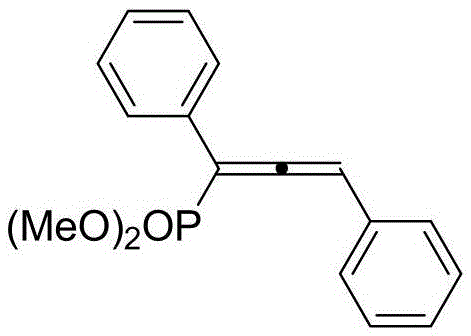
Preparation method of 1,1,3-tri-substituted divinyl dimethyl phosphate compound
A technology for dimethyl allenyl phosphate and compounds, which is applied in the field of preparation of 1,1,3-trisubstituted allenyl dimethyl phosphate compounds, and can solve complex synthesis of substrates, difficulty in obtaining raw materials, and limitations Problems such as the scope of application, to achieve the effect of low reaction cost, high reaction efficiency and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of Dimethyl 1,3-Diphenyldienyl Phosphate
[0042] Add 59mg (ie 0.26mmol) phenyl phosphodiester diazonium, 20mg (ie 0.2mmol) diisopropylamine, 7.6mg (ie 0.04mmol) cuprous iodide, 1mL1, 4-dioxane. After the reaction tube is sealed, replace the system with a nitrogen atmosphere, add 20mg of phenylacetylene (ie 0.2mmol), and react at 80°C for 3 hours. After the reaction, the solid insolubles in the system are filtered off with suction, and concentrated under reduced pressure using a rotary evaporator. 1,3-Diphenyldienyl dimethyl phosphate can be obtained by column chromatography purification using petroleum ether:ethyl acetate 1:1 as eluent with a yield of 85%. Its structure is shown in the following formula:
[0043]
[0044] Keeping other reaction conditions unchanged, reacting at 50°C for 5 hours, the yield was 83%; reacting at 40°C for 30 hours, the yield was 75%;
[0045] Keeping other reaction conditions unchanged, increase the amount of phenyl phospho...
Embodiment 2
[0054] Synthesis of Dimethyl 1-(4-Chlorophenyl)-3-Phenyldienyl Phosphate
[0055] Add 45mg (ie 0.26mmol) dimethyl diazonium phenylphosphate, 20mg (ie 0.2mmol) diisopropylamine, 7.6mg (ie 0.04mmol) cuprous iodide, 1mL1 to a 25mL long tubular reaction tube ,4-dioxane. After the reaction tube is sealed, replace the system with a nitrogen atmosphere, add 27mg of p-chlorophenylacetylene (0.2mmol), and react at 80°C for 3 hours. Concentrate, use petroleum ether: ethyl acetate 1:1 as eluent column chromatography to obtain 1-(4-chlorophenyl)-3-phenyldienyl phosphate dimethyl ester, its structure is as follows Shown:
[0056]
[0057] The compound is a colorless liquid with a yield of 86%, and its NMR data are as follows:
[0058] 1 HNMR (400MHz, CDCl 3 )δ7.61(d, J=8.0Hz, 2H), 7.27-7.38(m, 7H), 6.73(d, J=12.8Hz, 1H), 3.81(d, J=11.2Hz, 3H), 3.78( d,J=10.8Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ212.66(d, J=2.7Hz), 133.77(d, J=2.0Hz), 130.75(d, J=76Hz), 129.95(d, J=8.1Hz), 129.16(d, ...
Embodiment 3
[0060] Synthesis of Dimethyl 1-(4-Bromophenyl)-3-Phenyldienyl Phosphate
[0061] Add 59mg (ie 0.26mmol) dimethyl diazonium phenylphosphate, 20mg (ie 0.2mmol) diisopropylamine, 7.6mg (ie 0.04mmol) cuprous iodide to a 25mL long tubular reaction tube, 1mL1 ,4-dioxane. After the reaction tube is sealed, replace the system with a nitrogen atmosphere, add 36mg of p-bromophenylacetylene (0.2mmol), and react at 80°C for 3 hours. After the reaction, filter out the solid insoluble matter in the system, and use a rotary evaporator to reduce the pressure Concentrate, and use petroleum ether: ethyl acetate 1:1 as eluent column chromatography to obtain 1-(4-bromophenyl)-3-phenyldienyl phosphate dimethyl ester, its structure is as follows Shown:
[0062]
[0063] The compound is a colorless liquid with a yield of 80%, and its NMR data are as follows:
[0064] 1 HNMR (400MHz, CDCl 3 )δ7.61(d,J=8.0Hz,2H),7.47(d,J=8.0Hz,2H),7.30-7.38(m,3H),7.21-7.23(m,2H),6.71(d,J =12.2Hz,1H),3.80(d,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com