R 3 Ethyl (-CH 2 -CH 3 ).
[0060] For the polysubstituted indole compounds Carry out measurement analysis, its physical constant is:
[0061] 1 H NMR (400MHz, CDCl 3 )δ9.54 (d, J = 7.6Hz, 1H), 7.62 (d, J = 8.1Hz, 1H), 7.57 (d...
Embodiment 3
[0065] (1) Add 80mL of absolute ethanol to a 250mL single-necked flask, and then add 10mmol of And 10mmol of p-toluenesulfonylhydrazide, reflux reaction at 70°C for 24 hours to carry out dehydration condensation, recrystallization after cooling to room temperature to obtain
[0066] (2) Under nitrogen protection, add 5mmol of step (1) gained in 100mL three-necked flask 0.25 mmol of Pd 2 (dba) 3 , 0.5mmol of dppf, 20mmol of potassium carbonate, 20mL of tetrahydrofuran and 10mmol of After stirring and reacting at 90°C for 10 hours, cool to room temperature, remove the solvent, and separate by column chromatography to obtain the polysubstituted indole compound, whose structural formula is
[0067] Among them, the Middle R 1 for hydrogen, R 3 Ethyl (-CH 2 -CH 3 ).
[0068] For the polysubstituted indole compounds Carry out measurement analysis, its physical constant is:
[0069] 1 H NMR (400MHz, CDCl 3 )δ9.55(d, J=7.6Hz, 1H), 7.60(t, J=12.6Hz, 2H), 7.37(q, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com