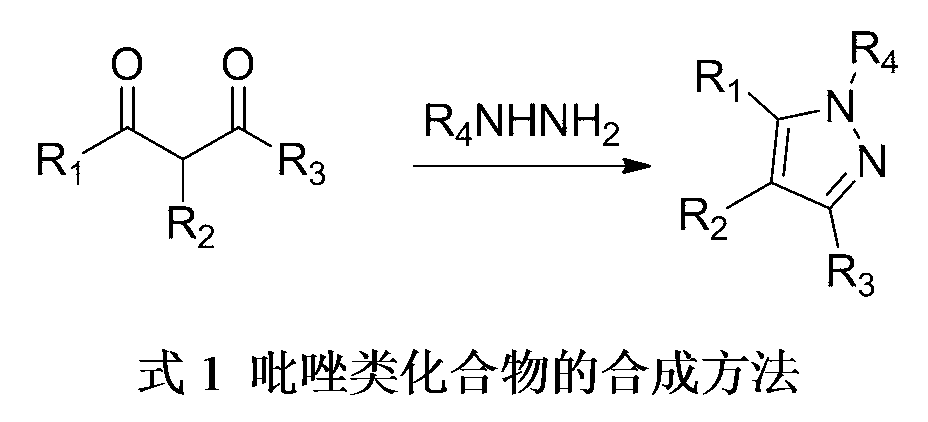
3,4,5-trisubstituted pyrazole compound and preparation method thereof
A compound and pyrazole technology, applied in the field of pyrazole compounds and their preparation, can solve problems such as difficulty in preparing 1,3-dione, and achieve the effects of high yield and single product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, 3-benzoyl-4,5-diphenyl-1H-pyrazole (m1)
[0034] Add 42.4 mg (0.4 mmol) of benzaldehyde, 74.4 mg (0.4 mmol) of p-toluenesulfonyl hydrazide, and 99.6 mg (0.4 mmol) of 1,3-diphenyl-2-azido-2-propen-1-one to In the reaction flask, add 2.0 ml of DMF (N,N-dimethylformamide) and 0.08 g (2.0 mmol) of sodium hydroxide. After the addition, stir and react at 60°C for 2 hours, and detect the reaction by TLC (PE:EA= 4:1, namely petroleum ether: ethyl acetate = 4:1 volume ratio). After confirming the completion of the reaction, the reaction solution was extracted three times with 20*3 mL ethyl acetate, the organic layer (on the upper layer) was combined, washed three times with 30*3 mL saturated brine, and then dried with anhydrous sodium sulfate (about 2.0 g) for 30 Minutes, concentrated (concentrated with a rotary evaporator), column chromatography (PE:AE=6:1), the specific process parameters of the column chromatography are as follows:
[0035] A silica gel column is...
Embodiment 2
[0054] Example 2, 3-benzoyl-4-phenyl-5-(4-bromophenyl)-1H-pyrazole (m2)
[0055] Replace benzaldehyde with p-bromobenzaldehyde, the molar weight is constant, and all the other are equal to Example 1. 143.5 mg of the product 3-benzoyl-4-phenyl-5-(4-bromophenyl)-1H-pyrazole was obtained as a white solid, with a yield of 89%.
[0056] Its structural formula is:
[0057]
[0058] White solid; mp: 217.0 – 217.7 °C; 1 H NMR (500 MHz, CDCl 3 ) δ 11.08 (br s, 1H), 7.74 (d, J = 7.0 Hz, 2H), 7.43 – 7.41 (m, 3H), 7.25 – 7.21 (m, 4H), 7.18 – 7.17 (m, 3H), 7.10 (d, J = 6.1 Hz, 2H); 13 C NMR (125 MHz, CDCl 3 ) δ 188.12, 136.75, 135.08, 132.97, 131.91 131.56, 130.60, 129.98, 129.69, 128.42, 128.12, 127.61, 122.77; HRMS (ESI): m / z calcd for C 22 h 15 BrN 2 O [M+H] + : 403.0441, found: 403.0438.
Embodiment 3
[0059] Example 3, 3-benzoyl-4-phenyl-5-(3-bromophenyl)-1H-pyrazole (m3)
[0060] Replace benzaldehyde with m-bromobenzaldehyde, the molar weight is constant, and all the other are equal to Example 1. 137.7 mg of 3-benzoyl-4-phenyl-5-(3-bromophenyl)-1H-pyrazole was obtained as a white solid, with a yield of 85%.
[0061] Its structural formula is:
[0062]
[0063] White solid; mp: 155.5 – 156.5 °C; 1 H NMR (500 MHz, CDCl 3 ) δ 11.81 (br s, 1H), 7.73 (d, J = 6.4 Hz, 2H), 7.60 (s, 1H), 7.43 – 7.37 (m, 6H), 7.25 – 7.13 (m, 2H), 7.11 – 7.08 (m, 3H); 13 C NMR (125 MHz, CDCl 3 ) δ 188.11, 136.64, 132.98, 131.41, 131.39, 131.03, 130.59, 130.09, 129.98, 128.39, 128.10, 127.63, 126.81, 123.02, 122.70 for CMS (zIcMS) : 22 h 15 BrN 2 O [M+H] + : 403.0441, found: 403.0442.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com