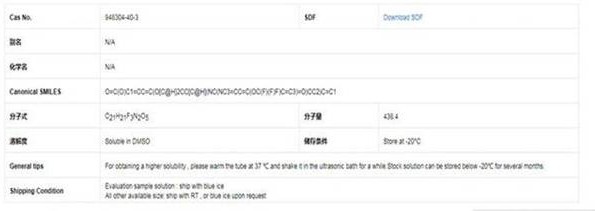
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "NS - Normal saline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Normal saline (NSS, NS or N/S) is the commonly used phrase for a solution of 0.90% w/v of NaCl, 308 mOsm/L or 9.0 g per litre.
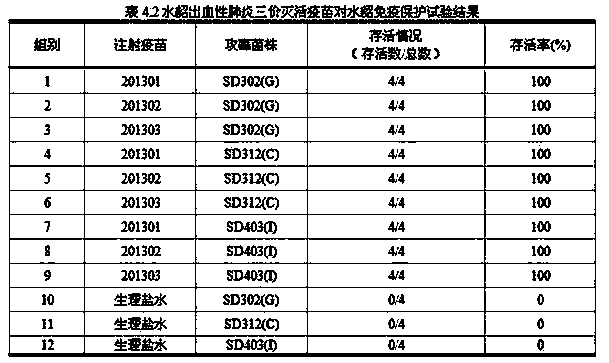
Method for preparing trivalent inactivated vaccine for preventing mink hemorrhagic pneumonia
InactiveCN103948918ALow costEasy to manufactureAntibacterial agentsBacterial antigen ingredientsThiomersalateNS - Normal saline
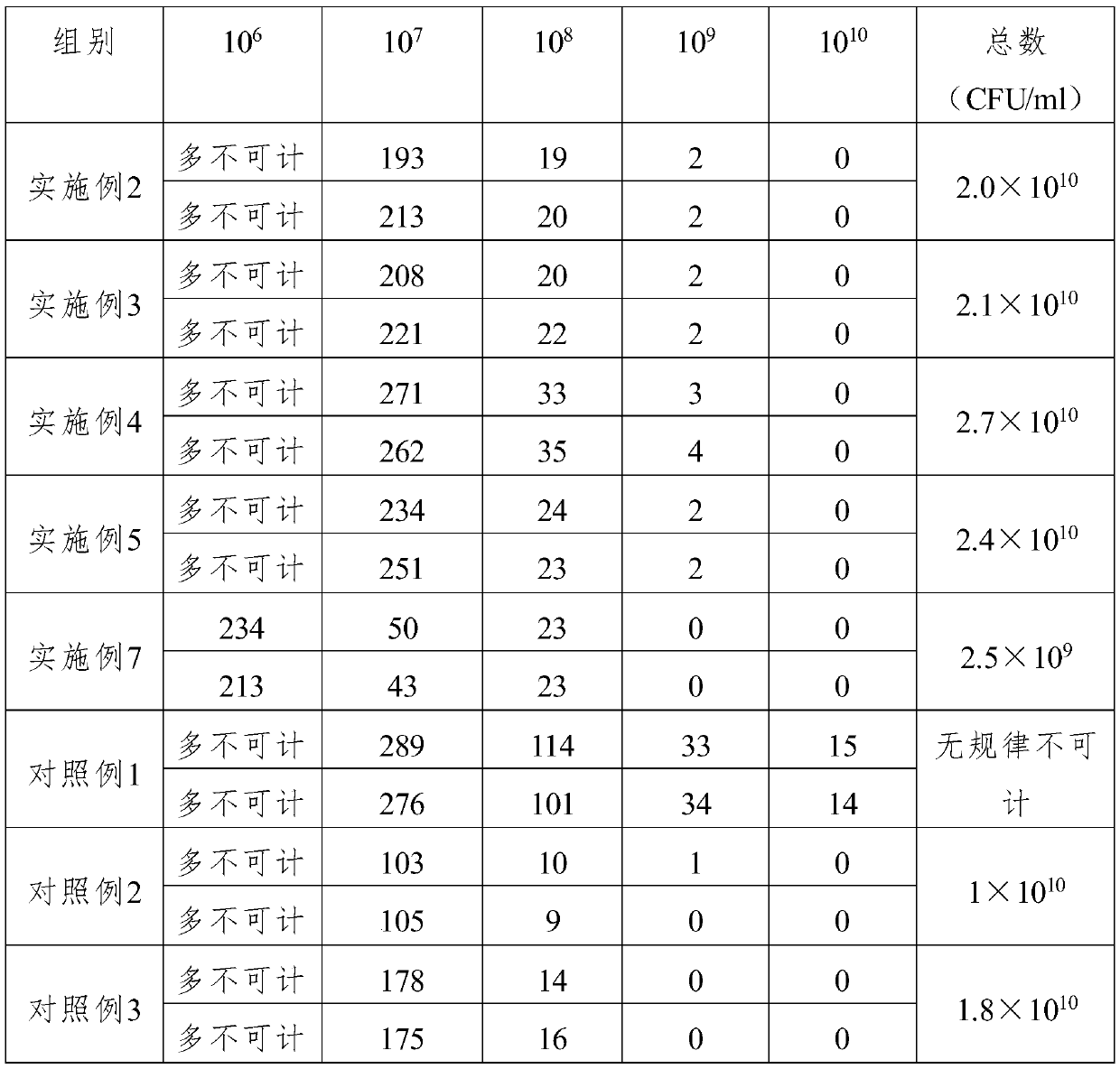
The invention relates to a method for preparing a trivalent inactivated vaccine for preventing mink hemorrhagic pneumonia. The method is characterized by comprising the following concrete steps: separating serum G pseudomonas aeruginosa SD302, serum C pseudomonas aeruginosa SD312 and serum I pseudomonas aeruginosa SD403 from an ill mink body; storing bacteria by adopting a freeze-drying mode; respectively putting the three serum pseudomonas aeruginosa into PYG culture medium containing 2% fetal calf serum to cultivate at 37 DEG C; then carrying out enlarge cultivation, and carrying out inactivated treatment on a bacterium solution; diluting the inactivated bacterium solution until the concentration is 8.0*10<9> CFU / mL by using normal saline according to the bacteria content; mixing the three diluted inactivated bacterium solutions with 40% aluminum glue solution according to the volume ratio of 1:1:1:1; adding thiomersalate of which the final concentration is 0.004%, and mixing to obtain the trivalent inactivated vaccine. The method is safe, multi-titer, good in immunogenicity, good in specificity and the like, and is moderate in price, and applicable to substratum or clinical prevention of the mink hemorrhagic pneumonia.
Owner:母连志
Polypeptide fragment for preparing DC (Dendritic Cell) vaccine and DC vaccine
InactiveCN109575118AImprove securityReduce manufacturing costCulture processCancer antigen ingredientsHuman serum albuminAmino acid
The invention discloses a polypeptide fragment for preparing DC (Dendritic Cell) vaccine and the DC vaccine. The polypeptide fragment is selected from at least one of amino acid sequences as shown inSEQ ID NO. 1 to SEQ ID NO. 6. The DC vaccine contains polypeptide fragment loaded DC. Specifically, the DC vaccine is prepared from the polypeptide fragment loaded DC, human serum albumin and normal saline, wherein the mass concentration of the human serum albumin in the normal saline is 5 to 20 percent, and the density of the polypeptide fragment loaded DC is (0.5-2)*10<6> per mL. According to the polypeptide fragment for preparing the DC vaccine and the DC vaccine, disclosed by the invention, human mammaglobin is selected as an attack target spot, an effective DC polypeptide vaccine preparedfrom the polypeptide fragment obtained through computer design and experimental screening can be used for treating patients suffering from HER2 (Human Epidermal Growth Factor Receptor-2) positive / negative breast cancer, and preventing recurrence and metastasis. The DC vaccine is capable of reducing the production cost while increasing the vaccine safety.
Owner:英普乐孚生物技术(上海)有限公司
Composition for protecting adipose tissue and preparation method and application of composition
ActiveCN106754676AReduce the rate of bacterial contaminationStrong activityCulture processDead animal preservationL-glutamineStem cell
The invention discloses an isolated adipose protectant which comprises normal saline, DMEM solution, L-glutamine, penicillin and streptomycin according to the proportion of 500ml to 500ml to (146.1mg-584.4mg) to 125000U to 125000U. The invention further discloses a preparation method and application of the protectant. The protectant has effect of effectively reducing adipose tissue culture stem cell contamination rate. Growth rate of the stem cells during culture of the adipose tissue is higher than that of stem cells growing without protective liquid, and culture time of primary cells can be shortened, culture efficiency is improved, and cost is saved.
Owner:中科领康(广州)医疗有限公司
Bacillus quantity detection method
InactiveCN110964775AIncrease surface tensionGood dispersionMicrobiological testing/measurementMicroorganism based processesBiotechnologyMicroorganism
The embodiment of the invention discloses a bacillus quantity detection method, and relates to the technical field of microbiological detection. The method comprises the following steps: diluting a bacillus-containing detection sample by adopting normal saline containing an emulsifier; coating a flat plate with the diluent, culturing to obtain a bacillus colony, and calculating the number of bacillus in the detection sample according to a statistical result of the bacillus colony. According to the detection method disclosed by the invention, the normal saline containing the emulsifier is usedfor diluting the detection sample; the emulsifier can improve the surface tension of the bacillus in different states in the normal saline, so that dispersion of the bacillus in different states in the normal saline is better promoted, the bacillus can be fully and uniformly distributed in the normal saline, and the accuracy of bacillus quantity detection is further improved.
Owner:ACAD OF NAT FOOD & STRATEGIC RESERVES ADMINISTRATION
Method applied to intra-abdominal pressure monitoring of critical patient
PendingCN111657911AThe monitoring results are accurateVolatility sensitiveCatheterSensorsNS - Normal salineEngineering
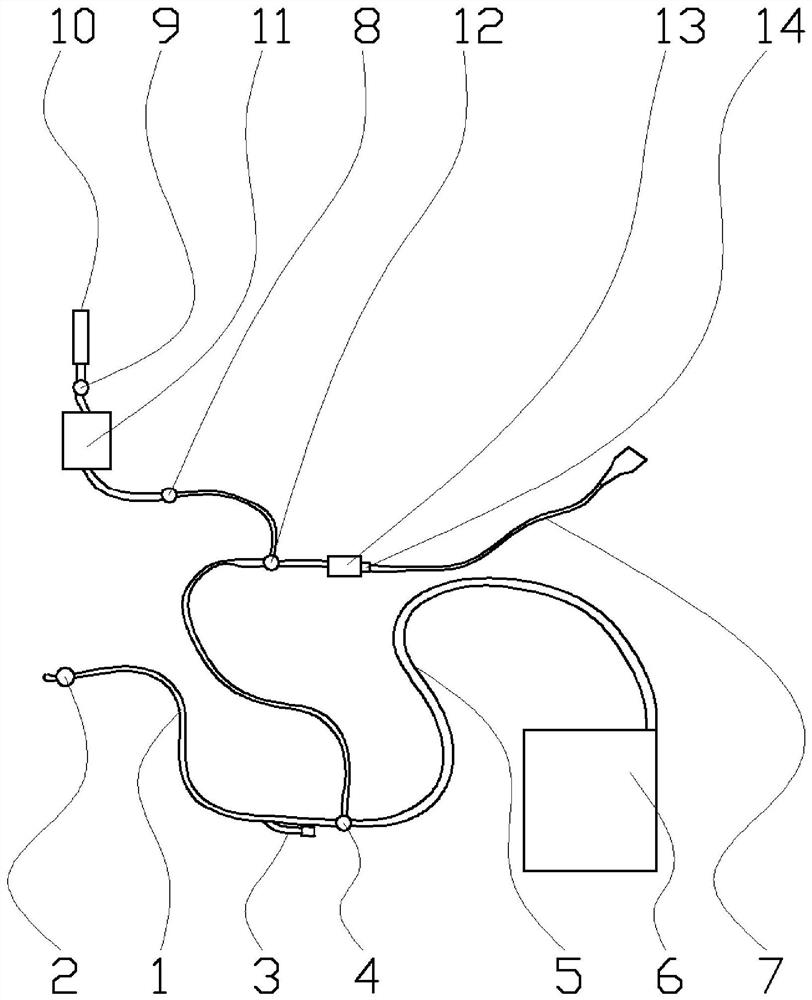
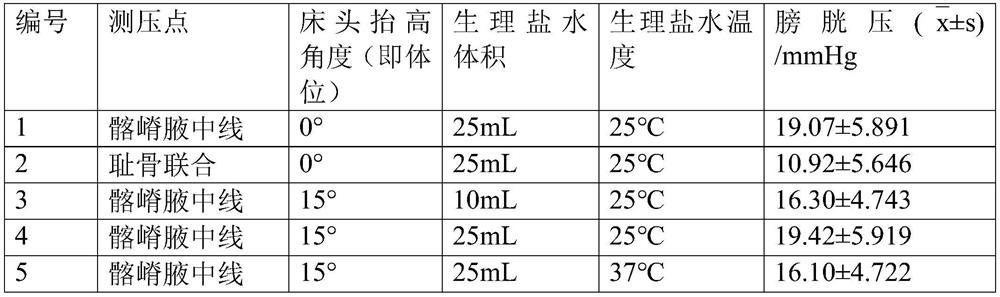
The invention relates to a method applied to intra-abdominal pressure monitoring of a critical patient. The invention specifically provides application of a bladder pressure monitoring instrument in monitoring of bladder pressure of the critical patient, and further provides a method for monitoring the bladder pressure of the critical patient. Under the conditions that a crista iliaca axillary midline serves as a pressure measuring point, the body position of the patient is 0-15 degrees, the volume of normal saline infused into the bladder is 10-25 mL, and the temperature of the normal salineis 25-37 DEG C, an intra-abdominal pressure monitoring result obtained through the monitoring method is not accurate and high in repeatability, but also is more sensitive to fluctuation of the bladderpressure of the patient and capable of reflecting the fluctuation condition of the bladder pressure of the patient more obviously. Particularly, for the critical patients difficult to lie flat, the 15-degree body position is selected, and the comfort level of the critical patients can be further improved. The bladder pressure monitoring method disclosed by the invention is particularly suitable for the critical patients difficult to lie flat, the comfort of the patient during monitoring can be improved, and the bladder pressure monitoring method has a good application prospect in timely and accurately monitoring fluctuation conditions of bladder pressure and intra-abdominal pressure of the critical patients.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Stem cell preparation and application of stem cell preparation in preparing drug for treating chronic nephritis
InactiveCN106265745AReduce urine proteinReduce in quantityMammal material medical ingredientsSolution deliveryRemission rateT lymphocyte
The invention relates to the technical field of medicines, in particular to a stem cell preparation and an application of the stem cell preparation in preparing a drug for treating chronic nephritis. The provided stem cell preparation is prepared through resuspension of umbilical cord mesenchymal stem cells by the aid of normal saline, the content of urine protein of a patient and the number of urine erythrocyte are reduced by inhibiting expression of T cell surface antigen CD8 and secretion of INF-gamma and up-regulating secretion of IL-4 of T lymphocyte of peripheral blood, so that the effect for treating chronic nephritis is realized, the effective rate of treatment can reach 88%, and the remission rate can reach 54%.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
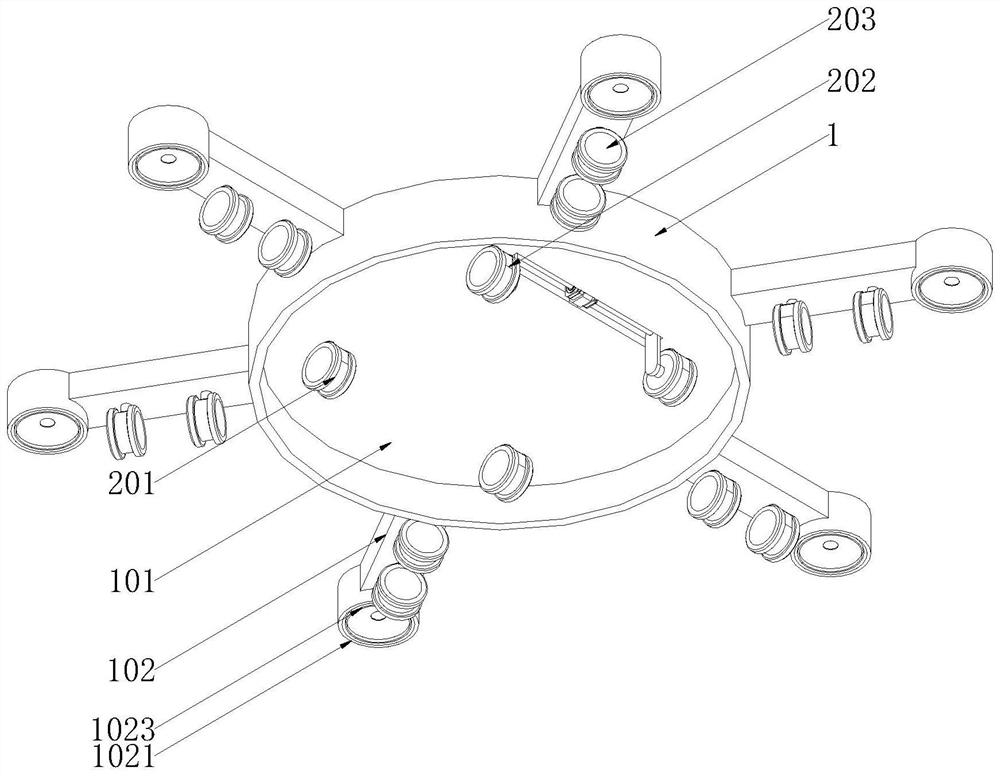
Female-rat vaginal-smear sample collection device
PendingCN107307887AImprove consistencyEnsure consistencySurgical needlesVaccination/ovulation diagnosticsSmear sampleObstetrics
The invention provides a female-rat vaginal-smear sample collection device. The female-rat vaginal-smear sample collection device comprises a vagina built-in head and a normal saline pusher. The quantitative normal saline pusher is adopted, and the consistency of normal saline for sampling is guaranteed. In the vagina built-in head, a cervix end and an orificium vaginae end are fixed for guaranteeing the consistency of a sampling part; a soft material is used in the cervix end, and operational friction stimulation is reduced; meanwhile, the flow direction of the normal saline entering the vagina can be changed to be outwards, the cervix is protected, infection caused when the normal saline reversely flows is avoided, and infection caused when cells of the inner wall of the vagina are injured is avoided in the mode that once washing is carried out with saline. In the operation process, the normal saline can be kept in the aseptic condition, and the animal infection rate in the operation process is reduced; the vagina built-in head can be disinfected and washed to be reused.
Owner:HUADONG HOSPITAL
Dermatological scurf sampling device
PendingCN113974701AKeep aliveAchieve clamping slide effectSurgical needlesPreparing sample for investigationDermatology departmentNS - Normal saline
The invention relates to a sampling device, in particular to a dermatological scurf sampling device. The invention provides a dermatological scurf sampling device capable of automatically scraping scurf, automatically dripping normal saline and automatically clamping a glass slide. The dermatological scurf sampling device comprises a handheld frame body; first support frames which are arranged at the top ends of the two first telescopic rods; a scraper which is rotationally connected between the two first support frames; and two first telescopic rods which are both wound with the first springs, wherein first telescopic rods are arranged on the two sides of the handheld frame body, and the two ends of the first springs are connected with the first support frame and the handheld frame body correspondingly. A flow guide pipe channel on the front side is opened through an electromagnetic valve, normal saline drips on the scurf through the flow guide pipe channel on the front side, so that the scurf is kept active through the normal saline, and the effect that the scurf activity is kept through the normal saline is achieved.
Owner:张小芳
Method for detecting plasma coagulase by kinetic turbidimetric assay to identify staphylococcus aureus
InactiveCN104745676AShort time limitLow costMicrobiological testing/measurementMicroorganism based processesAssayNS - Normal saline
Owner:NANCHANG UNIV
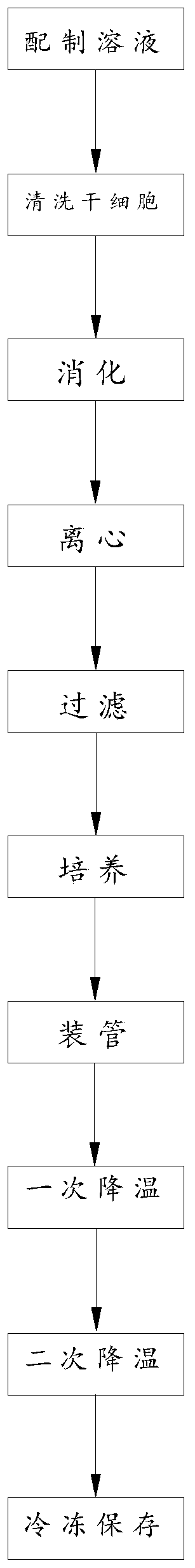
Stem cell freezing solution and stem cell freezing method thereof
InactiveCN110839616AAvoid affecting the recovery rate of heatingAvoid influenceDead animal preservationNS - Normal salineCentrifugation
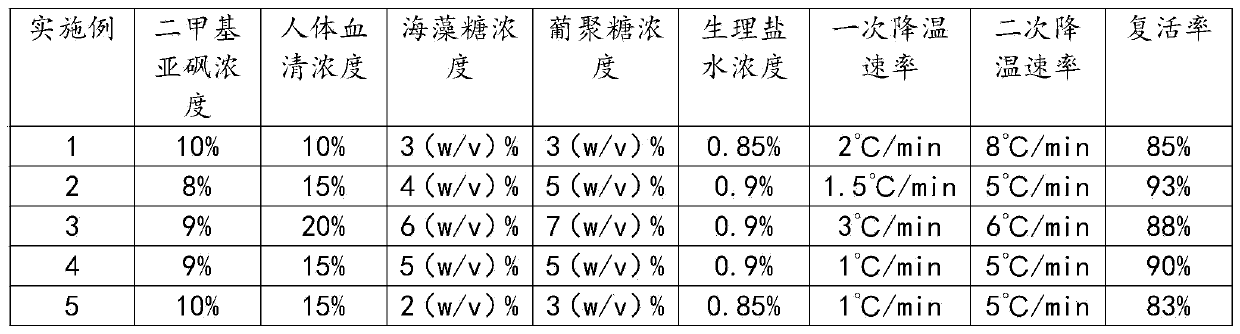
The invention discloses a stem cell freezing solution and a stem cell freezing method thereof, and the stem cell freezing solution comprises the following raw material components in percentage by concentration: 8-10% of dimethyl sulfoxide, 10-20% of human serum, 2-6 (w / v)% of trehalose, 3-7 (w / v)% of glucan and 0.85-0.9% of normal saline. The stem cell freezing solution comprises the following components in parts by weight: 1 part of the dimethyl sulfoxide, 2 parts of the human serum, 1 part of the trehalose, 1 part of the glucan and 5 parts of the normal saline. The stem cell freezing methodcomprises the steps of solution preparation, stem cell cleaning, digestion, centrifugation, filtration, culture, tubing, primary cooling, secondary cooling and cryopreservation. Stepped cooling and freezing is adopted, so that cytoplasm damage of stem cells due to fast temperature change is prevented, and the influence on the temperature rise reactivation rate is avoided; the prepared solution isstored in a 37 DEG C environment, so that the stem cells are transferred into the solution to simulate a human body environment, and the influence of different external environments on the stem cellsis avoided. The problem that in the prior art, after the stem cells are frozen, the temperature rise reactivation rate is low is solved.
Owner:ANHUI HUIEN BIOTECH
Detection method for oxidative stress functions of different organs of mice
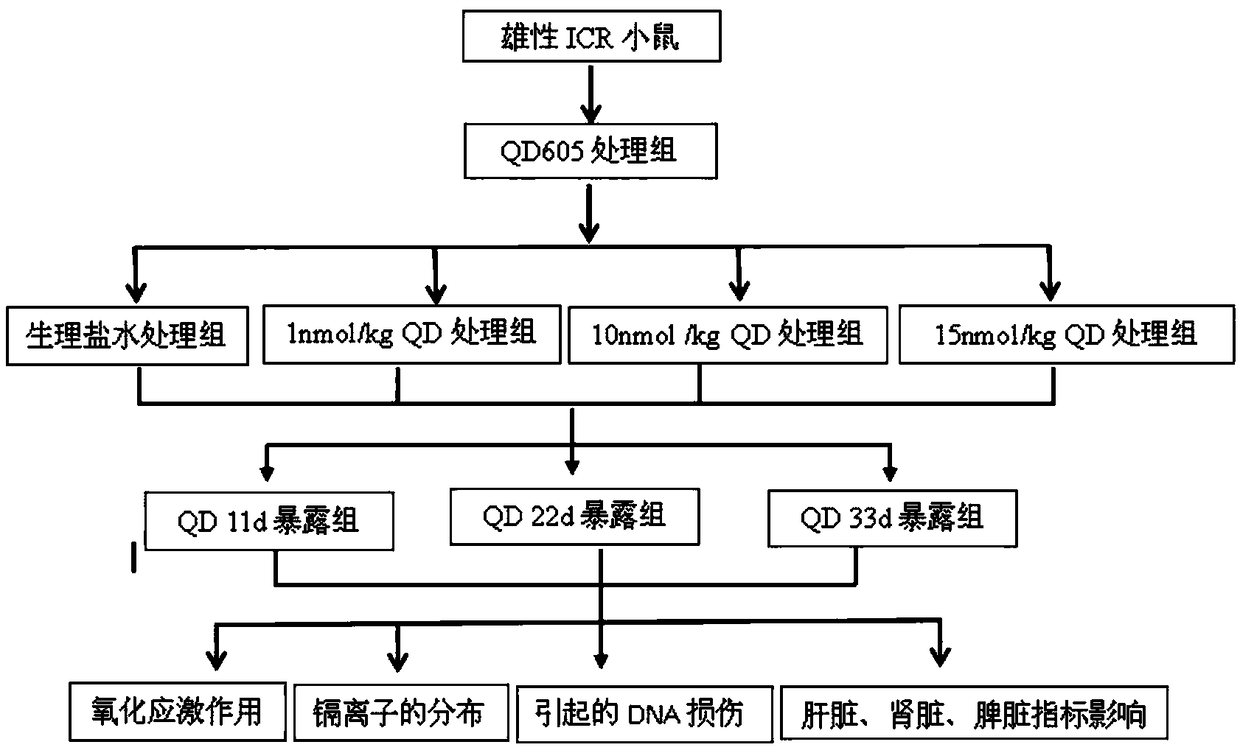
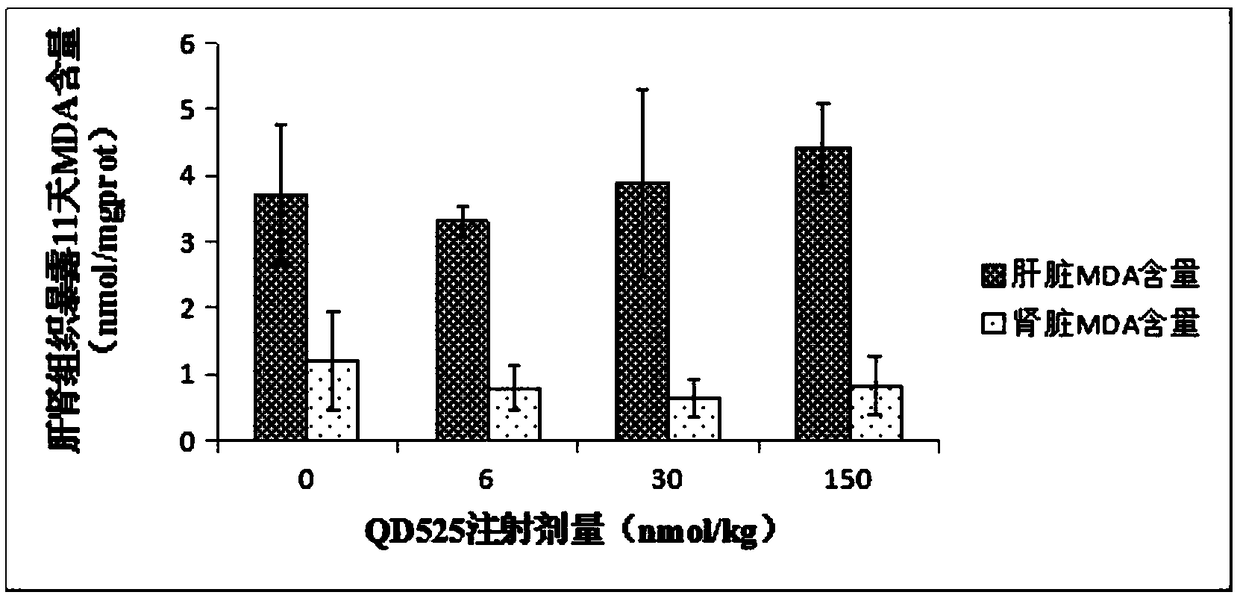
The invention belongs to the technical field of new species of vertebrates, and discloses a detection method for oxidative stress functions of different organs of mice. The clean male ICR mice with the weight of 20 g are selected, the mice are randomly grouped, and CdSe / ZnS QDs establishes a poisoned mouse model through tail intravenous injection; low-dosage groups, middle-dosage groups and high-dosage groups of quantum dots QD605 are prepared, and control groups, low-dosage groups, middle-dosage groups and high-dosage groups of quantum dots QD525 are prepared; all the control groups are treated by normal saline; the exposure times of the dosage groups are 11 days, 22 days and 33 days respectively. According to the method, by observing the influence of the quantum dots on genes and proteomes relevant to DNA impairment of the mice and liver and kidney antioxidase, the molecular toxicity effect of the CdSe / ZnS quantum dots on the animal bodies is further disclosed, and basic materials are provided for toxicology invention and bio-safety evaluation of the quantum dots.
Owner:XINXIANG MEDICAL UNIV
System and method for testing tensile property of soft hydrophilic material in normal saline
InactiveCN112683667AFix loading issuesTroubleshoot testing difficultiesMaterial strength using tensile/compressive forcesEngineeringTensile testing
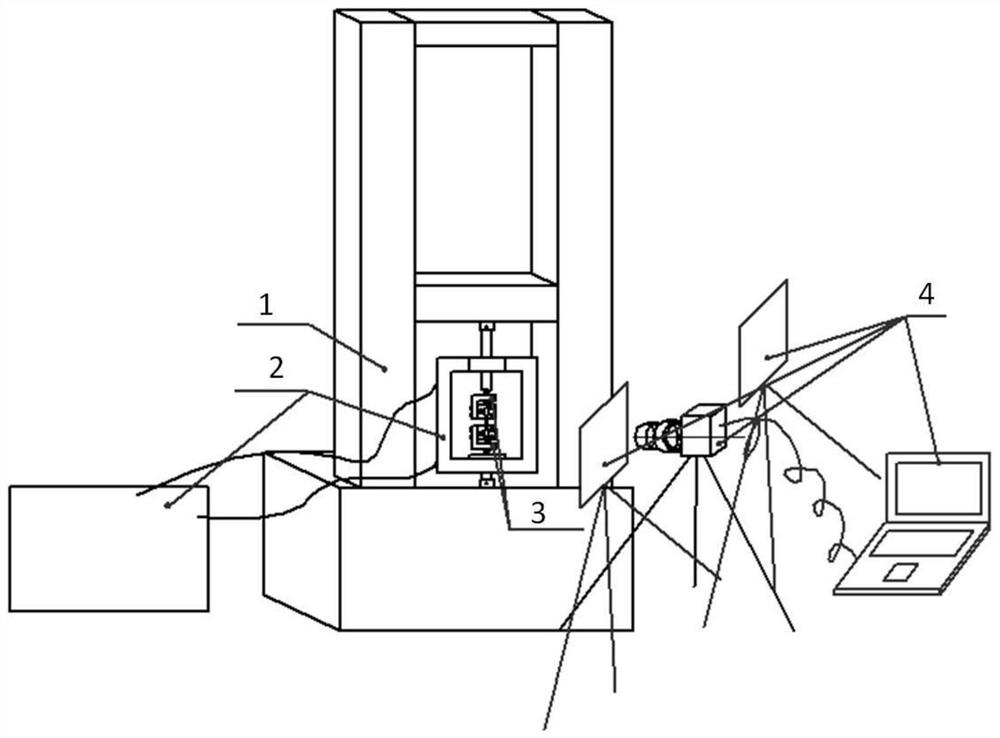
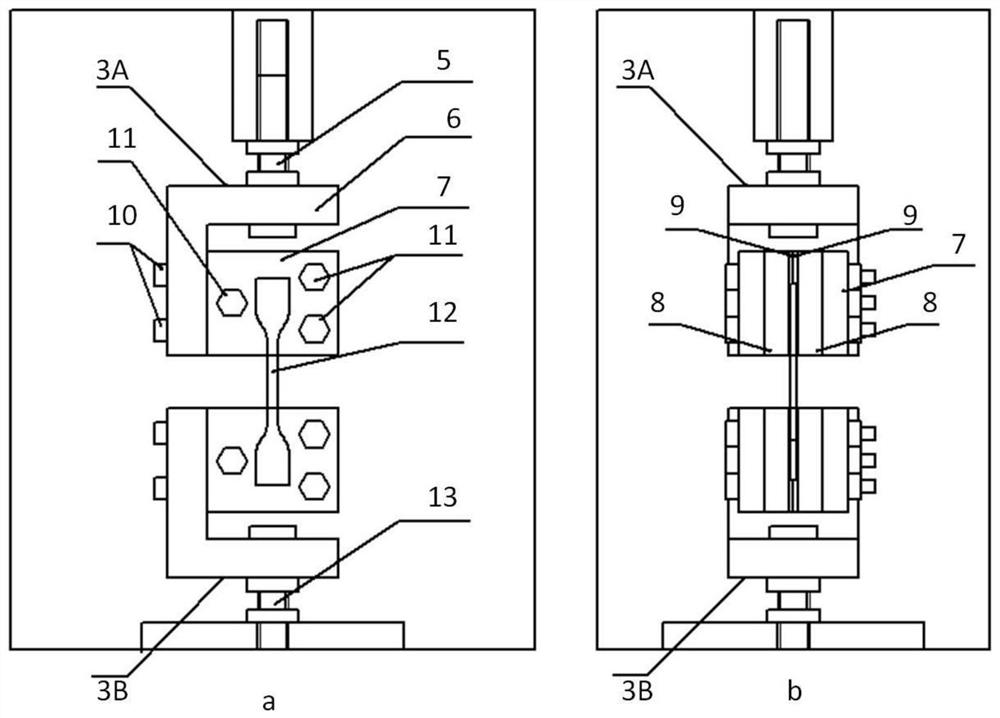
The invention relates to a system for testing the tensile property of a soft hydrophilic material in normal saline. The system comprises a microcomputer-controlled universal tensile testing machine (1), a salt bath box (2A), a temperature-controlled circulating water tank (2B), a salt bath system (2), a clamping device (3) and a digital image acquisition system (4). The invention further discloses a method for testing the elasticity modulus, the Poisson's ratio and the elongation at break of the soft hydrophilic material in the normal saline environment through the system. The device and the method are high in controllability, convenient to operate and easy to popularize.
Owner:BEIJING CENT FOR PHYSICAL & CHEM ANALYSIS
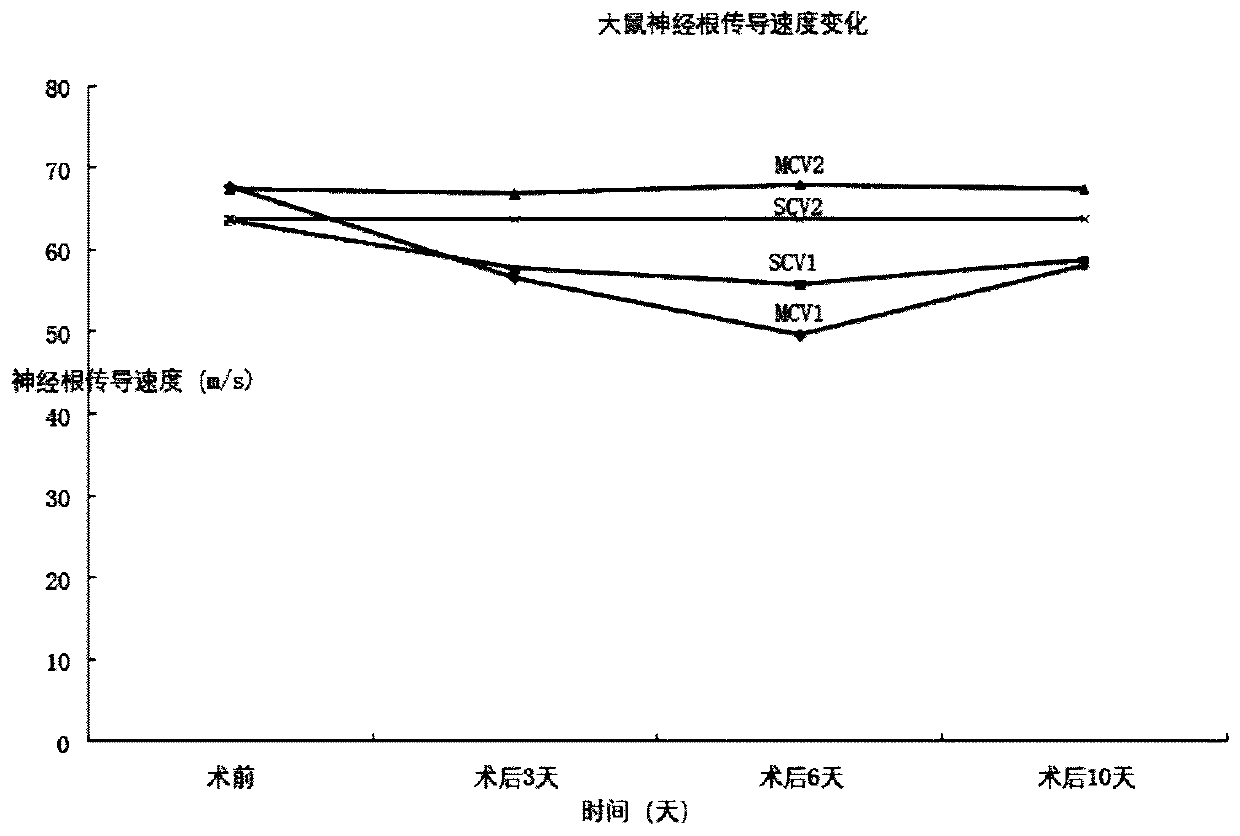
Establishment of animal model of non-compressive lumbar disc nucleus pulposus herniation
InactiveCN111357711AMorphological changesShorten speedAnimal husbandryLumbar spinal canalLumbar spine
The present invention discloses an establishment method of an animal model of non-compressive lumbar disc nucleus pulposus herniation. The method comprises the following steps: S1, cutting a rat tail1 cm from a root part of the rat tail and suturing wound to obtain rat caudal vertebra nucleus pulposus; S2, incising fibrous rings of the rat caudal vertebra nucleus pulposus, the nucleus pulposus isjelly-like, and taking 5 nucleus pulposus and 50 [mu]l normal saline to be fully mixed and diluted into a suspension; and S3, injecting 20 [mu]l of the nucleus pulposus suspension and 30 [mu]l of 2%of a lidocaine injection to epidural spaces of lumbar vertebra to enable the suspension to fill a periphery of nerve roots. Characteristics that the rat tail is thick and large and contains enough intervertebral disc tissue, and ithe nucleus pulposus is jelly-like, an epidural puncture technique is combined, the rat autologous nucleus pulposus suspension is injected into the epidural spaces of thelumbar vertebra to produce nucleus pulposus tissues which are exposed to a blood circulation and body immune system. The establishment method confirms that even if the nucleus pulposus suspension inlumbar vertebra canal of the rat does not compress the nerve roots, changes in nerve root functions and morphology also appear, and proves effectiveness of the model.
Owner:GENERAL HOSPITAL OF NINGXIA MEDICAL UNIV
A detection system for intestinal feces
ActiveCN110836808BHigh degree of intelligenceReduce work intensityPreparing sample for investigationFecesMedicine
The invention provides a detection system for intestinal feces. The detection method of the system includes: step 1, adding physiological saline and feces into the first centrifugal chamber; step 2, the stirring device of the first centrifugal chamber enters the stirring mode; step 3. The second liquid level sensor group starts to detect the liquid level height in the second centrifugal chamber; Step 4, the filtrate enters the second centrifugal chamber for centrifugation, and the primary centrifugal sediment after removing the supernatant enters the transfer chamber; Step 5, the second centrifugal chamber enters the emptying operation; Step 6, the secondary centrifugal sediment in the transfer chamber is returned to the first centrifugal chamber for centrifugation; Step 7, the transfer chamber enters the emptying operation; Step 8, the first control valve is opened again , the three centrifugal sediments enter the second centrifugal chamber; the physiological saline in the output storage bin flows into the second centrifugal chamber, and the second centrifugal chamber performs centrifugal work; Step 9, the fecal bacteria suspension that meets the requirements in the second centrifugal chamber enters the second centrifugal chamber Transit chamber storage.
Owner:张俊美
Hemoglobin storage method
InactiveCN104873960ASimple and fast operationLow costPeptide/protein ingredientsBlood disorderSaline waterMethemoglobin
The invention relates to a hemoglobin storage method which is characterized by comprising the following steps of adding a proper quantity of human blood into normal saline (a 0.9% NaCl solution) with the volume being 3-6 times that of the human blood, centrifuging at the temperature of 4 DEG C and the rotating speed of 2800r / min for 15-30 minutes to obtain a supernatant containing leukocytes, plasma proteins and platelets, extruding the supernatant, retaining deposited erythrocytes at the lower layer, repeating the washing process for more than 3-5 times until the supernatant is basically colorless, and removing other substances except for the erythrocytes to the maximum extent to obtain clean deposited erythrocytes; adding a certain quantity of deposited erythrocytes into equivoluminal sterilized high-purity deionized water to pre-expand for 5-15 minutes, finally, adding water with the volume being 2 times that of the deposited erythrocytes to carry out low permeation for 10-20 minutes, centrifuging at the rotating speed of 10000r / min for 30-60 minutes, and extracting the supernatant after centrifuging to obtain a hemoglobin solution; and subpackaging the purified hemoglobin solution into a 10ml sample bottle, sealing by using paraffin, and storing the purified hemoglobin solution in a brown bottle filled with a pyrogallic acid solution at the temperature of 4 DEG C, wherein the content of hemoglobin does not exceed 3% after the sample is stored for 4 months and is kept on the relatively-low level.
Owner:TIANJIN POLYTECHNIC UNIV
A biologically active immune preparation for enhancing sheeppox vaccine antibody and its preparation method
InactiveCN106552264BImprove immune antibody levelsObvious weight gainPeptide/protein ingredientsViral antigen ingredientsConnective tissue fiberNS - Normal saline
The invention relates to a biological immune preparation for strengthening a goatpox vaccine antibody and a manufacturing method of the biological immune preparation. The steps adopted in the method include that the fresh spleen of a healthy yak is selected, after fat, the envelope and the internal connective tissue are removed, the spleen is subjected to freezing preservation, the yak spleen obtained after freezing preservation is taken out and melted and is minced through a tissue stamp mill, after sterile tri-distilled water with the same weight is added, the high-speed tissue stamp mill is used for conducting homogenizing at a high speed for two times, and homogenizing is conducted 10 min each time; and prepared homogenate is frozen at the temperature of minus 20 DEG C, freezing and thawing are conducted repeatedly for five times, centrifuging is conducted after the last time of thawing, supernatant is taken and put into a dialysis bag, the supernatant is dialyzed overnight under the environment of 0 DEG C to 4 DEG C through pyrogen-free cold normal saline, dialysate outside the bag is collected and is subjected to filtration sterilization through a filtering film, and the dialysate is subjected to sterile subpackaging and is then subjected to freezing preservation. After the biological immune preparation enters an organism, the effect of the biological immune preparation can be rapidly developed and can last for several months or even longer, the immune antibody level of the vaccine is effectively improved, the biological immune preparation can be rapidly and completely absorbed by the organism without any residue, weight increasing is obvious after the biological immune preparation is injected into the organism, and a goat can be lively in spirit.
Owner:QINGHAI UNIVERSITY
Medicament formula for treating female vaginal relaxation syndrome
PendingCN112353817ARelief of Relaxation Syndrome SymptomsRelieve symptomsOrganic active ingredientsPeptide/protein ingredientsTissue repairUmbilical cord
The invention discloses a medicament formula for treating female vaginal relaxation syndrome. The medicament formula comprises mesenchymal stem cells and a filling agent, wherein the volume ratio of the mesenchymal stem cells to the filling agent is 1: 1-1: 3, the mesenchymal stem cells are parturient umbilical cord placenta or endometrium, the stem cell resuspension is normal saline containing exosome, and the filling agent is sodium alginate; the filling agent is any one or two of hyaluronate or collagen. In the formula of the medicament, the mesenchymal stem cells and the filler jointly play a role in filling in a short time, the symptom of female vaginal relaxation syndrome is relieved, the mesenchymal stem cells stimulate local tissue repair and angiogenesis in a microenvironment by secreting growth factors and cytokines at the same time, the contraction function of urethral sphincter is enhanced, and the effect of treating female vaginal relaxation syndrome is achieved; when thefiller and the allogeneic mesenchymal stem cells are gradually decomposed and absorbed by an organism, the newly-generated tissue achieves a purpose of relieving the female vaginal relaxation syndromefor a long time, so a problem of the female vaginal relaxation syndrome is better solved.
Owner:湖南科诺康美生命科技有限公司
Preparation method for hemoglobin
InactiveCN104804080ALow costSimple and fast operationHaemoglobins/myoglobinsPeptide preparation methodsWhite blood cellNS - Normal saline
The invention relates to a preparation method for hemoglobin. The method is characterized by comprising the following steps: taking a proper amount of human blood, adding normal saline (a 0.9% NaCl solution) with the volume being 3-6 times that of the human blood, and performing centrifugation at a speed of 2,800 rpm and a temperature of 4 DEG C for 15-30 minutes; obtaining supernatant liquid containing leukocytes, plasma proteins and platelets, extruding the supernatant liquid, remaining lower-layer packed red cells, repeating a washing process for 3-5 times until the supernatant liquid is basically colorless, removing other substances except the red cells to the maximum extent, and obtaining clean packed red cells; taking a certain amount of the packed red cells, adding sterilization and high-purity de-ionized water with the same volume for pre-expansion for 5-15 minutes, finally adding water with the volume being 2 times that of a pre-expansion solution for hyposmosis for 10-20 minutes, and performing centrifugation at a speed of 10,000 rpm for 30-60 minutes, wherein the centrifuged supernatant liquid is a hemoglobin solution.
Owner:TIANJIN POLYTECHNIC UNIV
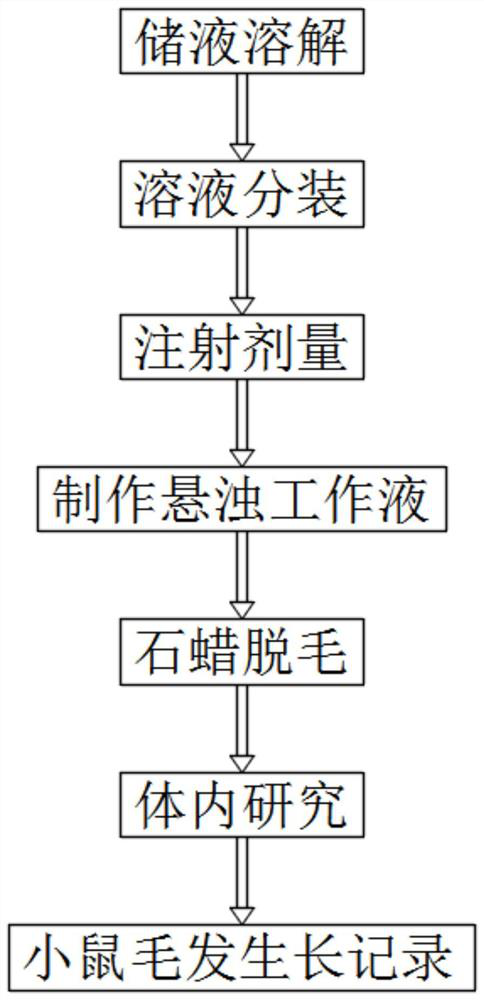
Method for inducing white hair model
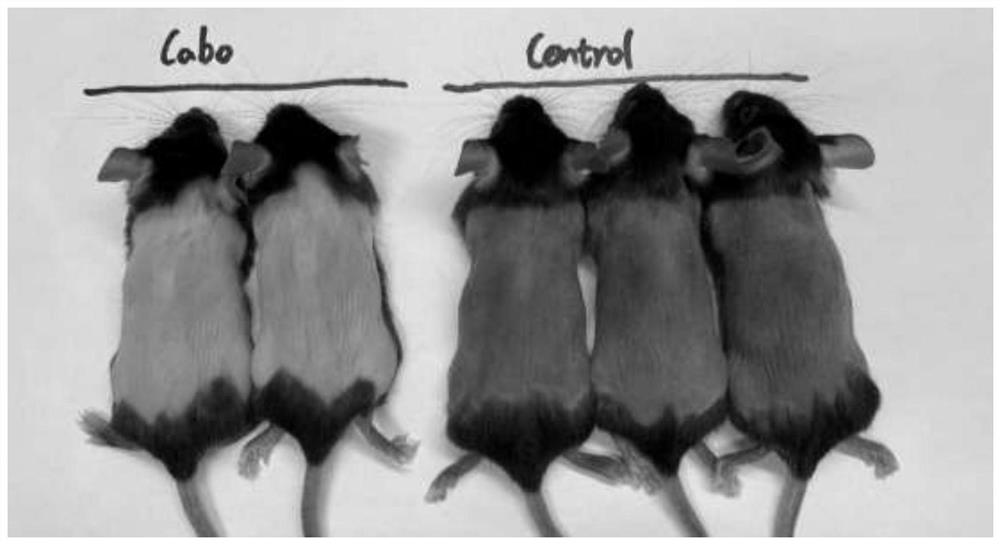
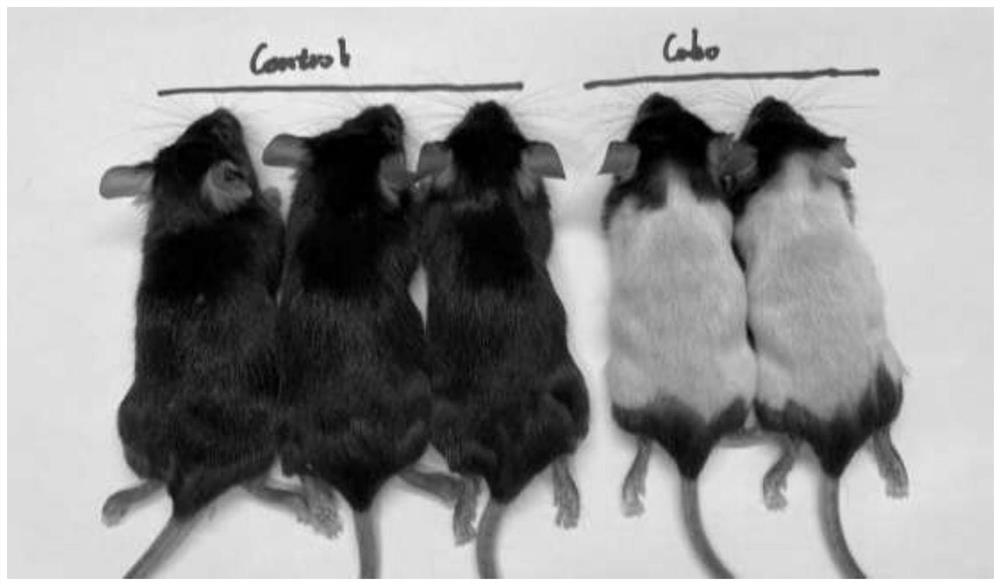
The invention discloses a method for inducing a white hair model. The method comprises the following steps: step 1, storing liquid and dissolving; step 2, sub-packaging the solution; step 3, injection dosage: according to the weight of the mouse, injecting a liquid substance into the body of the laboratory mouse according to the dosage of 60mg / kg; step 4, preparing a suspended working solution: taking one tube of 100 [mu] L storage solution, and adding normal saline of which the volume is 10-15 times that of the storage solution to prepare the suspended working solution; 5, paraffin unhairing, wherein paraffin unhairing is conducted on the mice of 8-9 weeks old on the 0th day; step 6, in-vivo research: according to the injection dosage of 60 mg / kg, 200 [mu] L of the working solution is continuously injected into the intraperitoneal cavity of a mouse of 8-9 weeks old and 25 g every day, and the time lasts for 2-3 weeks; and step 7, recording mouse hair growth. The Cabozantinib is used as a VEGFR2 (vascular endothelial growth factor receptor 2) inhibitor and can play a role in inducing white hair, and the white hair of a mouse grows densely in the twenty-fourth day after the mouse is injected with the Cabozantinib.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
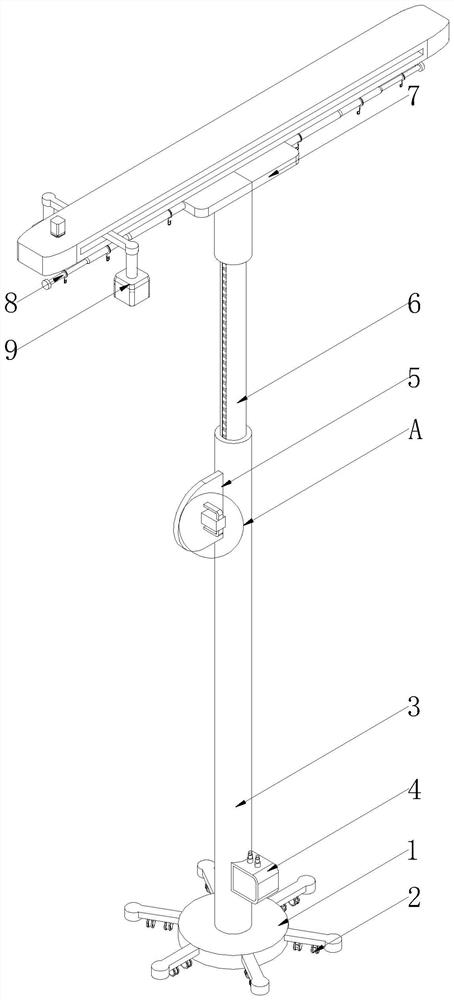
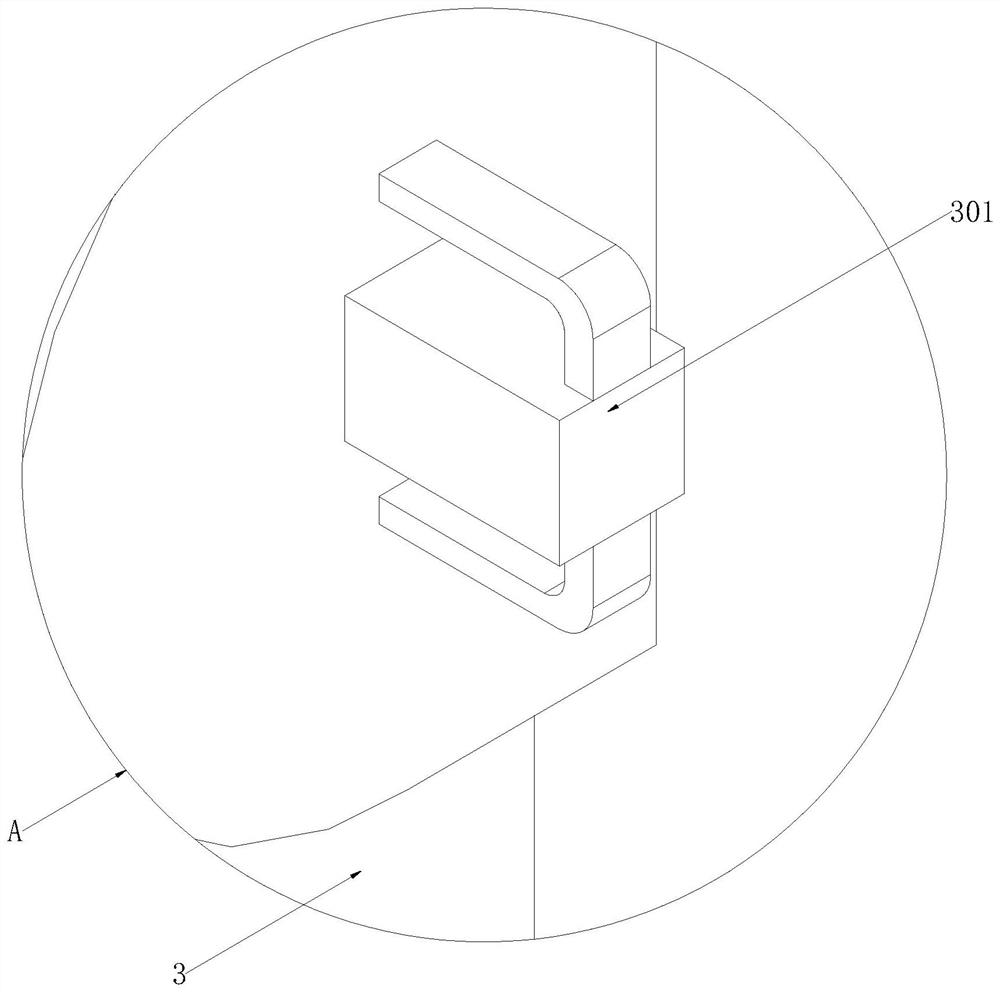
Auxiliary device for injection of normal saline filling bottle and use method of auxiliary device
PendingCN114848958AEnable mobilityImprove practicalityMedical devicesFlow monitorsNS - Normal salineMechanical engineering
The invention discloses an auxiliary device for injection of a normal saline filling bottle and a using method thereof.The auxiliary device comprises a base, a fixed rod, a movable rod and a top platform, a driving assembly is arranged at the bottom end of the base, the fixed rod is fixed to the top end of the base, a signal box is fixedly arranged at the bottom end of the side face of the fixed rod, and a control assembly is arranged at the top end of the side face of the fixed rod; the movable rod is movably connected to the top end of the fixed rod, the top platform is fixed to the top end of the movable rod, telescopic hanging assemblies are arranged on the two sides of the top platform, and a monitoring auxiliary assembly is arranged at the top end of the top platform. According to the auxiliary device for injection of the normal saline filling bottle and the using method of the auxiliary device, the capacity that an infusion support synchronously moves along with a patient is achieved, the actual height of the normal saline filling bottle can be controlled in cooperation with a telescopic hanging assembly and a control assembly, and the convenience of the device in use is improved; and then the alarm is matched to give an alarm when the liquid level is below the indication line, so that the practicability of the device is further improved, and the workload of medical staff is reduced.
Owner:福州安得尔卫生技术开发有限公司
Nimodipine fat emulsion concentrate and preparation method and use thereof
InactiveCN104706593ANo delaminationImprove liquidityOrganic active ingredientsSenses disorderDiseaseNimodipine
The invention relates to a nimodipine fat emulsion concentrate and a preparation method and use thereof, and belongs to the field of medicine and pharmacy. The nimodipine fat emulsion concentrate overcomes the defects of complex traditional fat milk preparation process and poor product stability. The nimodipine fat emulsion concentrate is simple in preparation process only needing simple physical mixing without homogenization process. The product can be sterilized through a 0.22 mum millipore filter film, and can spontaneously emulsify during clinical use by dilution with normal saline or glucose solution and other water solutions and slight oscillation, and under the optimal conditions, average particle size is about 0.2 mum, and fully shows injection fat milk properties. The nimodipine fat emulsion concentrate product has good liquidity, and may not be hung on the wall, the appearance is single-phase, transparent and clear, clarity detection is acceptable, and after repeated freezing and thawing, preparation stratification phenomenon does not occur. The nimodipine fat emulsion concentrate product is used for prevention and treatment of ischemic neurological damage caused by cerebral angiospasm after subarachnoid hemorrhage and senile brain function impairment, hemicranias, sudden deafness and other diseases.
Owner:天津迈迪瑞康生物医药科技有限公司
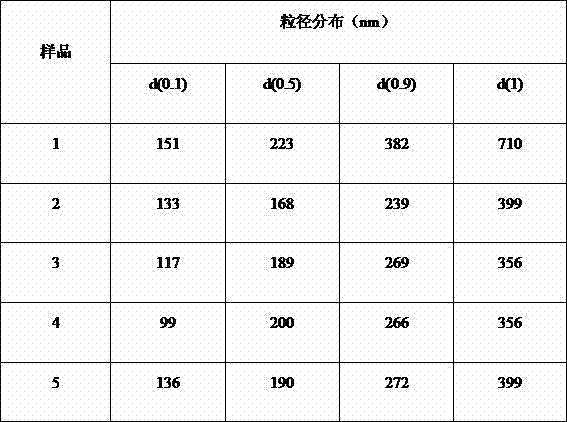
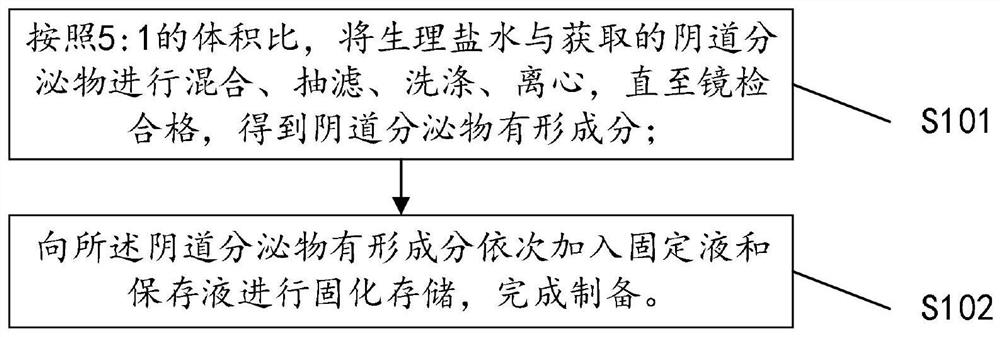
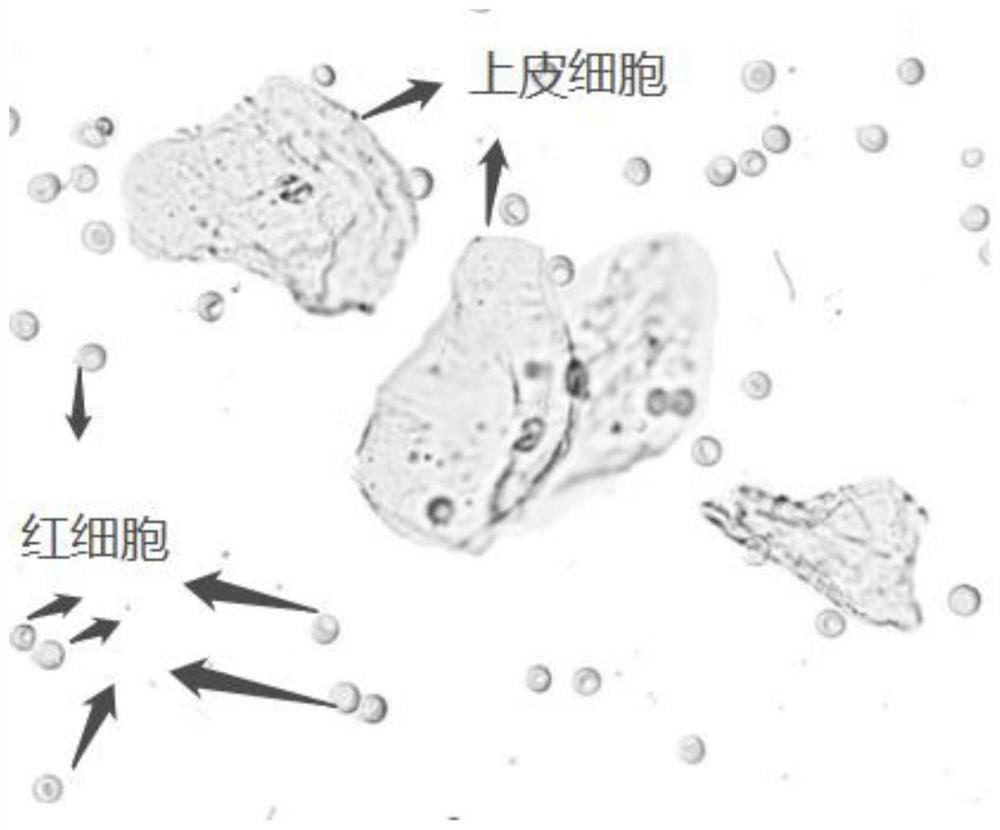
Quality control composition for analyzing visible components of vaginal secretion and preparation method of quality control composition
InactiveCN112881114AImprove accuracyPreparing sample for investigationMaterial analysis by optical meansBiotechnologyPeristaltic pump
The invention discloses a quality control composition for analyzing visible components of vaginal secretion and a preparation method of the quality control composition. 7% normal saline and obtained vaginal secretion are mixed according to the volume ratio of 5: 1; then, suction filtration is carried out on the mixed vaginal secretion by utilizing a peristaltic pump with a 22-micron filter membrane to obtain residues; then, the residues are washed and centrifuged for multiple times by using normal saline until microscopic examination is qualified, so as to obtain the visible components of the vaginal secretion; finally, stationary liquid and preserving liquid are sequentially added into the visible components of the vaginal secretion to solidify and store the visible components, and therefore, the preparation of the quality control composition is completed. The detection accuracy of the visible components of the vaginal secretion is improved.
Owner:URIT MEDICAL ELECTRONICS CO LTD
A kind of construction method of azoospermia mouse model
ActiveCN108815152BHigh molding rateImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismIntraperitoneal routeStaining
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Bee venom injection
PendingCN113925883AExpected treatment effectHas a blood pressure lowering effectOrganic active ingredientsNervous disorderBlood flowVitamin B12
The invention discloses a bee venom injection which is used for performing stinging treatment on a human body part, so that local hyperemia, redness and swelling can be realized, meridians and collaterals and skins of a human body can be stimulated to dredge the meridians and collaterals, meanwhile, bee venom has the effects of reducing blood pressure, resisting arrhythmia and improving cerebral blood flow and myocardial functions, proliferation of tumor cells is obviously inhibited, and the damage effect on tumor cells is larger than that of normal cells, local skin can be anesthetized through lidocaine, so that pain of a patient is relieved, the hematopoietic function of an organism is in a normal state through vitamin B12, meanwhile, carbohydrate and fat metabolism can be promoted, amino acid can be activated, protein synthesis can be promoted, and through the arrangement of glucose, the injected liquid is conveniently absorbed and utilized by a body, and meanwhile, under the synergistic effect of glucose and normal saline, bee venom, lidocaine and vitamin B12 can be diluted and uniformly mixed, so that the injection can achieve the treatment effect.
Owner:谢小来
Evaluation method of ADSCs for relieving filial generation rat hypertension and application
InactiveCN108853519AIncrease the number ofSuppress high blood pressureGeneral/multifunctional contrast agentsUnknown materialsIntraperitoneal routeVein
The invention relates to the technical field of biomedicine, in particular to an evaluation method of ADSCs for relieving filial generation rat hypertension and application. The method comprises the following steps of (1) cell culture: putting the ADSCs into a complete culture medium for culture; (2) cell marking: diluting 1mg / ml SPIO by a complete culture medium into 50 mug / mL; meanwhile, diluting polylysine into 1.5ug / ml; then mixing the two kinds of solutions according to a ratio of 1:1, wherein the final dilution concentration of the polylysine is 0.75 mug / mL; after the sufficient and uniform mixing, putting the materials into a cell culture box to be cultured; (3) pregnancy period modeling: building an LPS group, an LPS+ADSCs group and a control group (for the LPS group, injecting LPSinto the abdominal cavity of SD pregnant rats on the 8th day, 10-th day and 12-th day from the pregnancy; for the LPS+ADSCs group, injecting LPS into the abdominal cavity of SD pregnant rats on the 8th day, 10-th day and 12-th day from the pregnancy; meanwhile, injecting the ADSCs prepared in the step 2 into the caudal vein on the 8th day from the pregnancy; for the control group, injecting the equal volume normal saline into the abdominal cavity of SD pregnant rats on the 8th day, 10-th day and 12-th day from the pregnancy); 4, SPIO-ADSCs migration observation under MRI; (5) filial generation rat blood pressure change observation.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Stomach tube and nutrition tube integrated multifunctional pipeline structure for thoracic department
The invention discloses a stomach tube and nutrition tube integrated multifunctional pipeline structure for the thoracic department, and particularly relates to the field of the thoracication.The stomach tube and nutrition tube integrated multifunctional pipeline structure comprises a catheter, an air bag is arranged on the outer surface of the catheter, and a buffer tube is arranged on the portion, located above the air bag, of the outer surface of the catheter. When a nutrient solution or normal saline is injected, one end of an injector is connected with the butt joint pipe at one end of the top cover, the top cover is screwed into one end of the connector, the connector is in threaded connection with the top cover, in the screwing-in process, the inner ring pushes the inner plug to open the through hole in the outer surface of the inner pipe, after conveying is completed, the top cover is rotated, the top cover is slowly taken out, and the connector is screwed into the connector. In the process that the top cover is separated from the connector, the spring pushes the inner plug upwards, and after the top cover is completely separated from the connector, the inner plug closes the through hole in the surface of the inner tube, so that air is prevented from entering and polluting the catheter, meanwhile, the difficulty of cleaning the stomach tube is reduced, the service life of the stomach tube is prolonged, and the stomach tube is practical.
Owner:河南省胸科医院
Biological degreasing agent and preparation method thereof
InactiveCN106755122AEfficient removalQuick removalMicroorganismsNon-surface-active detergent compositionsSaline waterLiquid medium
The invention discloses a biological degreasing agent and a preparation method thereof. The method comprises the steps of inoculating lactobacillus into a liquid medium for culturing, and then diluting by using normal saline; sequentially adding Na2CO3 and hydrochloric acid; and carrying out standing and centrifuging and collecting supernate to obtain the biological degreasing agent. According to the prepared biological degreasing agent, greasy dirt on tableware can be effectively removed within an extremely short period of time; and the oil washing rate reaches 83.3-92.3%. Compared with the existing degreasing agent, the biological degreasing agent has the advantages of being mild in property, environment-friendly, nontoxic, short in degreasing time and high in efficiency.
Owner:广州舒国生物科技有限公司
Preparation method of human anatomy viscera specimen
InactiveCN109377846BHas a bactericidal effectImprove permeabilityDead animal preservationEducational modelsHuman anatomyHuman body
The invention relates to a method for preparing a human anatomical visceral specimen. The method comprises the following steps: step one, pouring normal saline into a viscus through an artery for washing and cleaning; step two, pouring visceral and immersing the visceral artery into pretreatment liquid for 12 to 24 hours, and then carrying out vacuum treatment; step three, carrying out pre coolingon the viscus for 3 to 5h at a temperature of 2 DEG C, and immersing the cooled viscus into a dehydration solution for 16 to 24h; step four, immersing the viscus into a sealed container with preservation solution for 36 to 72h and carrying out gradient vacuum treatment; step five, removing excess material of the visceral outer layer to obtain a softened specimen; and step six, placing the softened specimen into a sealed box filled with a curing agent for 72 to 96 hours to obtain a cured specimen. The method is simple and easy. Since the pretreatment liquid and the preservation liquid are notirritating, the operator is protected from being injured; the softened visceral specimen is not easy to damage and can be used for long time; and the visceral specimen has the natural color and soft texture and has no mildew phenomenon.
Owner:BAOTOU MEDICAL COLLEGE OF INNER MONGOLIA UNIV OF SCI & TECH
Application of hydrolase inhibitor t-TUCB in Alzheimer disease
InactiveCN113509458AImprove pathological featuresImprove oral bioavailabilityOrganic active ingredientsNervous disorderDiseaseStaining
The invention discloses an application of a hydrolase inhibitor t-TUCB in Alzheimer's disease, which comprises the following steps: S1, performing intragastric administration on 6-month-old 5XFAD mice with different doses of t-TUCB (0.5 mg / kg, 1mg / kg and 2mg / mg) for 8 weeks, and dissolving the t-TUCB in a 40% PEG-400 solution prepared from normal saline; S2, then killing the mice, taking out the head, freezing and slicing, detecting and analyzing the levels of Abeta in hippocampus and motor cortex in the mice of the administration group and the mice of the solvent group by using immunofluorescence staining (6E10), and result analysis shows that compared with the mice of the solvent group, the percentage of the area fraction of plaques in a 1mg group, a 2mg group, a hippocampus and a motor cortex is remarkably reduced. The modern compound t-TUCB prepared by the invention can show good oral bioavailability and pharmacokinetics, is easier to prepare, and can obviously play a role in improving AD pathological characteristics, thereby further improving learning memory and cognitive competence.
Owner:广州市中西医结合医院
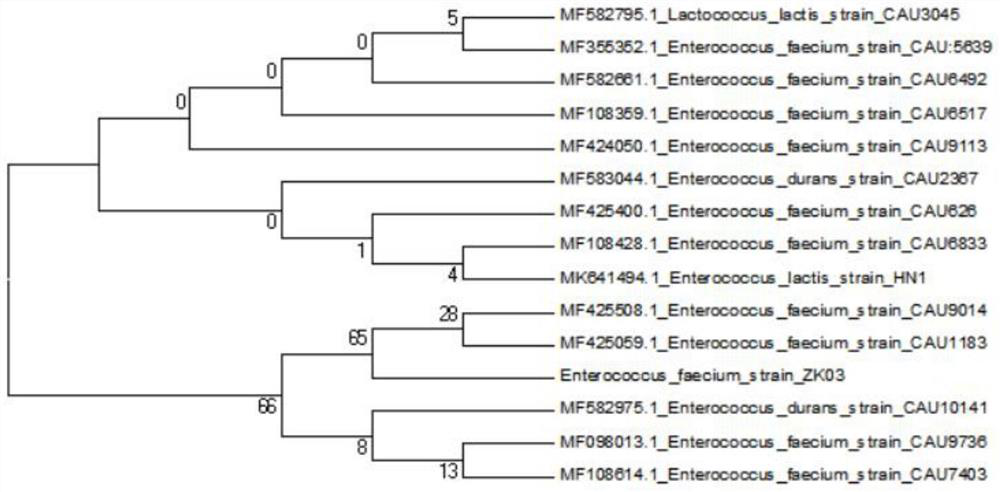
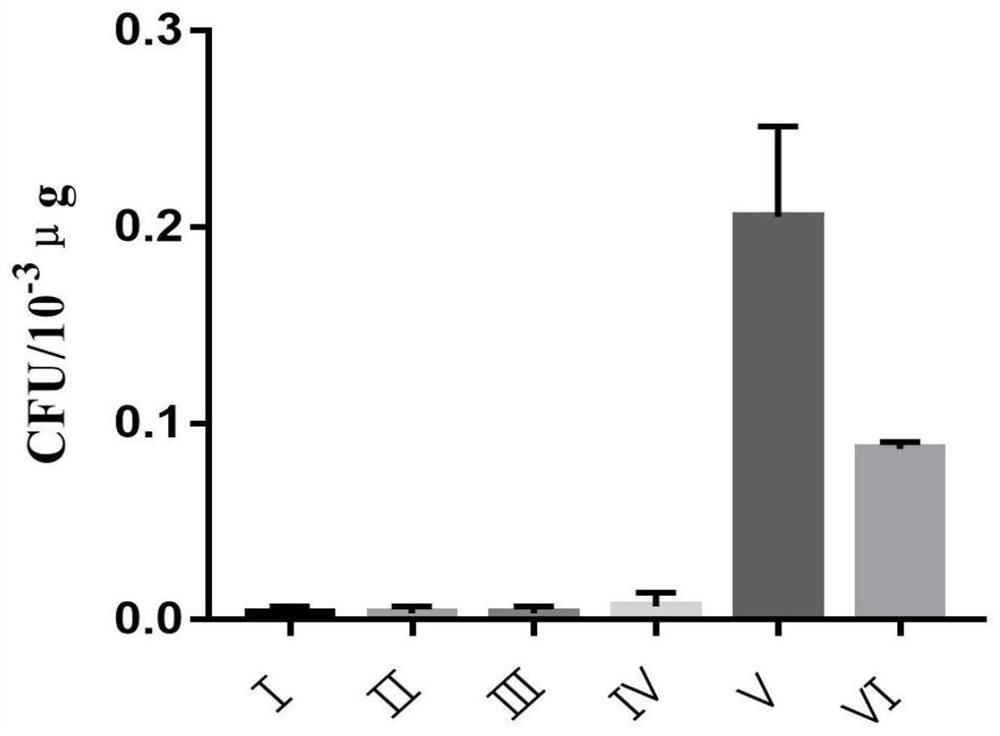
Wild enterococcus faecium ZK03 and electric shock transformation method and application thereof
PendingCN114480211AGood resistance to pig bile saltsImprove high temperature resistanceBiocideBacteriaBiotechnologyStaphyloccocus aureus
The invention discloses wild enterococcus faecium ZK03 as well as an electric shock transformation method and application thereof, and belongs to the field of microorganisms. The strain is preserved in Guangdong Microbial Culture Collection Center on January 5, 2022, and the preservation number is GDMCC (China General Microbiological Culture Collection Center) No. 62192. The strain does not contain enterococcus virulence factors such as Asa1, cylA, efaA, esp, gelE and hyl. The strain has the characteristics of good acid resistance, swine bile salt resistance, high temperature resistance and normal saline resistance, and can survive for a long time in normal saline at room temperature; the strain has a certain inhibition effect on staphylococcus aureus, salmonella and escherichia coli, can be used for preparing a bacteriostatic agent, and can also be used as a feed additive for fermentation production of feed. The electric shock transformation method of the wild enterococcus faecium ZK03 is simple to operate, high in transformation efficiency and strong in repeatability.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com