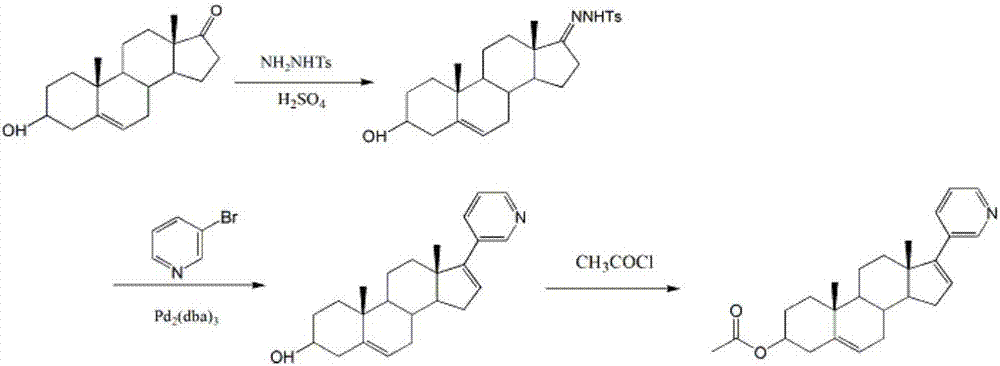
Synthesis method of abiraterone acetate
A technology of abiraterone acetate and a synthetic method, applied in directions such as steroids, organic chemistry, etc., can solve problems such as long steps, reduce the generation of double bond by-products, low yield, etc., and achieve mild reaction conditions and production costs. Low cost, easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of synthetic method of abiraterone acetate, comprises the steps:
[0022] A. Add 0.95g (5mmol) p-toluenesulfonyl chloride and 15mL toluene to a 100ml round-bottomed flask in turn, stir and dissolve thoroughly to obtain solution X, slowly add the amount according to the substance at 15°C (solution X is cooled to 15°C) Add 1.5mL (10mmol) 80% hydrazine hydrate to solution X at a ratio of 1:2, drop it within 5 minutes; continue to react for 30 minutes, and a white precipitate appears in the flask. TLC analysis confirmed the reaction endpoint. After completion of the reaction, add 30ml of cold water at 10°C, stir, filter with suction, wash the filter cake 3-5 times with purified water, and dry to obtain 0.82g of white p-toluenesulfonyl hydrazide crystals, with a yield of 86.3% (document value: yield rate of 92%).
[0023] B, the synthesis of dehydroepiandrosterone-17-p-toluenesulfonylhydrazone
[0024] Add 0.75g (3mmol) dehydroepiandrosterone, 25mL methanol, and 0....
Embodiment 2
[0030] A. Add 1.02g (5.3mmol) p-toluenesulfonyl chloride and 15mL toluene to a 100ml round-bottomed flask in turn, stir and dissolve thoroughly to obtain solution X, slowly add 1.7mL (12mmol) 80% hydrazine hydrate dropwise to the solution at 15°C In X, the dripping was completed within 5 minutes; the reaction was continued for 30 minutes, and a white precipitate appeared in the flask. TLC analysis confirmed the reaction endpoint. After completion of the reaction, add 30ml of 10°C water to stir, filter with suction, wash the filter cake 3-5 times with purified water, and dry to obtain 0.93g of white p-toluenesulfonyl hydrazide crystals, with a yield of 86.7% (literature value: yield 92%).
[0031] B, the synthesis of dehydroepiandrosterone-17-p-toluenesulfonylhydrazone
[0032] Add 0.97g (3mmol) dehydroepiandrosterone, 25mL methanol, 1.08g p-toluenesulfonyl hydrazide to a 100ml round-bottomed flask in turn, stir well at room temperature to dissolve, add 0.1mL 0.2mol·L -1 Sul...
Embodiment 3
[0038] A. Add 1.08g (5.7mmol) p-toluenesulfonyl chloride and 15mL toluene to a 100ml round-bottomed flask in turn, stir and dissolve thoroughly to obtain solution X, slowly add 1.8mL (13mmol) 80% hydrazine hydrate dropwise to the solution at 15°C In X, the dripping was completed within 5 minutes; the reaction was continued for 30 minutes, and a white precipitate appeared in the flask. TLC analysis confirmed the reaction endpoint. After completion of the reaction, add 30ml of 10°C water to stir, filter with suction, wash the filter cake 3-5 times with purified water, and dry to obtain 0.94g of white p-toluenesulfonyl hydrazide crystals, with a yield of 85.6% (literature value: yield 92%).
[0039] B, the synthesis of dehydroepiandrosterone-17-p-toluenesulfonylhydrazone
[0040] Add 1.18g (3.3mmol) dehydroepiandrosterone, 25mL methanol, and 1.28g p-toluenesulfonyl hydrazide to a 100ml round-bottomed flask in turn, stir and dissolve at room temperature, add 0.1mL 0.2mol·L -1 S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com