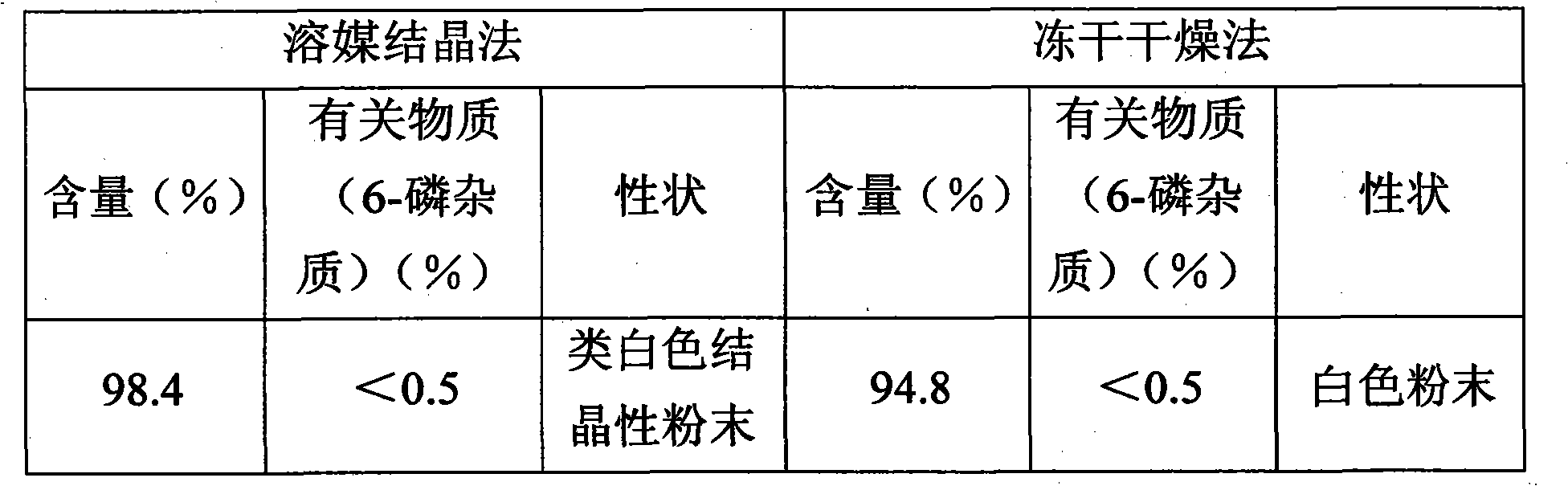
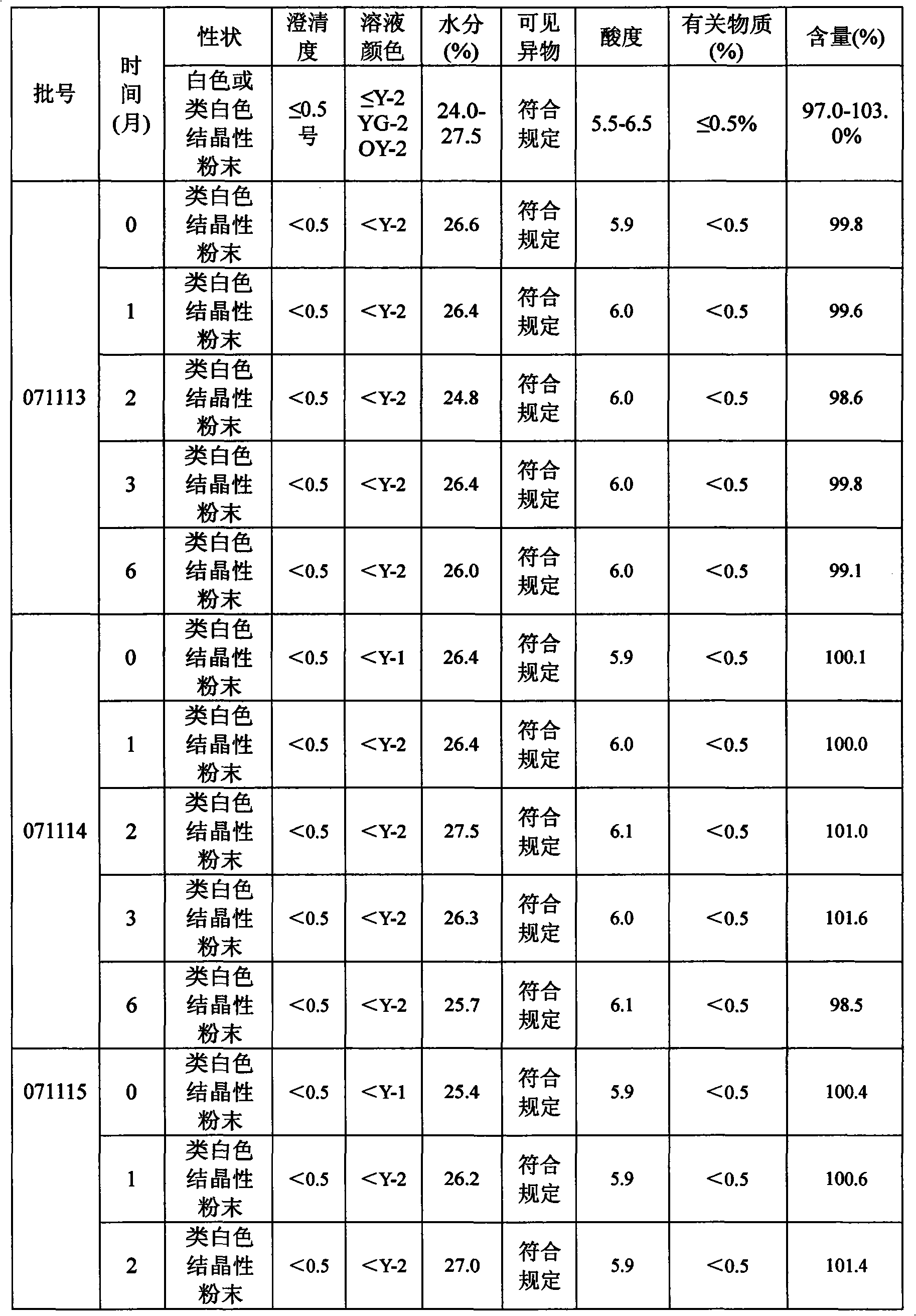
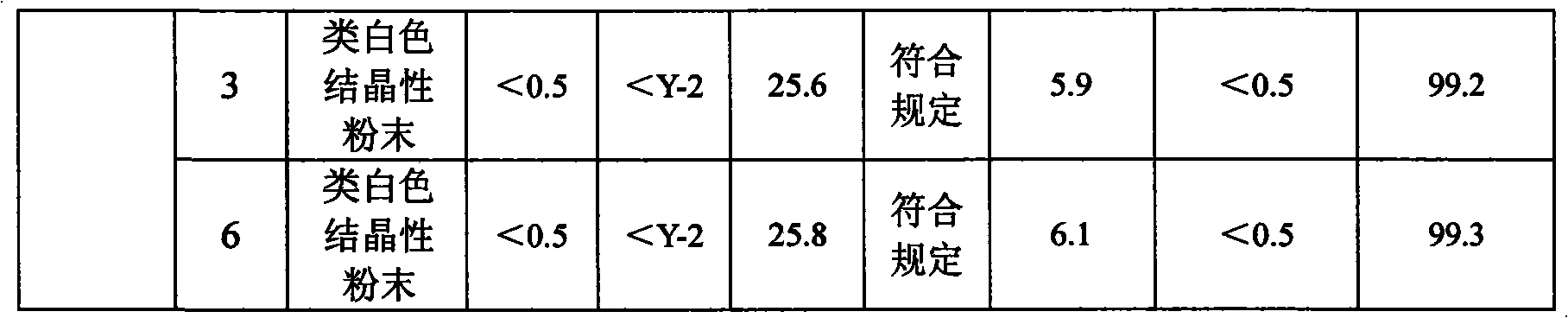
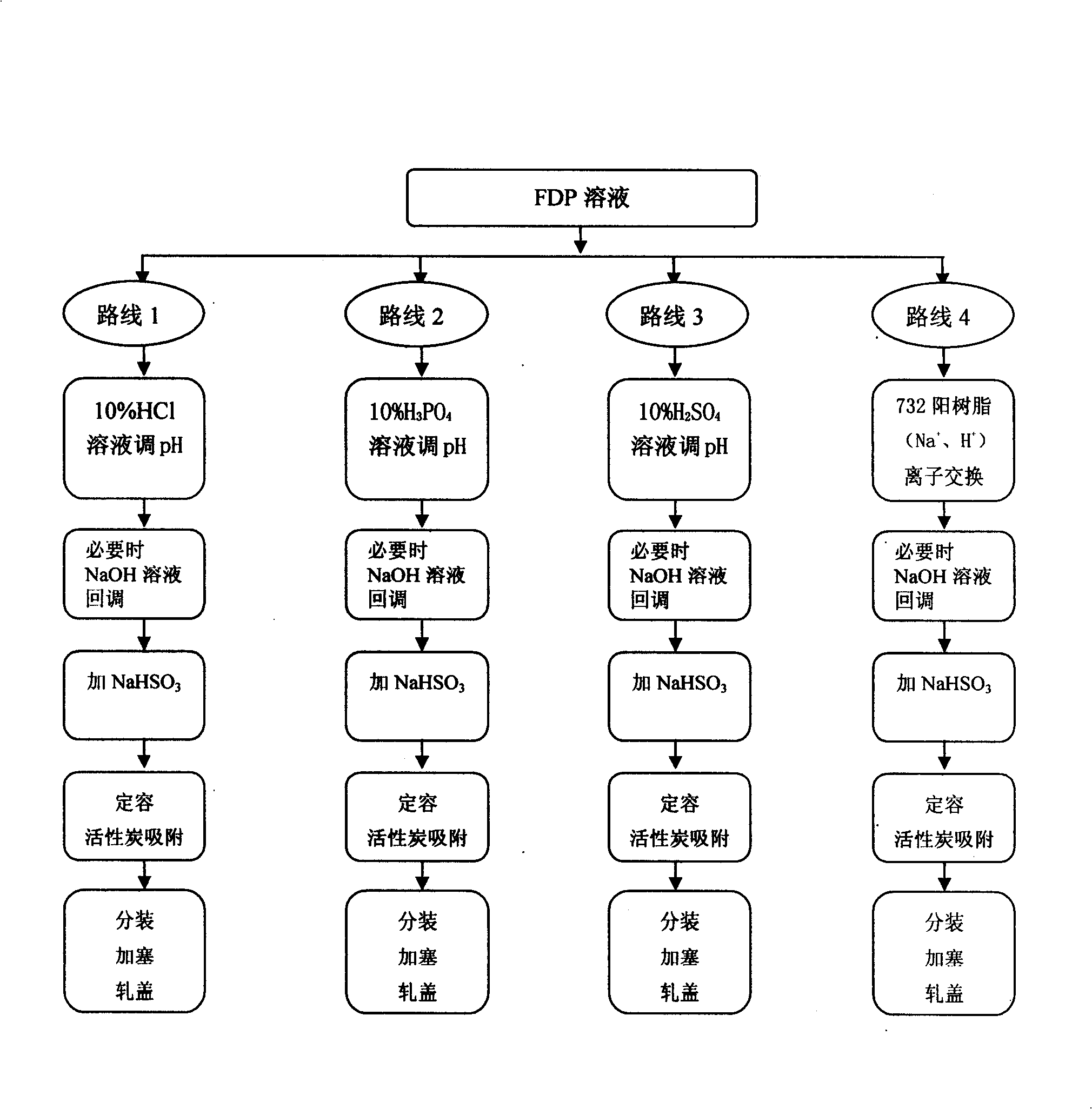
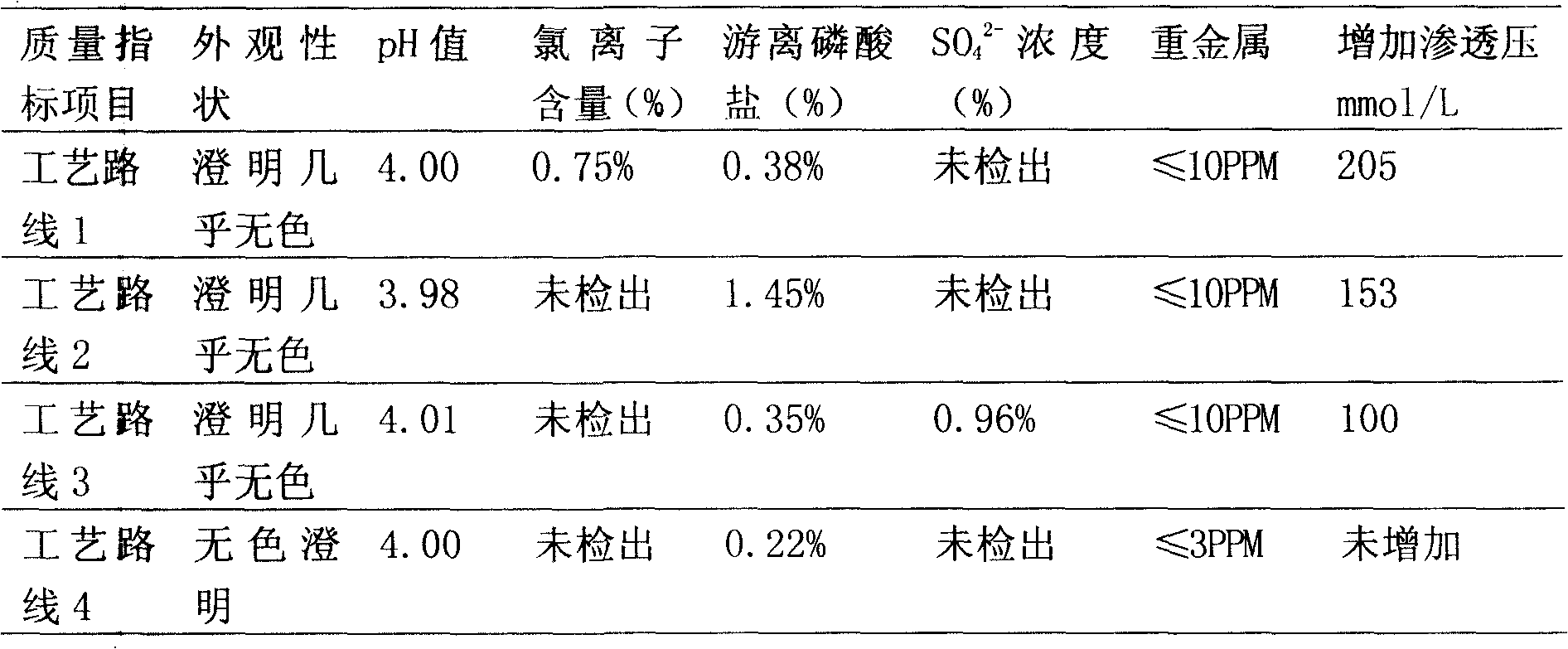
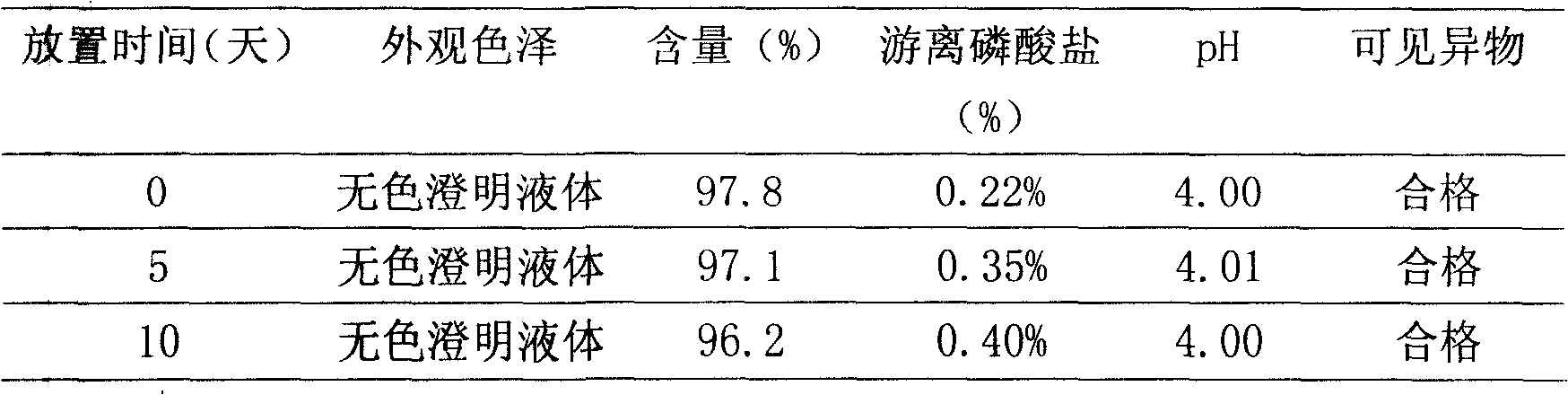
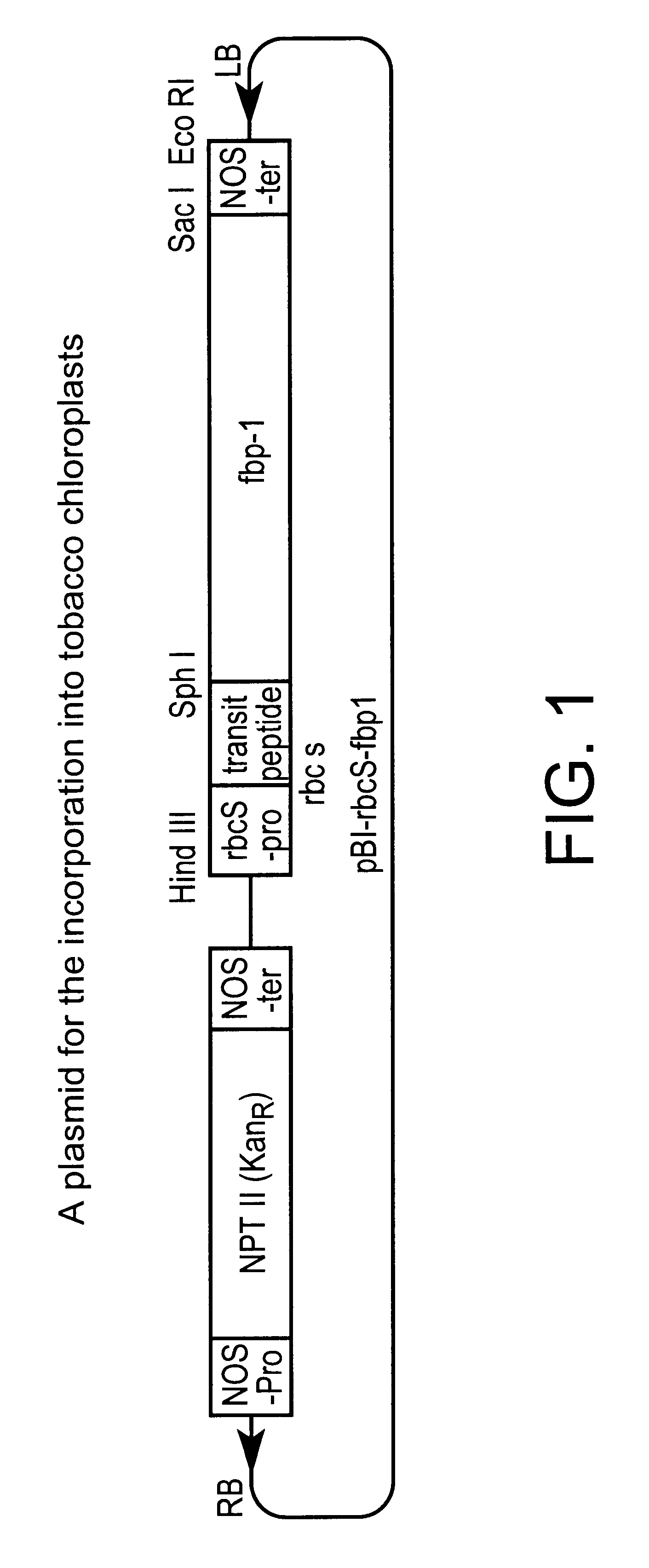
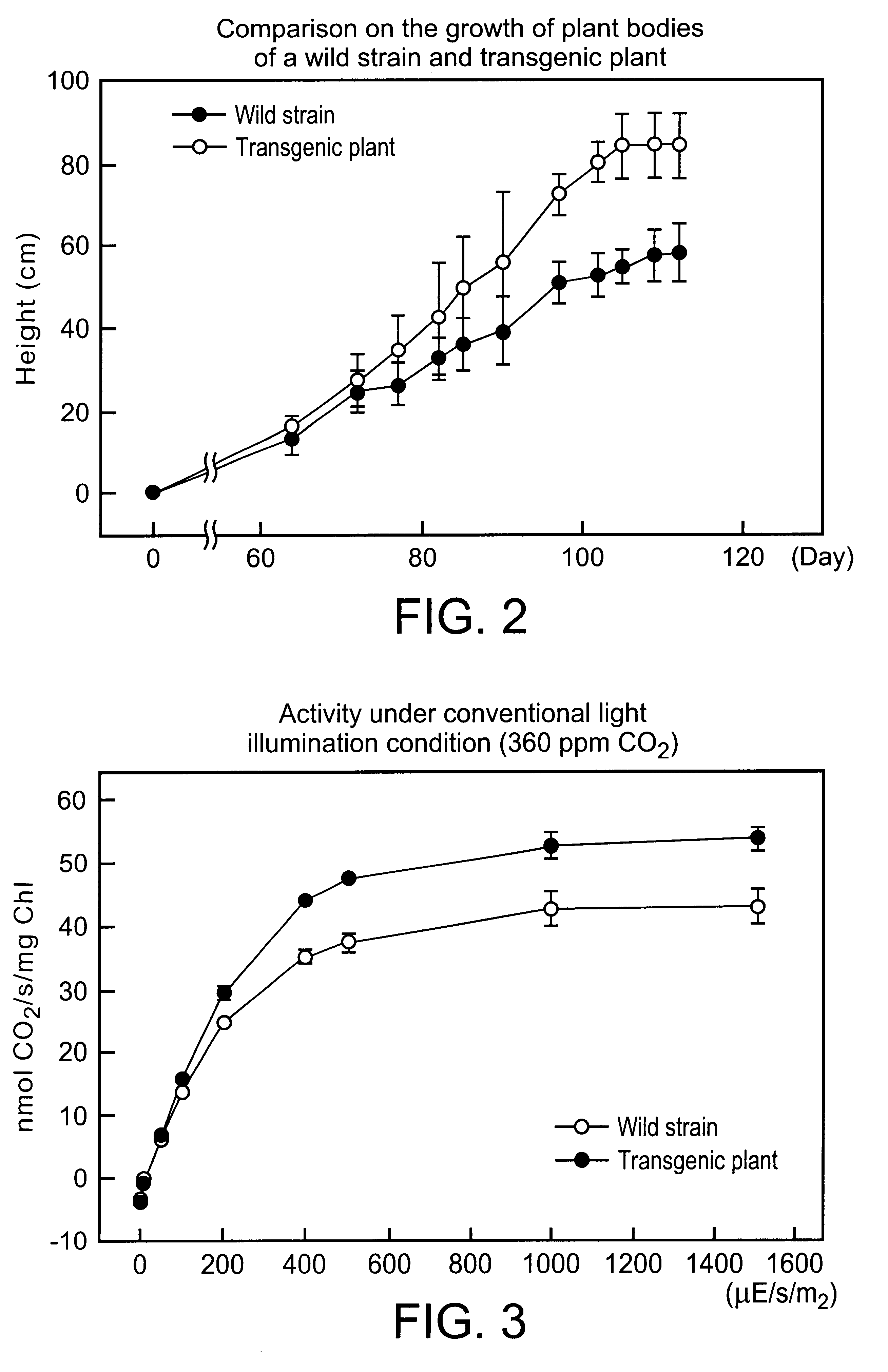
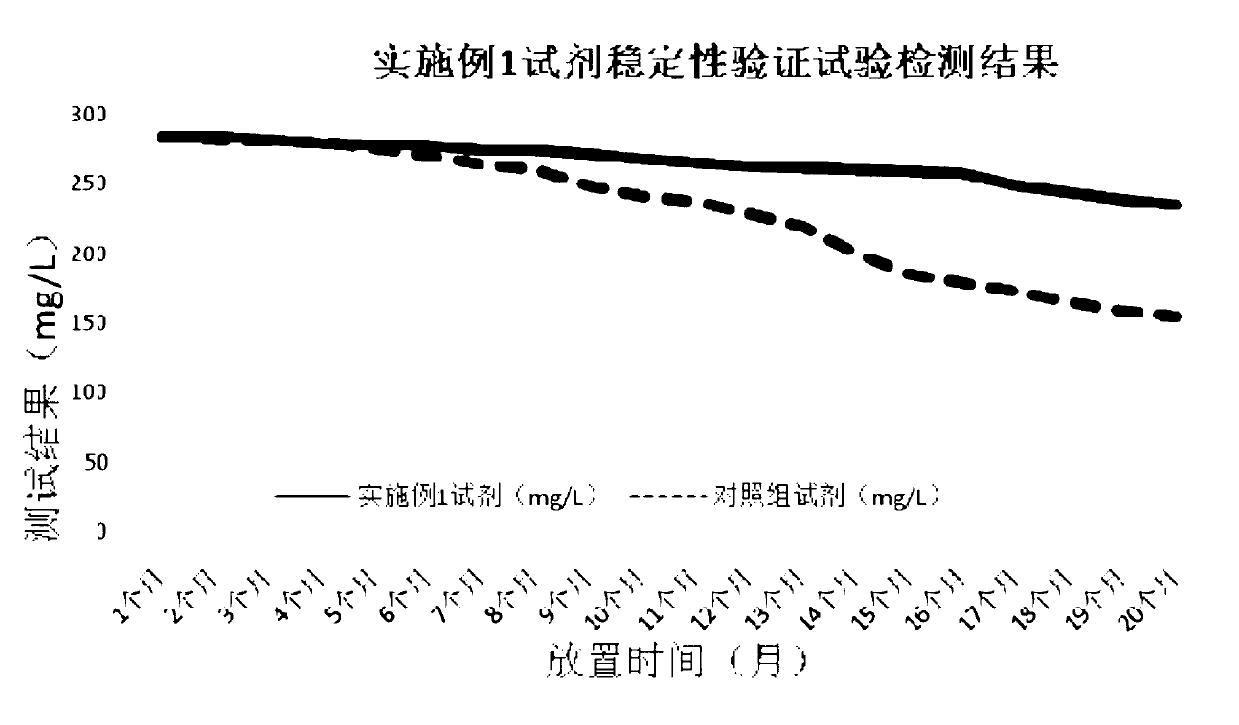
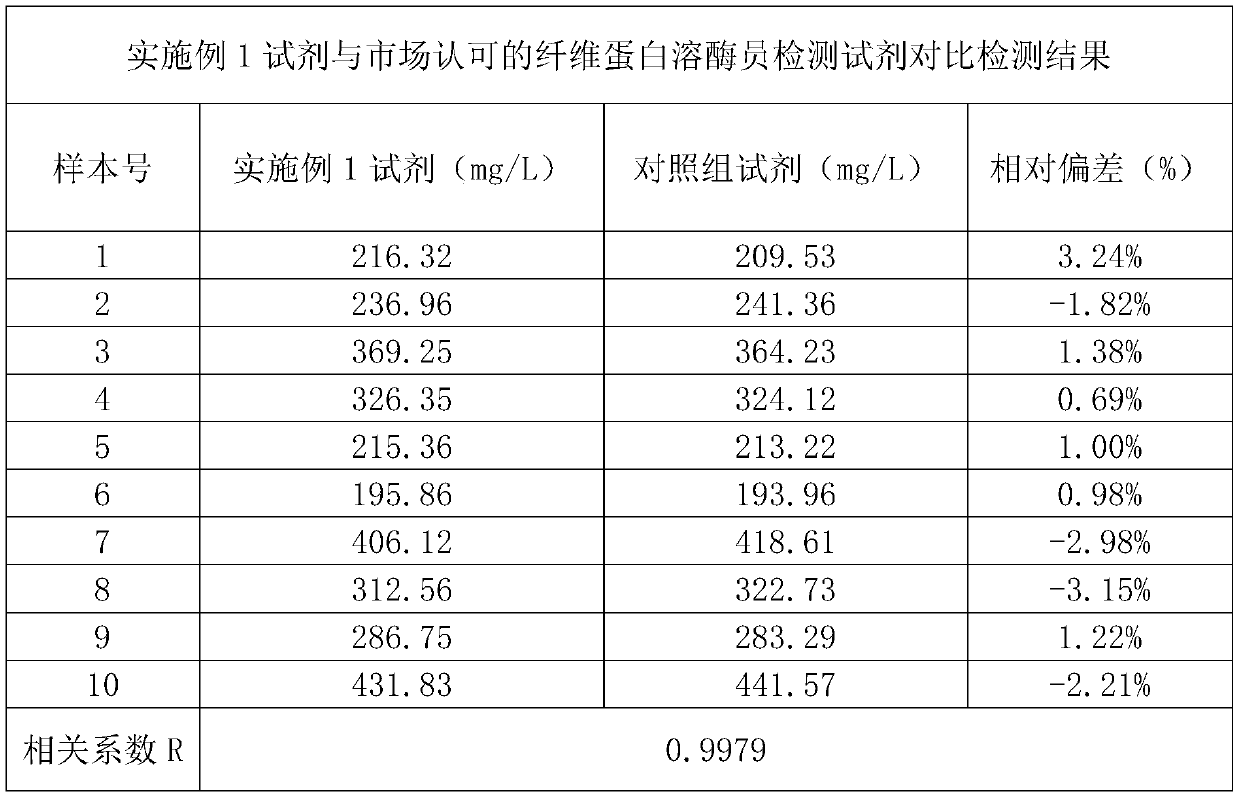
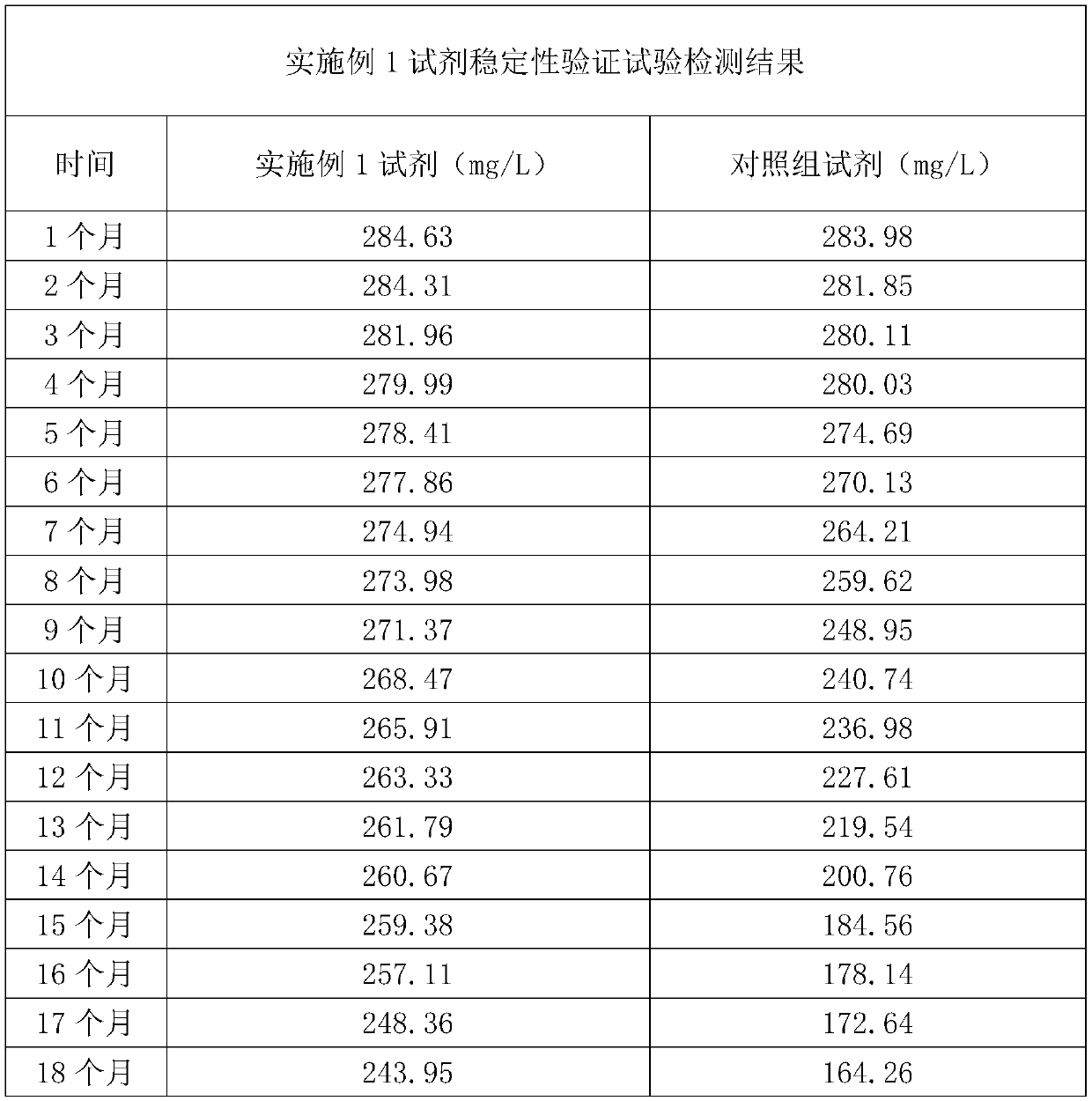
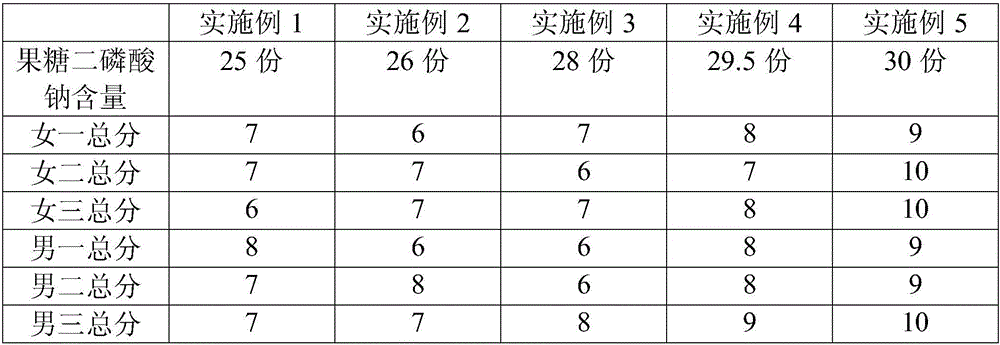
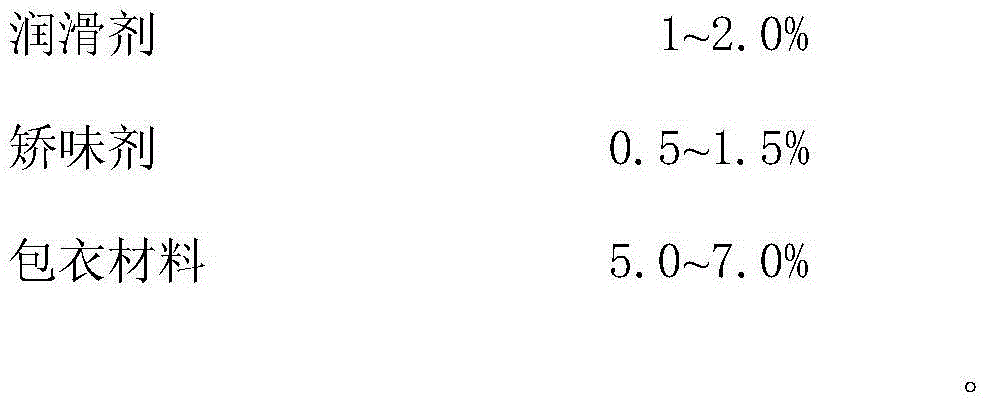Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
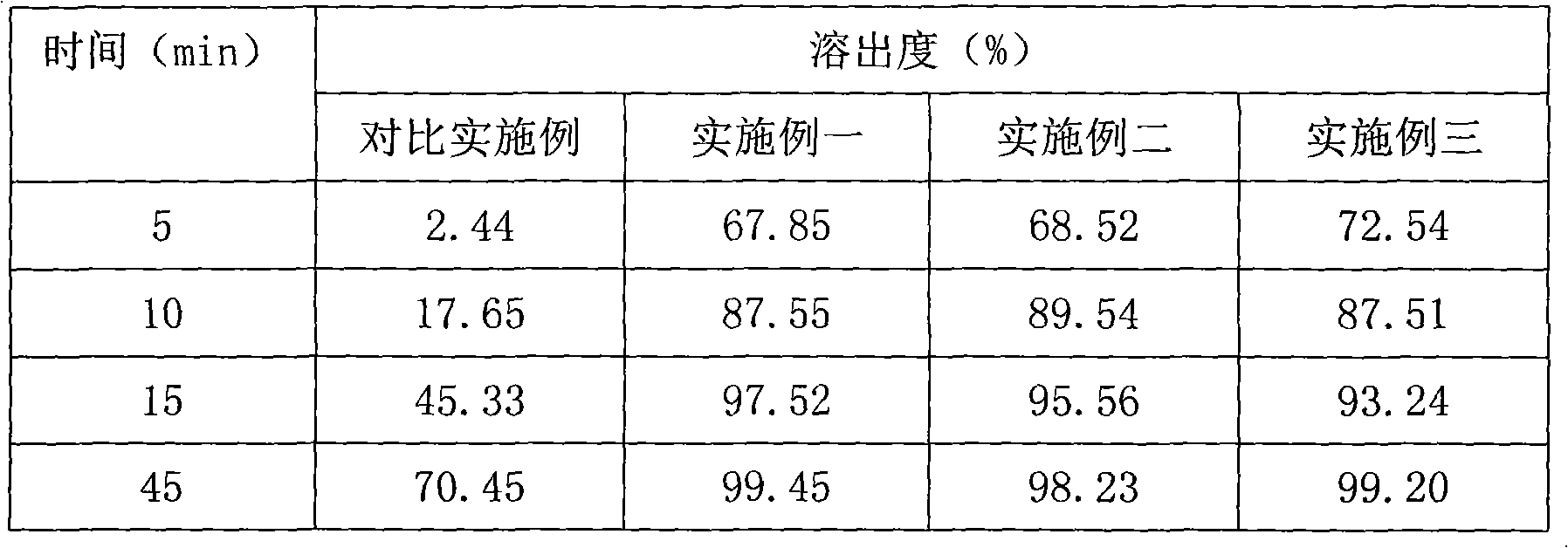
89 results about "Fructosediphosphates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
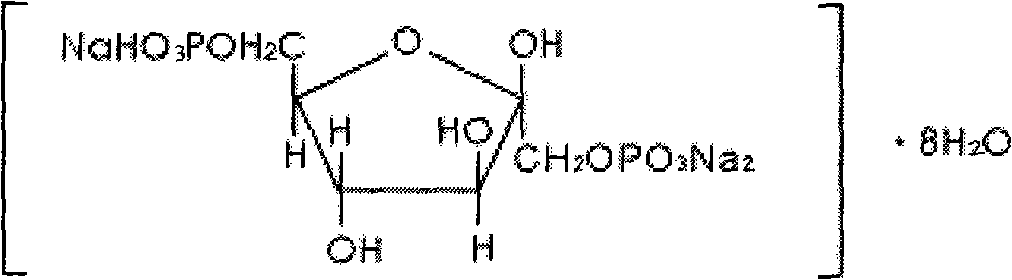
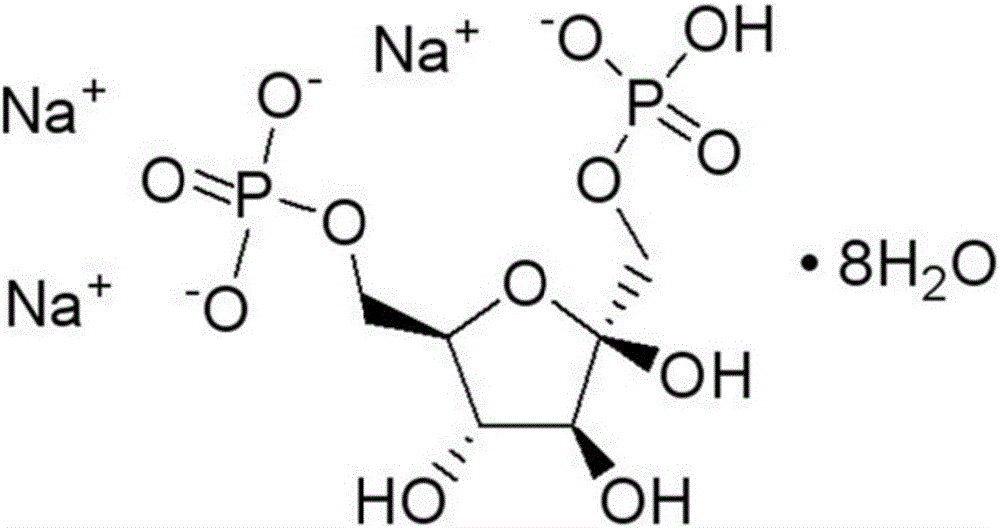
Diphosphoric acid esters of fructose. The fructose-1,6- diphosphate isomer is most prevalent. It is an important intermediate in the glycolysis process.
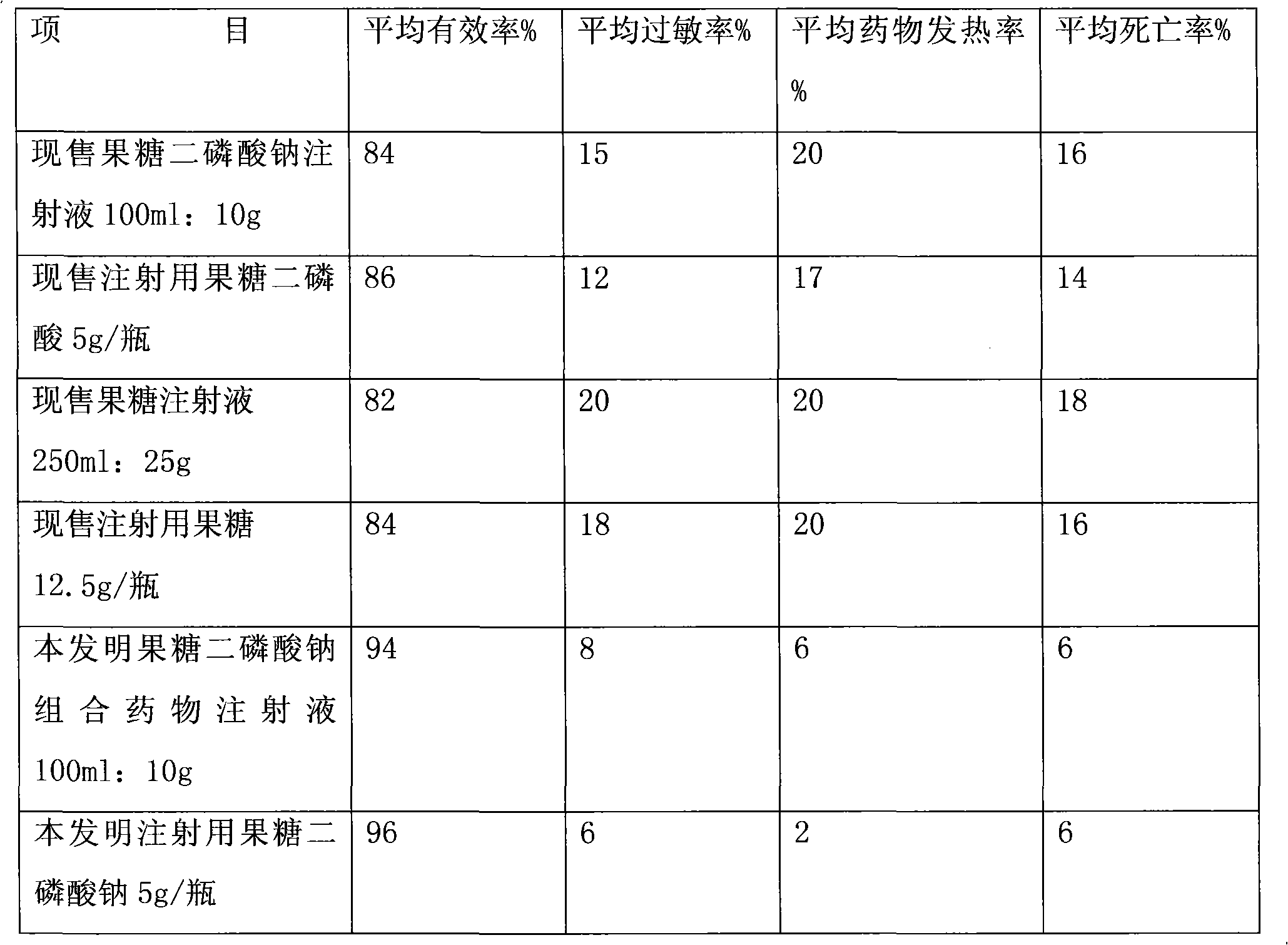
Preparation method of fructose diphosphate sodium powder injection
InactiveCN101953793AImprove stabilityEasy to produceOrganic active ingredientsPowder deliveryPunchingProcess conditions
The invention discloses a preparation method of a fructose diphosphate sodium powder injection, comprising the following steps of: decoloring, degerming, roughly filtering and finely filtering a fructose diphosphate sodium water solution; and punching, separating and crystallizing with ethanol or acetone and filling to obtain the aseptic powder injection. The fructose diphosphate sodium powder injection prepared by the method has higher purity, fewer impurities and more stable quality compared with the traditional products and is convenient to store and transport. The invention is simple to operate, has mild process condition and low cost and is suitable for industrial production.
Owner:SHANGHAI NEW ASIA PHARMA +2
Sodium fructose diphosphate granule agent and its preparation method
InactiveCN1650871AImprove stabilityExtended storage timeCarbohydrate active ingredientsGranular deliveryHeart diseasePharmacology
Owner:FUKANGREN BIO PHARMA
Method for preparing sodium fructose diphosphate injection
InactiveCN101244071ALow purityReduce osmotic pressurePharmaceutical delivery mechanismCarbohydrate active ingredientsCLARITYImpurity
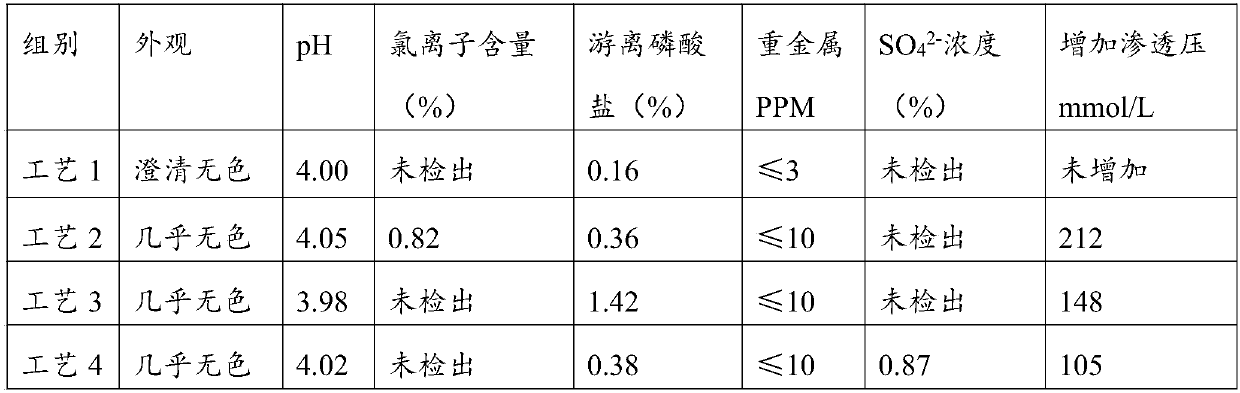
The invention relates to a preparation method of fructose sodium diphosphate injection, belonging to the technical field of medicine. The preparation method is: medical solution is treated by cation exchange resin, which can reduce acidity, remove metal cation, absorb colorful impurities and pyrogens, increase the clarity of the medical solution, avoid the defects of decreasing the product purity, increase osmotic pressure, and influencing medication safety caused by acid radical ion when acid is used to regulate acidity, and provides a stable fructose sodium diphosphate injection with high purity. The fructose sodium diphosphate injection is applied to intravenous injection or intravenous drip in clinical practice, and is suitable for low phosphorus acidemia. The preparation method has the advantages: the preparation method is simple and controllable, and the prepared product quality is stable and safe for clinical use.
Owner:广东宏远集团药业有限公司
Method for improving productivity of higher plants
InactiveUS6528705B1Improve productivityImprove abilitiesSugar derivativesHydrolasesSynechococcusPrimary metabolism
The present invention relates to a method to improve the primary metabolism of a higher plant having chloroplasts by expressing fructose-1,6-bisphosphatase / sedoheptulose-1,7-bisphosphatase derived from Cyanobacterium synechococcus in the chloroplasts. The invention further relates to transgenic plants comprising DNA encoding fructose-1,6-bisphosphatase / sedoheptulose-1,7-bisphosphatase derived from Cyanobacterium synechococcus.
Owner:NARA INSTITUTE OF SCIENCE AND TECHNOLOGY
Preparation method of fructose diphosphate sodium injection
ActiveCN107595770AReduce acidityAvoid problems such as reduced purity and increased osmotic pressureOrganic active ingredientsMetabolism disorderDrug productIon-exchange resin
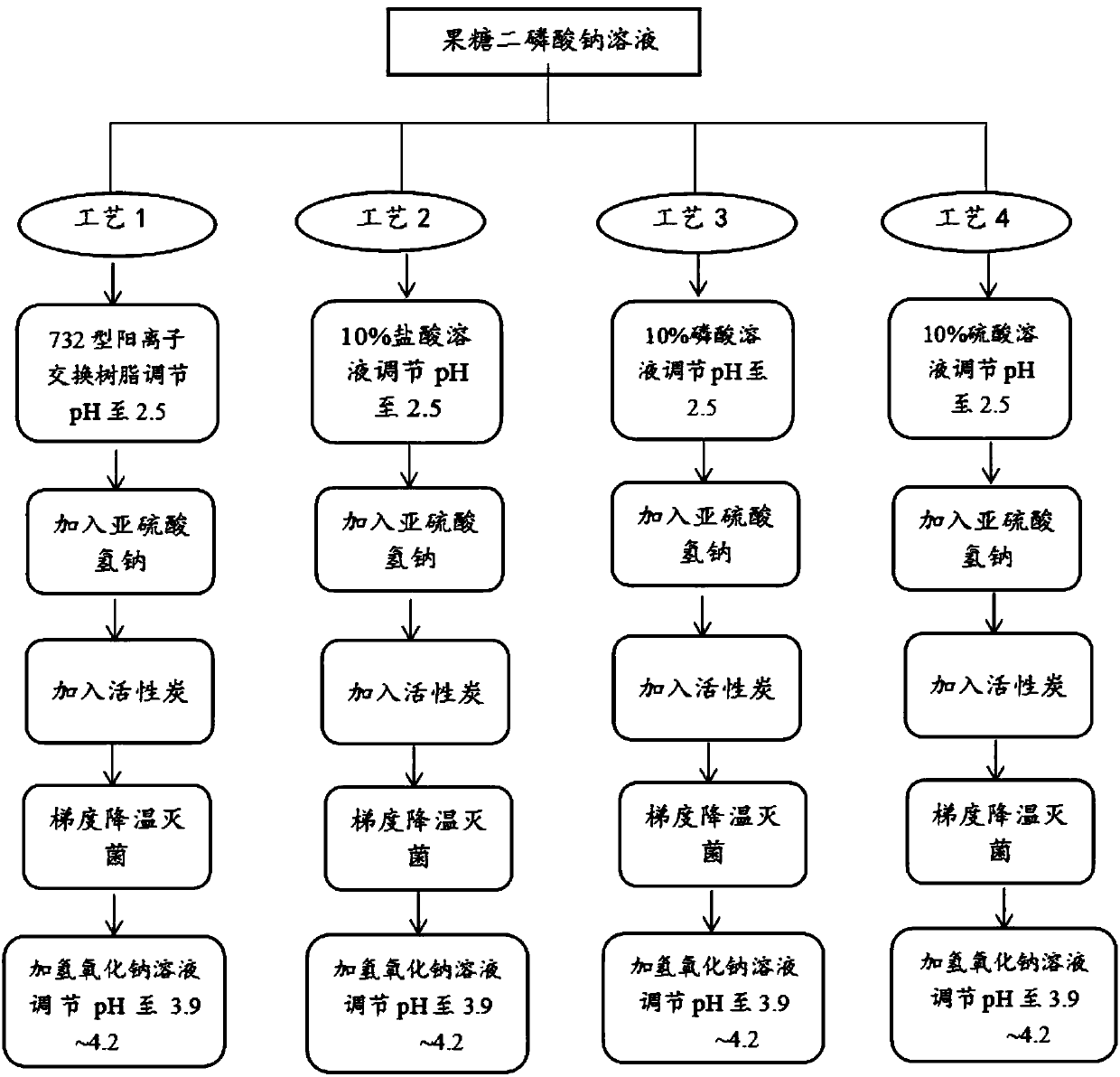
The invention belongs to the field of medicine, and more specifically relates to a preparation method of a fructose diphosphate sodium injection. The method mainly comprises the following steps: liquid medicine passes through 732 type cation exchange resin, after the pH value of the liquid medicine is reduced to 2.0-3.0, gradient cooling and disinfection are carried out. One key point of the technology is utilization of cation exchange resin for reducing acidity of the liquid medicine, and the problems of reduction of injection purity due to introduction of acidity conditioning agents, increasing of osmotic pressure, and the like are avoided, at the same time, impurities in the liquid medicine are removed, and quality and safety of the medicine are substantially improved. The other key point is that utilization of cation exchange resin reduces pH value of the liquid medicine to the largest extent, and at the same time, gradient cooling and disinfection are combined in order to solve the problem that the content of injection free phosphate exceeds the standard due to high temperature disinfection, further safety and purity of the medicine are improved.
Owner:广东宏远集团药业有限公司
Production process flow of fructose diphosphate sodium
InactiveCN102154399AGuaranteed purityReduce decompositionMicroorganism based processesFermentationReaction temperaturePotassium
The invention discloses a production process flow of fructose diphosphate sodium, relating to the technical field of biology. The production process flow comprises the following production process steps of: reacting 700kg of glucose, 375kg of sodium dihydrogen phosphate, 75kg of calcined soda, 27kg of magnesium chloride, 16kg of ammonium chloride, 14kg of potassium chloride, 2.5 tons of fresh beer yeast, 80kg of toluene and 2 tons of process water in a reaction kettle at the pH of about 6.0 and at a reaction temperature of 36-38 DEG C; performing cation-anion exchange; removing impurities from a concentrated solution; and crystallizing to obtain a product. The production process flow has the advantages of short reaction time, easiness of operation, high transformation ratio, short crystallizing and drying time, uniform crystallization particles, difficulty in agglomeration and low impurity content.
Owner:张家港市华天药业有限公司
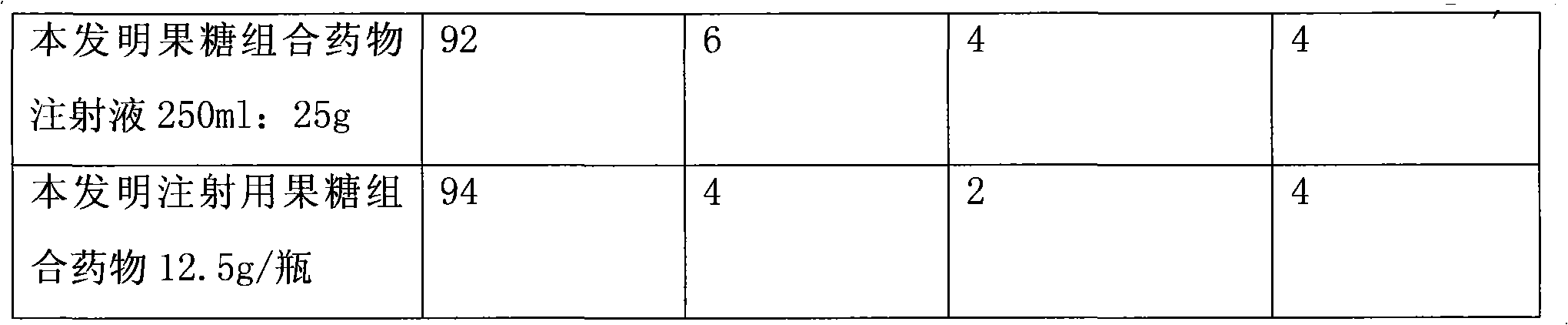
Fructose combination medicament
InactiveCN101926807AReduce adverse reactionsReduce allergiesOrganic active ingredientsMetabolism disorderExtracorporeal circulationParenteral nutrition
The invention provides a fructose combination medicament or a fructose diphosphate sodiumcombination medicament. The fructose combination medicament or the fructose diphosphate sodium combination medicament is characterized by comprising the following activated pharmaceutical ingredients in part by weight: 3.75 to 50 parts of fructose, 0.01 to 0.05 part of composition consisting of vitamin C and glutathione in the weight ratio of 1:1 and 0.00015 to 0.00020 part of disodium edentate. The invention also provides a method for preparing the fructose combination medicament. The fructose combination medicament and the fructose diphosphate sodium combination medicament are mainly used for treating shock, coronary heart diseases, angina pectoris, acute myocardial infarction and ischemia myocardial, heart failure, peripheral vascular diseases, acute adult respiratory distress and anesthetic accidents, and used for parenteral nutrition therapy for critical patients, heart surgery extracorporeal circulation and the adjuvant therapy for patients requiring multiple times of blood transfusion.
Owner:吴赣英
Preparation formula of novel alcohol-relieving capsules and preparation method
The invention discloses a preparation formula of novel alcohol-relieving capsules and a preparation method. The alcohol-relieving capsules are prepared by adopting a biological enzymatic technology, a high-tech crushing technology and a refine purification technology and by extracting effective essence in natural plants and preparing a mixture according to a scientific proportion. The alcohol-relieving capsules are prepared from the following ingredients: fructose diphosphate sodium (FDP) with the purity which is required to reach over 95 percent, puerarin with the puerarin content which is required to reach over 80 percent, and wall-broken spore powder of Ganoderma lucidum with the wall-broken rate which is more than or equal to 99 percent. The alcohol-relieving capsules are purified from essence of green food, are nontoxic and harmless, have no side effect, have low molecular weight, are easy to decompose and absorb, and can quickly relieve discomfort symptoms after drinking.
Owner:赵军 +1
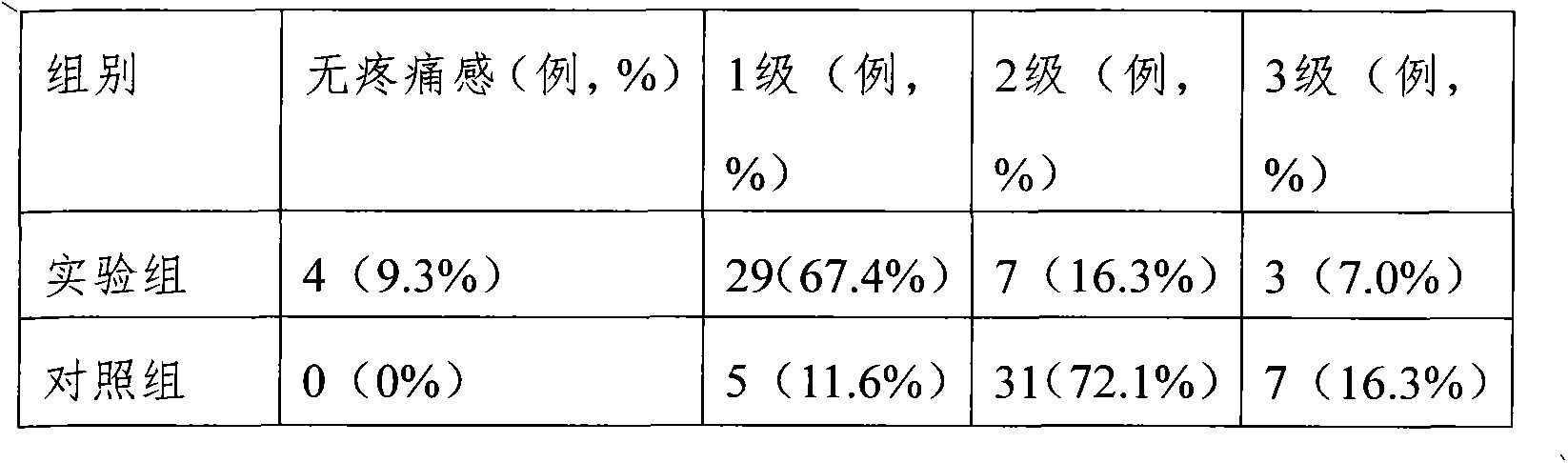
Medicine composition containing fructose sodium diphosphate compound
ActiveCN103054883ASimple prescriptionImprove stabilityOrganic active ingredientsInorganic non-active ingredientsHydrogenMANNITOL/SORBITOL
The invention provides a medicine composition containing a fructose sodium diphosphate compound, comprising fructose sodium diphosphate, sodium hydrogen sulfite, mannitol and water for injection. A preparation method for the medicine composition comprises the following steps of: sequentially dissolving fructose sodium diphosphate, sodium hydrogen sulfite and mannitol with the water for injection by stirring; adding active carbon to decolour; filtering and removing carbon; and filtering and removing bacteria. The fructose diphosphate medicine composition provided by the invention is simple in prescription, high in stability, capable of alleviating pain during injection, and conducive to application and popularization for fructose sodium diphosphate injection solution clinically. Additionally, high-temperature sterilization is avoided in the preparation method disclosed by the invention, the generation of related substances can be reduced, and the safety is improved.
Owner:罗诚
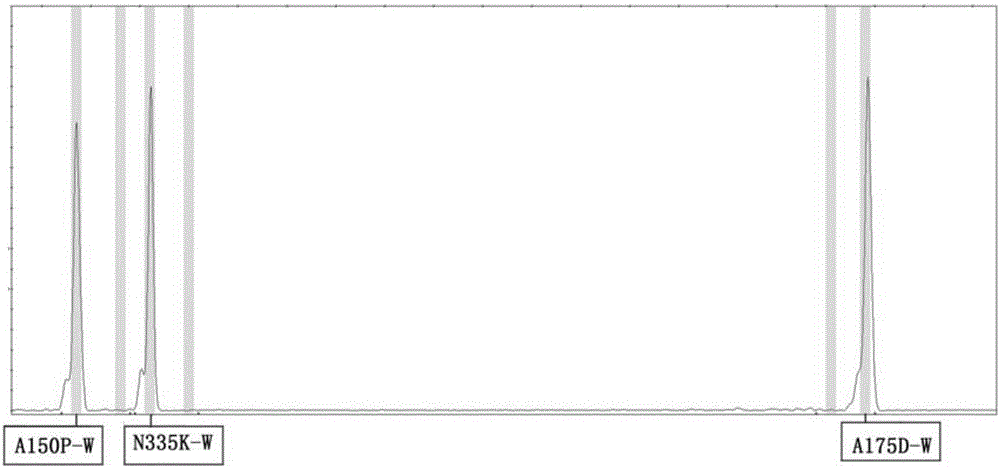
Method and kit for detecting human fructosediphosphate aldolase B gene
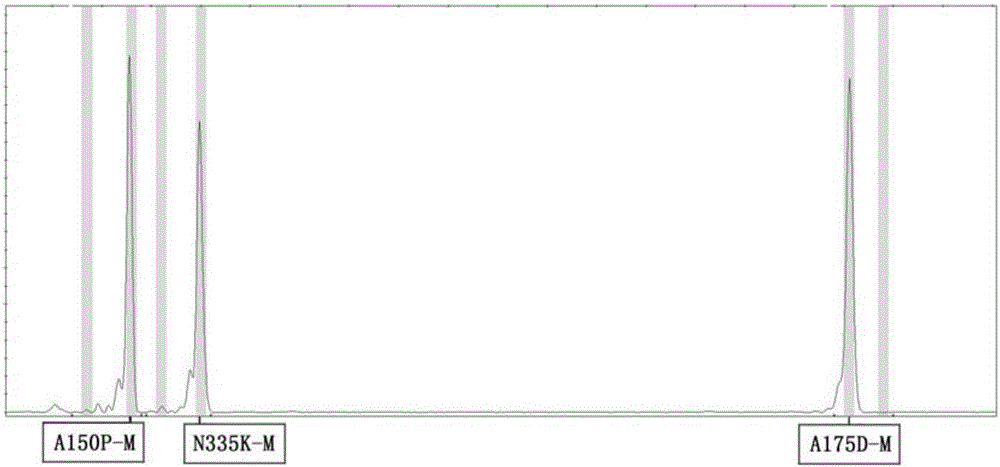
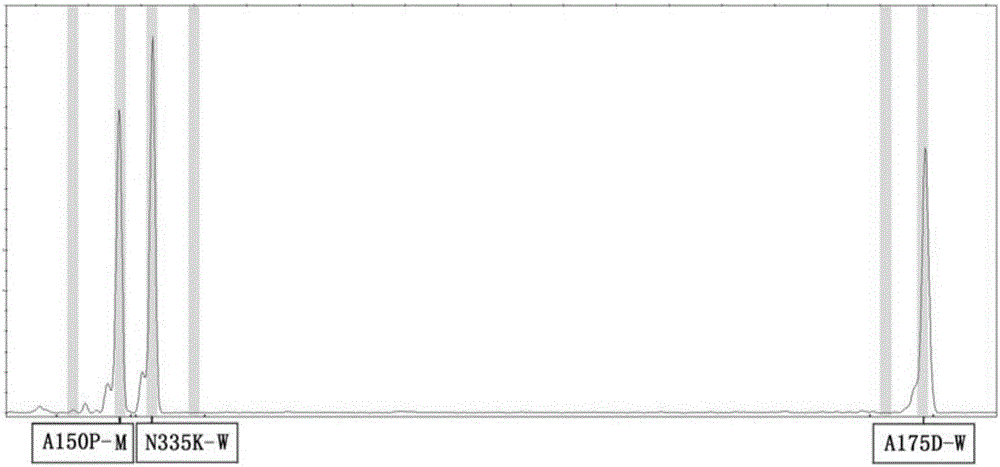
ActiveCN106399487AIncrease template volumeReduce false positivesMicrobiological testing/measurementFructosediphosphate AldolaseType specific
The invention provides a method and a kit for detecting the human fructosediphosphate aldolase B gene. The detection kit comprises a primer pair for specifically amplifying three polymorphic sites on the human fructosediphosphate aldolase B gene, wherein the three polymorphic sites are respectively A150P, A175D and N335K, the primer pair comprises a common upstream primer and genotyping downstream primers, and the genotyping downstream primers comprise a wild type specific primer and a mutant type specific primer. With the adoption of the kit, the genotypes of the three polymorphic sites on the human fructosediphosphate aldolase B gene can be rapidly and efficiently detected, and the kit belongs to the kit for screening the human fructosediphosphate aldolase B gene, which is rapid, easy and convenient to operate, and is economical and efficient.
Owner:广东安科华南生物科技有限公司
Fructose diphosphate sodium particle and preparation method thereof
InactiveCN101190187ACorrect salty tasteSweet tasteOrganic active ingredientsGranular deliveryPolyvinylpyrrolidoneHydroxypropylmethyl cellulose
The invention provides a fructose diphosphate sodium granule and a preparation of the fructose diphosphate sodium granule. The fructose diphosphate sodium granule of the invention comprises effective amount of fructose diphosphate sodium and medical subsidiary substances. The medical subsidiary substances comprise thinner, bond and flavoring agent; wherein, the thinner can be one or a plurality of substances among amylum pregelatinisatum, lactose, crystallite fibrin or mannitol; the bond is one or a plurality of substances among polyvinylpyrrolidone K30, ethyl cellulose or hydroxypropyl methyl cellulose; the flavoring agent is one or a plurality of substances among aspartame, citric acid, stevioside, lemon essence or orange essence. And the weight ratio of the medical subsidiary substances is: the fructose diphosphate sodium: the thinner: the bond: the flavoring agent is equal to 100: 30 to 200: 1 to 30:1 to 30. The invention also provides the preparation method of the fructose diphosphate sodium granule. The invention has the advantages that medicine-taking is convenient; the manufacturing cost is low; special equipment are not needed; taking and delivering are convenient and stable, etc.
Owner:何晶
Fructose diphosphate sodium sterile powder injection and preparation method thereof
ActiveCN104758258ANot easy to stickImprove liquidityOrganic active ingredientsPowder deliveryVitamin COrganic solvent
The invention provides fructose diphosphate sterile sodium powder injection comprising fructose 60-100 parts of diphosphate sterile sodium, 5-10 parts of 15-hydroxystearic acid macrogol ester and 1-5 parts of vitamin C. The invention further provides a preparation method of the fructose diphosphate sterile sodium powder injection. The preparation method comprises: dissolving diphosphate sterile sodium, 15-hydroxystearic acid macrogol ester and glucose in injection water by stirring, decoloring, filtering, sterilizing and drying to obtain the fructose diphosphate sterile sodium powder injection. The fructose diphosphate sterile sodium powder injection provided by the invention is unlikely adhered on the surface, and is good in liquidity and small in hygroscopicity in the air, thereby improving the medicine stability and facilitating the preparation production; no organic solvent is used, thereby saving resources, lowering the cost and reducing the environmental pollution; the fructose diphosphate sterile sodium powder injection is simple to prepare and is suitable for industrial production.
Owner:HAINAN GENERAL & KANGLI PHARMA
Nanofiber membrane with whitening and tightening effects and preparation method of nanofiber membrane
ActiveCN111743785AOvercome the technical problem of difficult dissolutionPromote absorptionCosmetic preparationsMonocomponent protein artificial filamentHydroxyprolineSpinning
The invention relates to a nanofiber membrane with whitening and tightening effects and a preparation method of the nanofiber membrane. The nano-fiber membrane is characterized by simultaneously containing pterostilbene, dipalmitoyl hydroxyproline and prinsepia utilis royle oil which serve as oil-soluble whitening and tightening active components and fructose diphosphate trisodium, asiaticoside, hydrolyzed elastin and gynostemma pentaphylla leaf extract which serve as water-soluble whitening and tightening active components, the oil-soluble whitening and firming active component and the water-soluble whitening and firming active component are wrapped by hydrogenated lecithin to prepare a whitening and firming nano-composition, and then the whitening and firming nano-composition is mixed with succinyl glycan, rhizobium gum and deionized water to prepare the nano-fiber membrane through an electrostatic spinning process. The product has the advantages of being good in skin friendliness, good in stability, fast in absorption, convenient to use and the like, meanwhile, the oil-soluble whitening and firming active matter and the water-soluble whitening and firming active matter are loaded, the technical defect that only one of the oil-soluble whitening and firming active matter and the water-soluble whitening and firming active matter can be loaded in the prior art is overcome, and the whitening and firming effect is better.
Owner:PROYA COSMETICS
1,6-diphospho-d-fructose ester arginine salt and its pharmaceutical uses
InactiveCN101007829AInhibit aggregationInhibition releaseEsterified saccharide compoundsOrganic active ingredientsSide effectArginine
The invention disclose the arginine salt of 1, 6- diphosphoric acid- D- fructose esters in hydrate type, and one or more than one of the four function groups is salinized by arginine. The salt precipitation with 1, 6- diphosphoric acid- D- fructose esters and arginine solves problems of strong side effect of sodium fructose diphosphate, and starting circuit, high natremia risk. It is demonstrated through pharmacological test that arginine salt can cooperate with drug and the toxcity is low.
Owner:GUANGDONG ZHONGKE DRUG R&D
Leech capsule
The invention relates to a leech capsule, and belongs to the technical field of leech deep processing. The preparation method comprises the following steps: removing excrements in leeches, soaking leeches in brine, after leeches die, fishing out leeches, blowing off impurities on the surface of leeches; grinding dried leeches, soaking grinded leeches in ethanol, carrying out sealed soaking; aftersealed soaking, concentrating and drying obtained substance to remove ethanol in the substance; mixing the substance with fructose sodium diphosphate and starch, sieving the mixture by a sieve with asize of 10-20 meshes, drying the mixture at a temperature of 30 to 45 DEG C, carrying out granulation and sterilization, and finally filling granules into capsules. At first, leeches are soaked in brine to change the tension force of the surface cells of leeches, then leeches are grinded, soaked in ethanol, concentrated, and dried so that hirudin is dissolved or to be dissolved, and the problem that the dissolution rate of hirudin in grinded leeches of a conventional oral leech capsule is low is solved.
Owner:广西鹿帅仁生物科技有限公司
Rapid-release fructose diphosphate sodium tablet and preparation method thereof
InactiveCN102038656AEasy to makeRelease quickly and completelyInorganic non-active ingredientsCarbohydrate active ingredientsSucroseAluminum silicate
The invention provides a rapid-release fructose diphosphate sodium tablet, which comprises the active constituent of fructose diphosphate sodium as well as one or more of a filler, a disintegrating agent, a binder, a lubricant, a flow aid, a flavoring agent, a smelling agent and a coloring agent, and further comprises a release accelerator for accelerating the release of the active constituent. The release accelerator can be selected from the following substances of lauryl sodium sulfate, poloxamer, tweens, brominated hexadecane trimethylamine, sodium lauryl sulfate, stearyl alcohol sulfonate sodium, polyoxyethylene higher fatty alcohol, sucrose ester, sorbitol fatty ester, soyabean lecithin, alginic acid, sodium alginate and colloidal magnesium aluminum silicate, and the dosage of the release accelerator accounts for 0.1-5% of the total weight of the tablet.
Owner:SHANGHAI TIANLONG PHARMA
Method For Improving Productivity of Plant By Chloroplast Technology
InactiveUS20090044300A1High photosynthesis activityPromote growthHydrolasesMicroorganismsNucleotidePollen
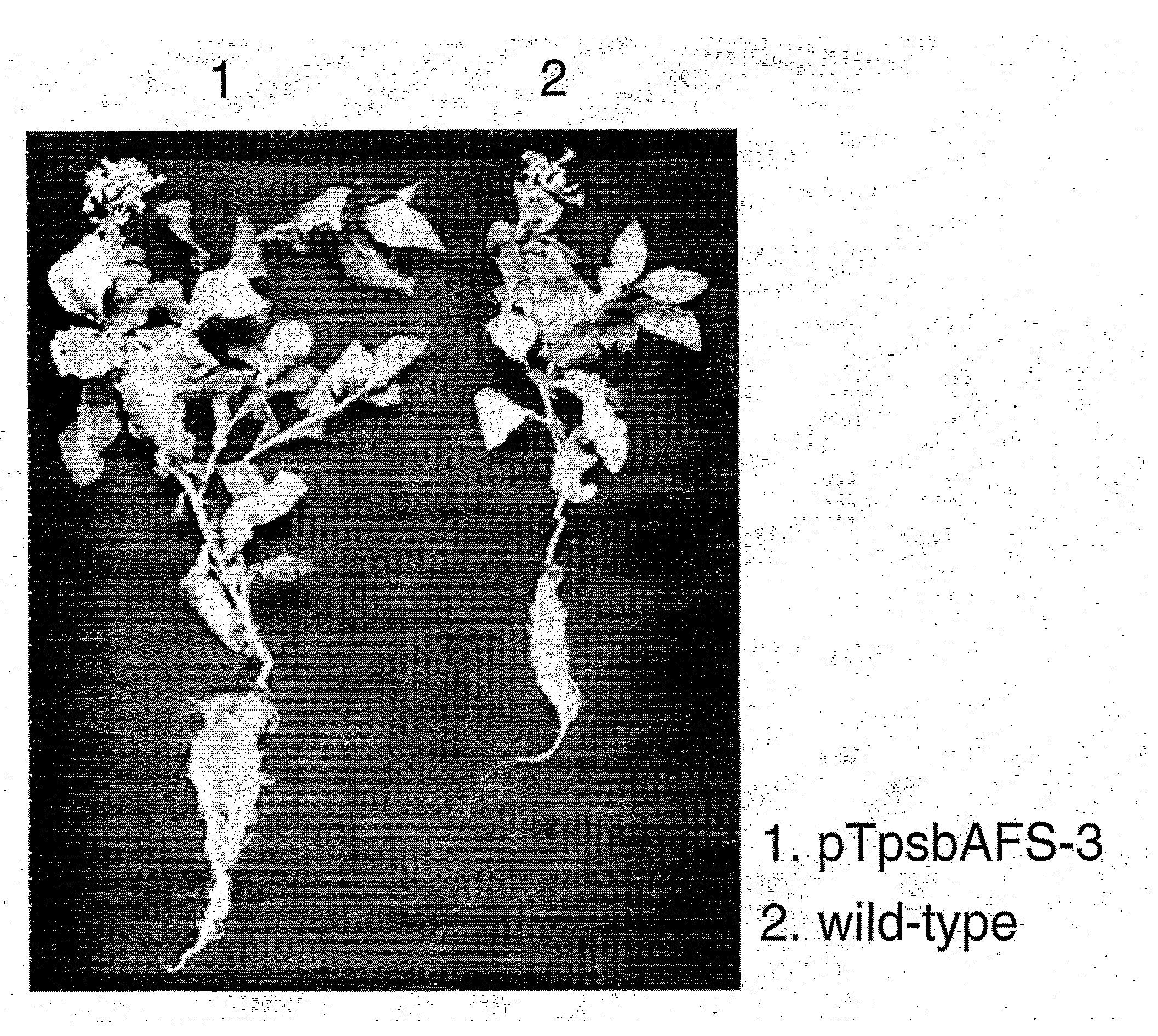
An object of the present invention is to provide a transformed plant which has high photosynthesis activity, and has promoted growth and productivity as compared with a wild strain, and has no fear of diffusion of an introduced gene by pollens, by expressing a trait of a specified gene by chloroplast technology in a higher plant. According to the present invention, there is provided a transformed plant using a gene recombinant vector having an expression cassette for enhancing photosynthesis activity, containing a DNA fragment comprising a gene encoding a protein having fructose-1,6-bisphosphatase sedoheptulose-1,7-bisphosphatase activities between a nucleotide sequence complementary to the chloroplast gene rbcL and the chloroplast gene aacD.
Owner:NATIONAL UNIVERSITY +1
Compound sodium fructose diphosphate and fructose orally disintegrating tablets and preparation method thereof
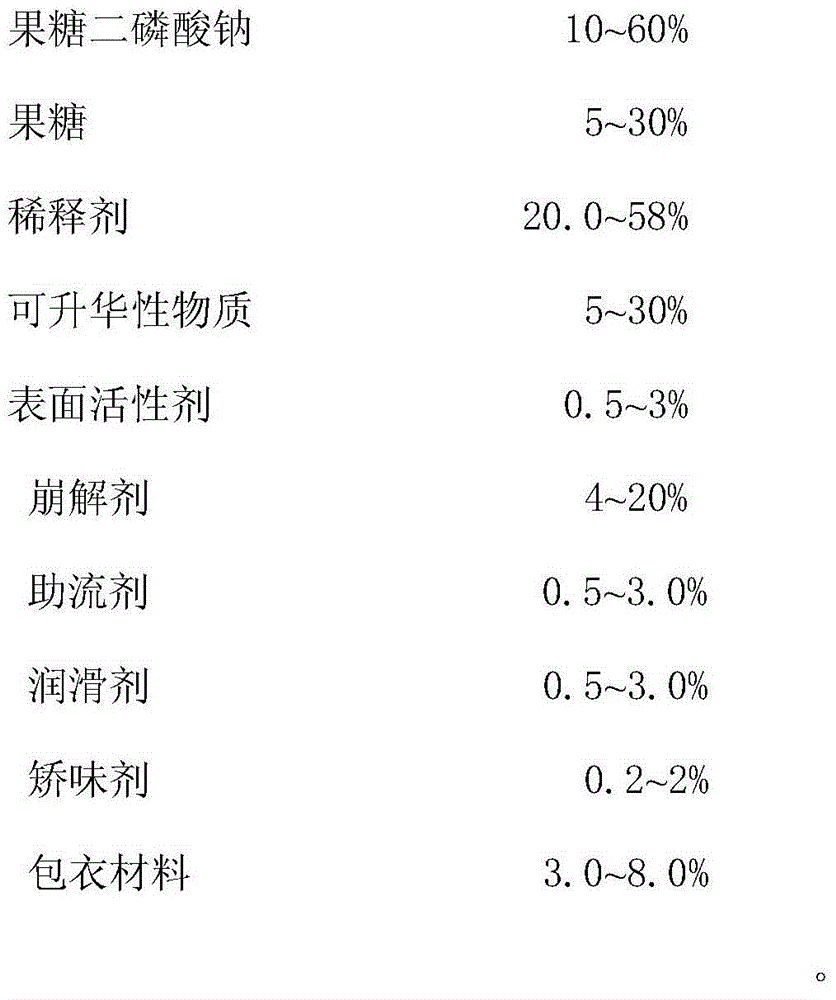
ActiveCN105769887ADisintegrates quicklyImprove support strengthOrganic active ingredientsNervous disorderOrally disintegrating tabletBULK ACTIVE INGREDIENT
The invention provides compound sodium fructose diphosphate and fructose orally disintegrating tablets. The compound sodium fructose diphosphate and fructose orally disintegrating tablets are prepared from the following ingredients in percentage by weight: 10-60% of sodium fructose diphosphate, 5-30% of fructose, 5-30% of sublimable substance, 0.5-3% of a surfactant, 4-20% of a disintegrating agent, 0.5-3.0% of a flow aid, 0.5-3.0% of a lubricant, 20.0-58% of a diluent, 0.2-2% of a corrigent, and 3-8% of a coating material. The invention further provides a preparation method of the compound sodium fructose diphosphate and fructose orally disintegrating tablets. The preparation method comprises the following steps: mixing the active ingredients, namely sodium fructose diphosphate and fructose, and pharmaceutical excipients, wherein the sublimable substance is added into the pharmaceutical excipients, tabletting the mixture, coating the obtained semi-finished product tablets, heating the semi-finished product tablets after coating, and drying, thus the sublimable substance sublimates, a plurality of holes are formed in the tablets, and finally, the tablets capable of rapidly disintegrating and with certain strength are obtained. The orally disintegrating tablets can rapidly disintegrate, so that good clinical effects can be obtained; the preparation method is easy and convenient to operate, is suitable for industrial production, and has a large application value.
Owner:SHANGHAI SUNTECH PHARMA +1
Method for preparing fructose diphosphate injection
ActiveCN103735498ASimple processImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonMicrofiltration membrane
The invention discloses a method for preparing a fructose diphosphate injection. The method comprises the following steps: (1) cooling injection water to below 40 DEG C for later use; (2) weighing a pH regulating agent, and stirring with injection water to dissolve; (3) weighing 20-80 percent by weight of injection water, introducing nitrogen into water, weighing the prescribed fructose diphosphate, adding, stirring and dissolving the fructose diphosphate, regulating the pH value to be 3-4 by using the pH regulating agent solution, and adding an auxiliary material to prepare q liquid medicine; (4) adding activated carbon into the liquid medicine, stirring and adsorbing for 10-40 minutes, filtering and decarburizing, adding the injection water to a prescribed amount, and simultaneously regulating the pH value the same as that in the step (3) by using the pH regulating agent solution; (5) sampling to inspect, filtering with a microfiltration membrane of 0.22 micron after the sample is qualified, filling into a glass bottle which is baked and sterilized at high temperature, plugging, sterilizing, capping and packaging to obtain a finished product. Compared with the prior art, the fructose diphosphate injection prepared by the method is simple in process, safe and reliable, good in stability and convenient in clinical application.
Owner:CISEN PHARMA
Preparation method of fructose sodium diphosphate injection
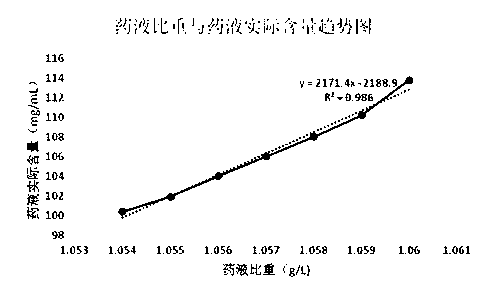
ActiveCN109223709ALess impuritiesReduce usageOrganic active ingredientsMetabolism disorderDrugs solutionPhosphoric acid
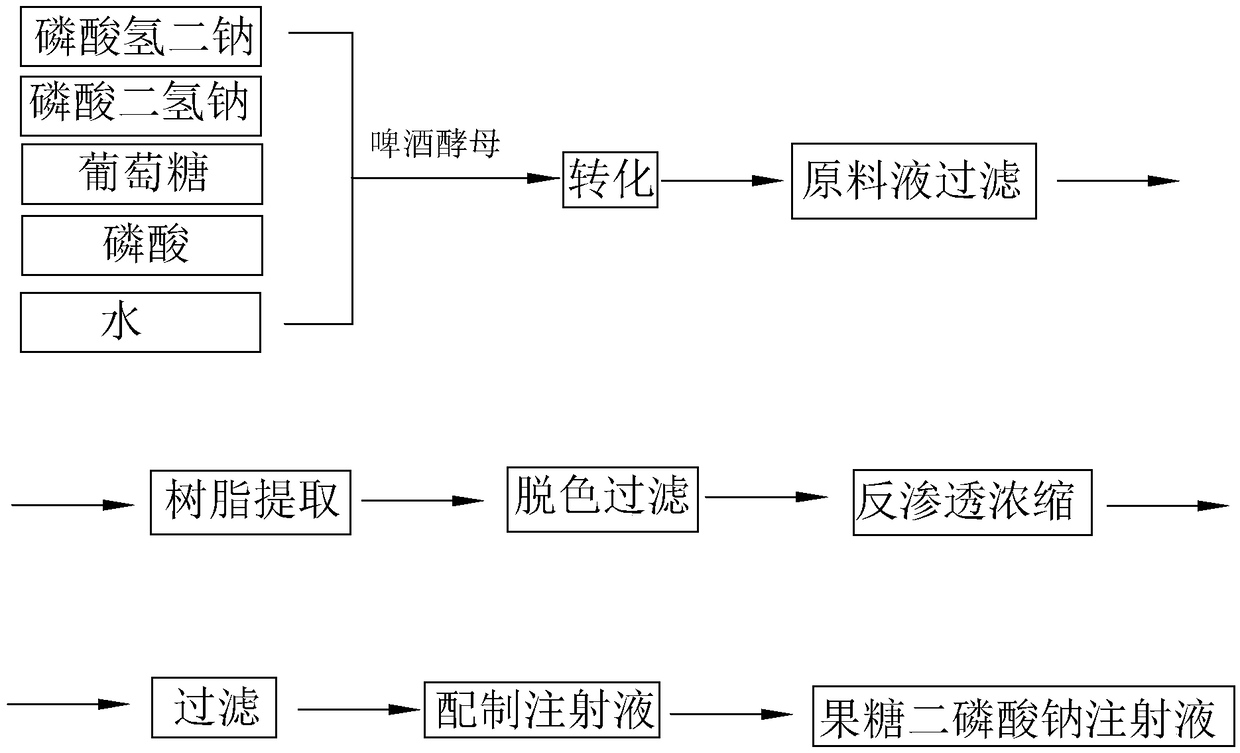
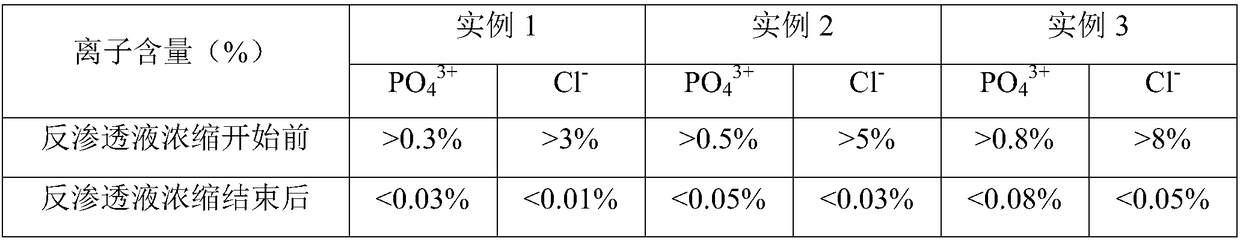
The invention discloses a preparation method of fructose sodium diphosphate injection, which comprises the following steps: S1, dissolving disodium hydrogen phosphate, sodium dihydrogen phosphate, glucose and phosphoric acid in water to prepare a reaction liquid, mixing with beer yeast, stirring and reacting to obtain a raw material liquid containing fructose sodium diphosphate; 2, filter that rawmaterial liquid plate frame; S3, adsorbing and purifying the solid-liquid separated raw liquid in S2 by anionic resin to obtain resin extract containing fructose sodium diphosphate; 4, decolorizing and filtering, decolorize that resin extract solution by activated carbon, and filtering and decarburizing; 5, carrying out initial concentration on that decolorization filtrate in S4, washing and desalinate the decolorization filtrate with water, and finally pressurizing the decolorization filtrate to concentrate the specific gravity of the decolorization filtrate to more than 1.05 g / ml; 6, filterthat concentrated drug solution to obtain RO solution; 7, filter that RO solution in S6, diluting, filter, and filling to obtain sterile fructose sodium diphosphate injection. The invention has the advantages of greatly reducing the use amount of the organic solvent in the preparation process and reducing the production cost.
Owner:BEIJING HUAJIN PHARM CO LTD
Fructose diphosphate compound and preparation method thereof
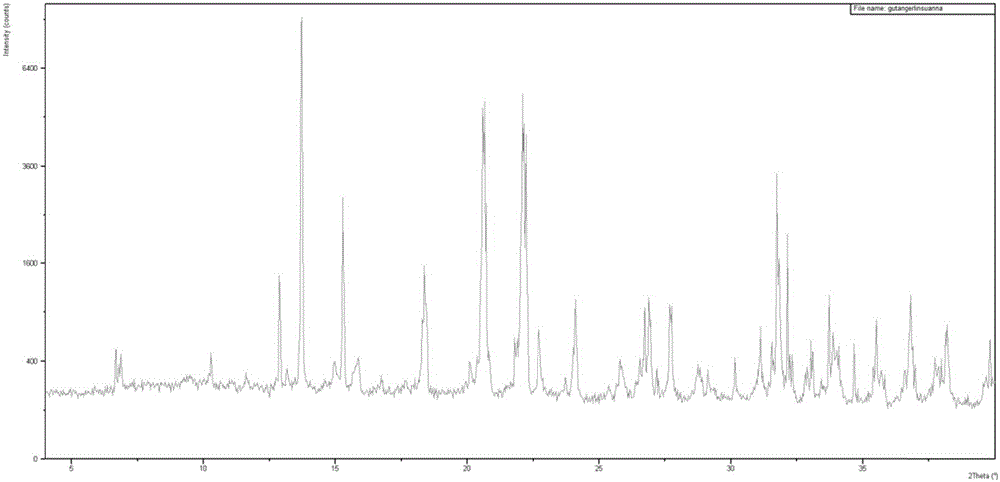
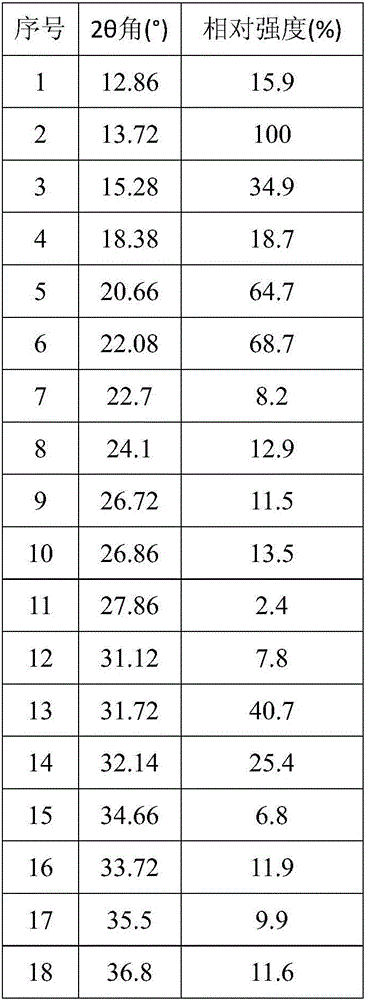
ActiveCN106810583AGood corrosion performanceHigh mechanical strengthEsterified saccharide compoundsOrganic active ingredientsIon exchangePhosphoric acid
The invention relates to the field of medicine, in particular to a fructose diphosphate compound. An X-ray diffraction spectrum of the fructose diphosphate compound is shown as Figure 1. The invention further provides a preparation method of the fructose diphosphate compound. The preparation method includes: adopting raw materials including beer yeast, glucose, phosphoric acid and magnesium carbonate for fermentation; filtering, and performing ion exchange; decoloring, filtering, crystallizing and drying to obtain 1, 6-fructose diphosphate trisodium hydrate. The fructose diphosphate compound produced by the method is lower in hygroscopicity, higher in stability and conducive to production and application of injections; due to unique crystal form of the fructose diphosphate compound, oral administration bioavailability is improved, and the fructose diphosphate compound is suitable for production and application of tablets.
Owner:ZHUHAI TONGYUAN PHARMA CO LTD
Exosome secretion culture medium and culture and separation method of umbilical cord mesenchymal stem cell exosomes
ActiveCN112920996AEase of mass productionImprove biological activityCell dissociation methodsCulture processSodium phosphatesCell culture media
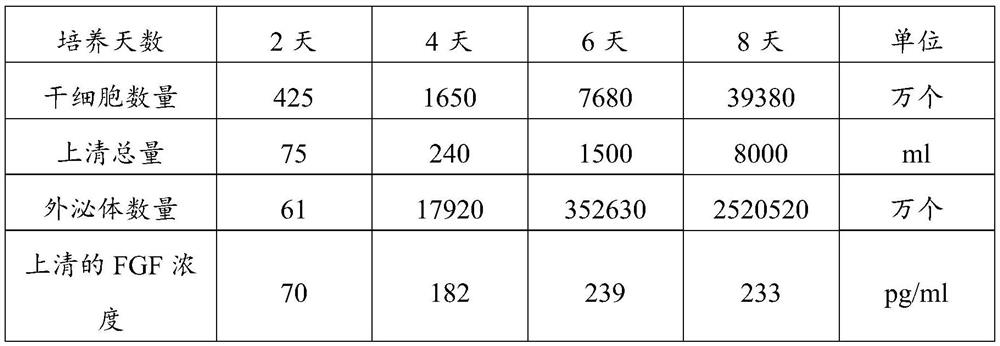
The invention discloses an exosome secretion culture medium and a culture and separation method of umbilical cord mesenchymal stem cell exosomes, and relates to the technical field of exosome enrichment and separation. A serum-free stem cell culture medium is used as a basic culture medium; and the following components are added: EGF with the concentration of 100 to 300ng / ml, TGF with the concentration of 50 to 150ng / ml, coenzyme Q10 with the concentration of 10 to 30mu g / ml, potassium salt with the concentration of 300 to 600mu g / ml, fructose sodium diphosphate with the concentration of 0.3 to 0.9 mu g / ml and histamine dihydrochloride with the concentration of 100 to 300 mu g / ml. By adopting the exosome secretion culture medium provided by the invention, more exosomes can be obtained under the condition of the same number of culture cells. The exosome secretion culture medium is beneficial to large-scale production of umbilical cord mesenchymal stem cell exosomes with good biological activity, further promotes wide application of the exosomes in medical treatment, anti-aging, beauty treatment and the like, and has good economic benefits.
Owner:广州研华生物科技有限公司
New crystalline technology of fructose-1, 6-trisodium diphosphate
InactiveCN1651448ALess irritatingImprove uniformityEsterified saccharide compoundsSugar derivativesSodium acetateAlcohol
A process for crystallizing pectose-1,6-trisodium biphosphate includes such steps as slowly and proportionally adding sodium acetate and alcohol to FDP solution, stirring at 20-40 deg.C while crystallizing, filtering, washing with alcohol and vacuum drying.
Owner:NANJING UNIV OF TECH
Effervescent tablet containing fructose sodium diphosphate
InactiveCN102077948AEasy to storeImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismEffervescent tabletSugar
The invention relates to an effervescent tablet containing fructose sodium diphosphate, comprising the fructose sodium diphosphate, citric acid, baking soda, oligosaccharide or other edible sugar in a weight ratio of (10-20):30:7:200. In the invention, the effervescent tablet is prepared with the fructose sodium diphosphate as a functional component, and compared with the other dosage forms, the effervescent tablet is easy to preserve, dissolve and absorb, has good stability and taste, and can be directly drunk after being soaked in water without stir.
Owner:张家港市华菱化工机械有限公司
Production method of fructose diphosphate sodium sterile powder
ActiveCN102838640AReduce labor intensityReduce exposureEsterified saccharide compoundsSugar derivativesAlcoholLiquid temperature
The invention relates to a production method of fructose diphosphate sodium sterile powder. The production method comprises the following steps of: dissolving fructose diphosphate sodium non-sterile powder with injection water, and then filtering by using carbon rods; filtering sequentially through a filter with bore diameters of 0.2-0.4 micrometer and a sterilization and filter device; then pressing sterilized compressed air into a crystallizing tank, and adding sterilized alcohol by controlling the liquid temperature to be 28-30 DEG C; adding a qualified fructose diphosphate sodium sterile raw drug as seed crystal to crystallize; after crystallization is finished, compressing a solid-liquid mixture into a vacuum rotary type drier by using sterile air to sequentially separate, wash and dry; and then oscillated screening, weighing, inside packaging and outside packaging to obtain the fructose diphosphate sodium sterile powder. The production method has the advantages of simple operation, lower cost and good quality of the fructose diphosphate sodium sterile powder.
Owner:张家港市华天药业有限公司
Levocarnitine freeze-dried composition for injection and preparation method thereof
InactiveCN103976960AImprove stabilityAvoid degradationPowder deliveryOrganic active ingredientsFreeze-dryingPowder injection
The invention discloses a levocarnitine freeze-dried composition for injection and a preparation method thereof. The composition contains levocarnitine, fructose diphosphate and lactic acid. Preferably, the weight ratio of levocarnitine to fructose diphosphate is 1:(0.6-2.0). By adding a proper freeze drying propping agent, a stable levocarnitine freeze-dried powder injection is provided. By using the preparation, the transformation of levocarnitine into a dextroisomer side product can be retrained to the maximum extent, degradation of medicaments is avoided, and the stability of the preparation is enhanced.
Owner:广东泽盛药业有限公司
Fructose diphosphate sodium liposome solid preparation and novel application thereof
InactiveCN101874779AUnexpected effectSolve AbsorbencyOrganic active ingredientsPill deliveryCholesterolAdditive ingredient
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Plasminogen measuring kit and preparation method thereof
InactiveCN110763841AImprove stabilityImprove thermal stabilityBiological material analysisAntiendomysial antibodiesSodium phosphates
The invention relates to a plasminogen measuring kit and a preparation method thereof. the measuring kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises the raw materials of80-120 mmol / L of citrate buffer solution, 8-15 mmol / L of preservative, 0.5-1.0mmol / L of surfactant, 0.8-3g / L of stachyose, 0.8-3g / L of fructose diphosphate, 0.05-0.5g / L of sodium hexametaphosphate, 40-60mmol / L of sodium chloride and 4-6g / L of bovine serum albumin (BSA); and the reagent R2 comprises the raw materials of 80-120 mmol / L of citrate buffer solution, 0.5-1% of latex particles (by mass volume), 8-15mmol / L of sodium azide, 1.8mg / ml of goat anti-human plasminogen antibody and 4-6g / L of bovine serum albumin (BSA). The invention provides a fibronectin measuring kit with high sensitivity and high detection precision and wide application range, and a preparation method thereof.
Owner:浙江爱康生物科技有限公司
Facial mask containing fructose sodium diphosphate and preparation method thereof
InactiveCN106667787AAnti agingAvoid damageCosmetic preparationsToilet preparationsFruit juiceWheat germ
The invention relates to facial masks, and discloses a facial mask containing fructose sodium diphosphate. The facial mask is prepared from t he following components in parts by weight: 25 to 30 parts of fructose sodium diphosphate, 10 to 15 parts of wheat germ extract, 10 to 15 parts of corn extract, 15 to 20 parts of cherry tomato juice, 8 to 10 parts of honeysuckle flower leaf extract, 12 to 20 parts of dried potato powder and 20 to 50 parts of deionized water. According to the facial mask disclosed by the invention, by taking the fructose sodium diphosphate as the main material and matching a certain proportion of wheat germ extract rich in vitamin E and a certain proportion of cherry tomato juice rich in glutathione, the obvious effects of resisting cellular oxidation, whitening and rapidly repairing cells can be achieved. The invention also discloses a preparation method of the facial mask containing the fructose sodium diphosphate. The preparation method disclosed by the invention is simple in operation, is environment-friendly, economic, efficient and non-toxic, and has wide application prospect. The facial mask prepared by the invention is capable of repairing, whitening, moisturizing and nourishing skin and delaying skin aging.
Owner:HANGZHOU ETK INTELLIGENT TECH CO LTD
Fructose diphosphate containing novel pharmaceutical composition injection for treating circulation system diseases
The invention provides an injection prepared from a novel composition which contains three medicines such as fructose diphosphate and the like, wherein the injection consists of the following components according to effective doses: 10-50g of the fructose diphosphate, 0.01-0.05g of isosorbide dinitrate and 100-1000ml of sodium hydrogen sulfite. The invention also provides preparation steps and a preparation method. The pharmaceutical composition is applicable to shock, coronary heart disease, angina, acute myocardial infarction, myocardial ischemia, cardiac failure and surrounding lesions. By adding the isosorbide dinitrate, myocardial oxygen consumption is reduced, oxygen supply is enhanced and the angina is relieved. In cooperation with and by expanding the application of the fructose diphosphate to clinical treatment, the medicine is safer and is more stable, and the medicine is capable of relieving irritant pain on a human body and enhancing a curative effect on treating circulation system related diseases. The preparation method comprises the following steps: sequentially stirring and dissolving the fructose diphosphate, the sodium hydrogen sulfite and the isosorbide dinitrate by virtue of injection water, decoloring with the addition of activated carbon, filtering and removing carbon; and filtering, sterilizing, filling and sterilizing.
Owner:邓学峰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com