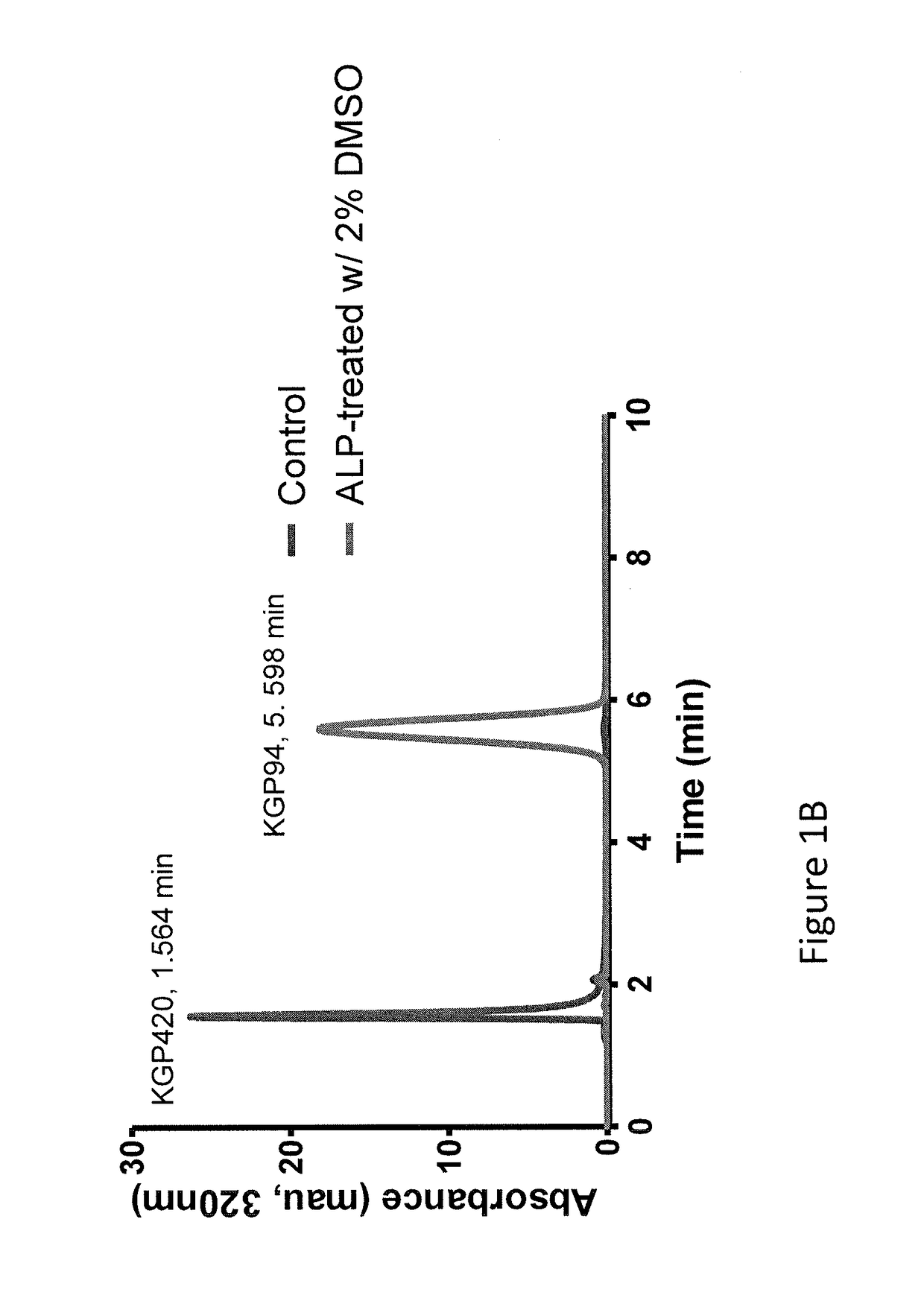
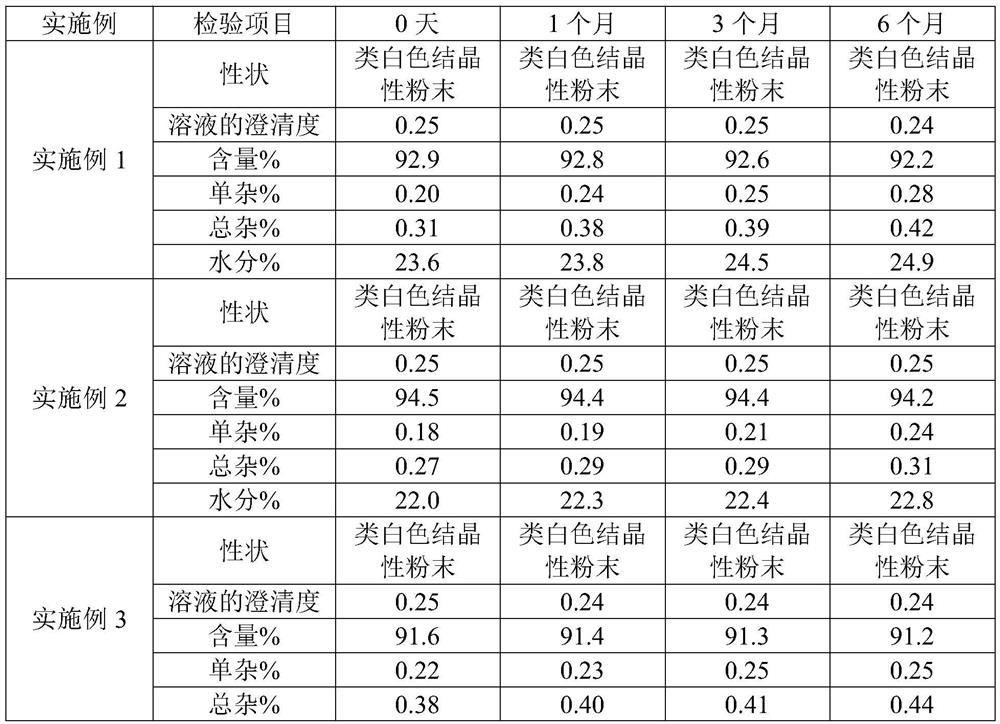
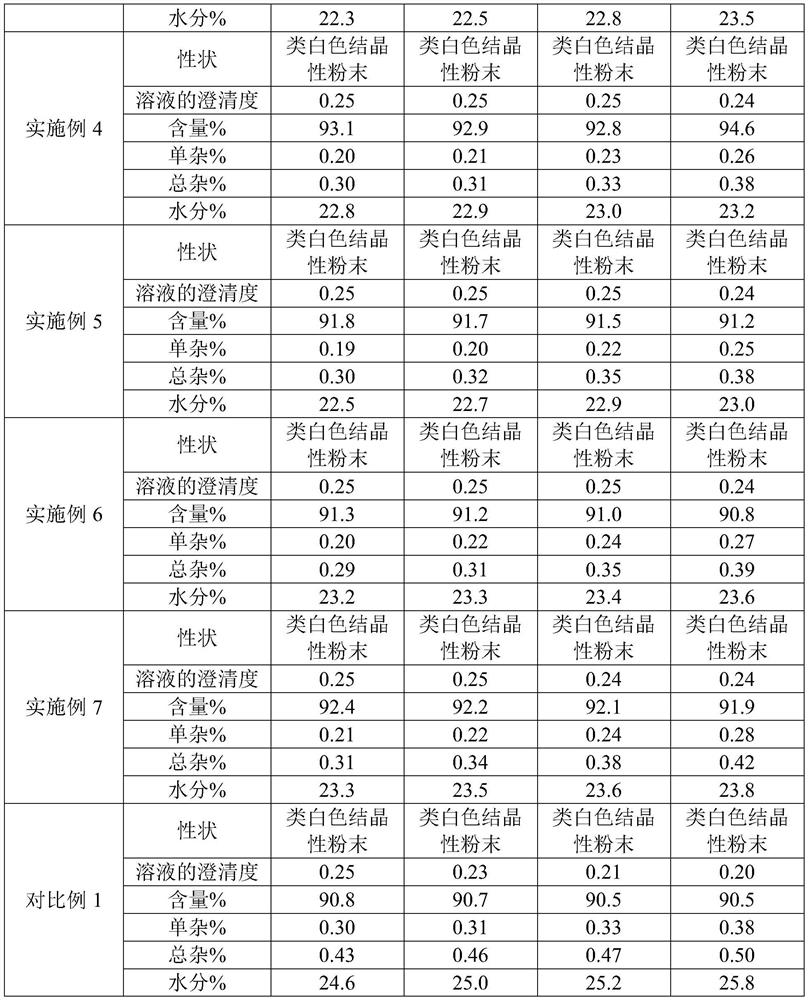
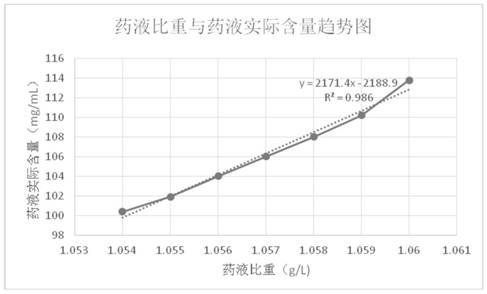
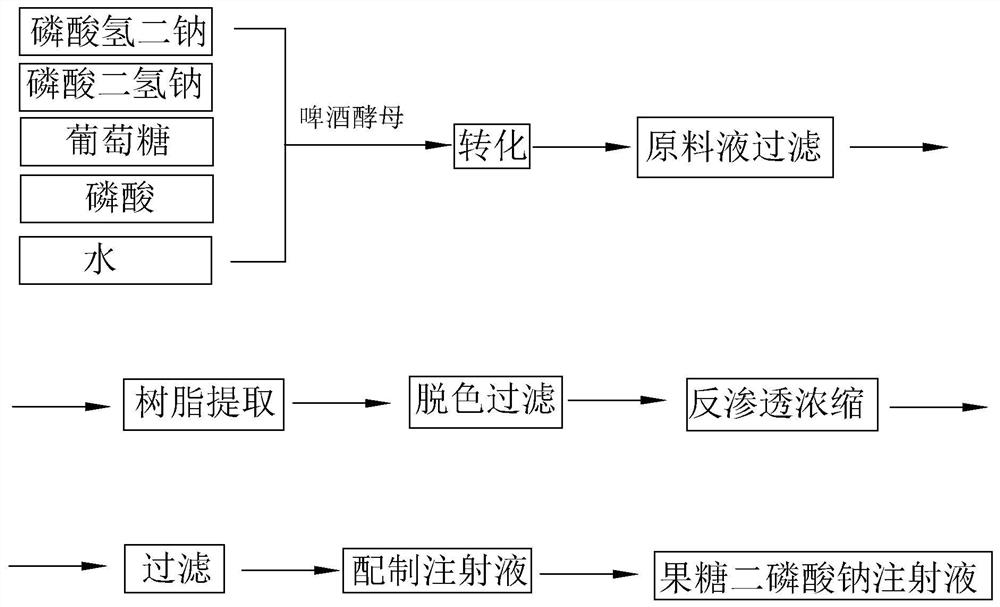
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
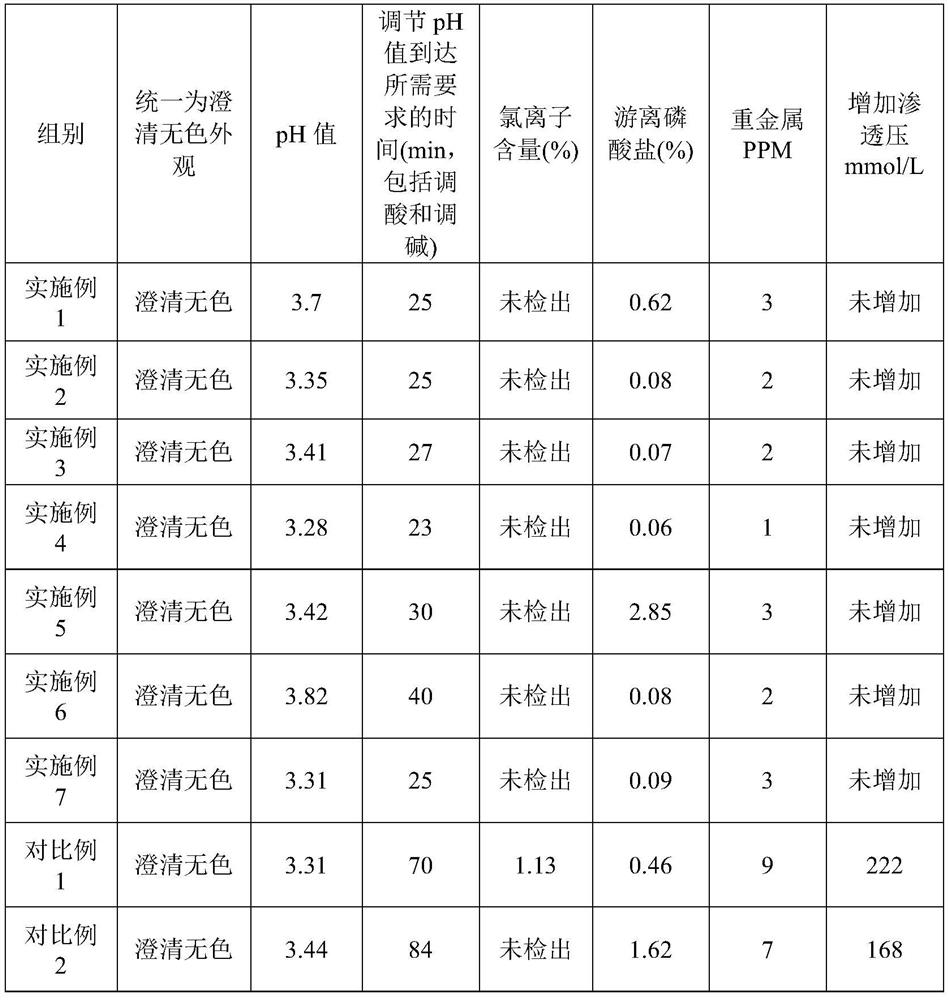
38 results about "Sodium diphosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
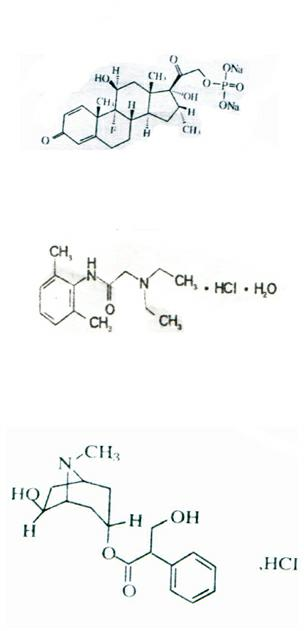
Disodium diphosphate also is known as disodium dihydrogen diphosphate, disodium dihydrogen pyrophosphate and disodium pyrophosphate. It also has the name sodium acid pyrophosphate. This chemical is an odorless white powder and, because it has a valance of greater than two, it can bond to many other chemicals.
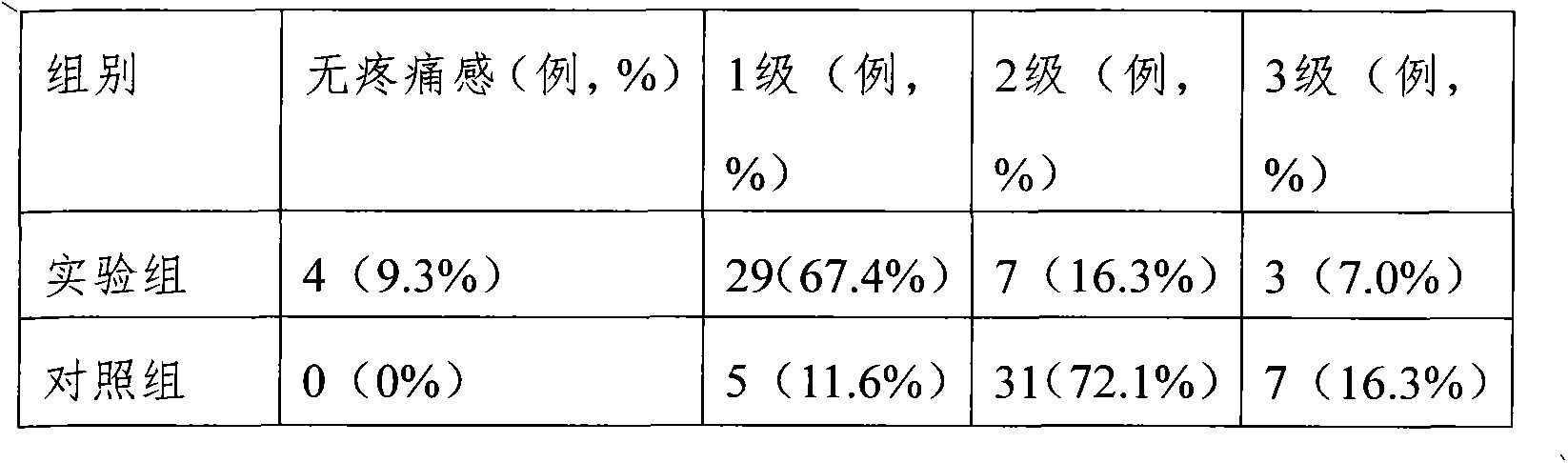
Medicament formula for relieving pain caused by infusion medicament and preventing phlebitis
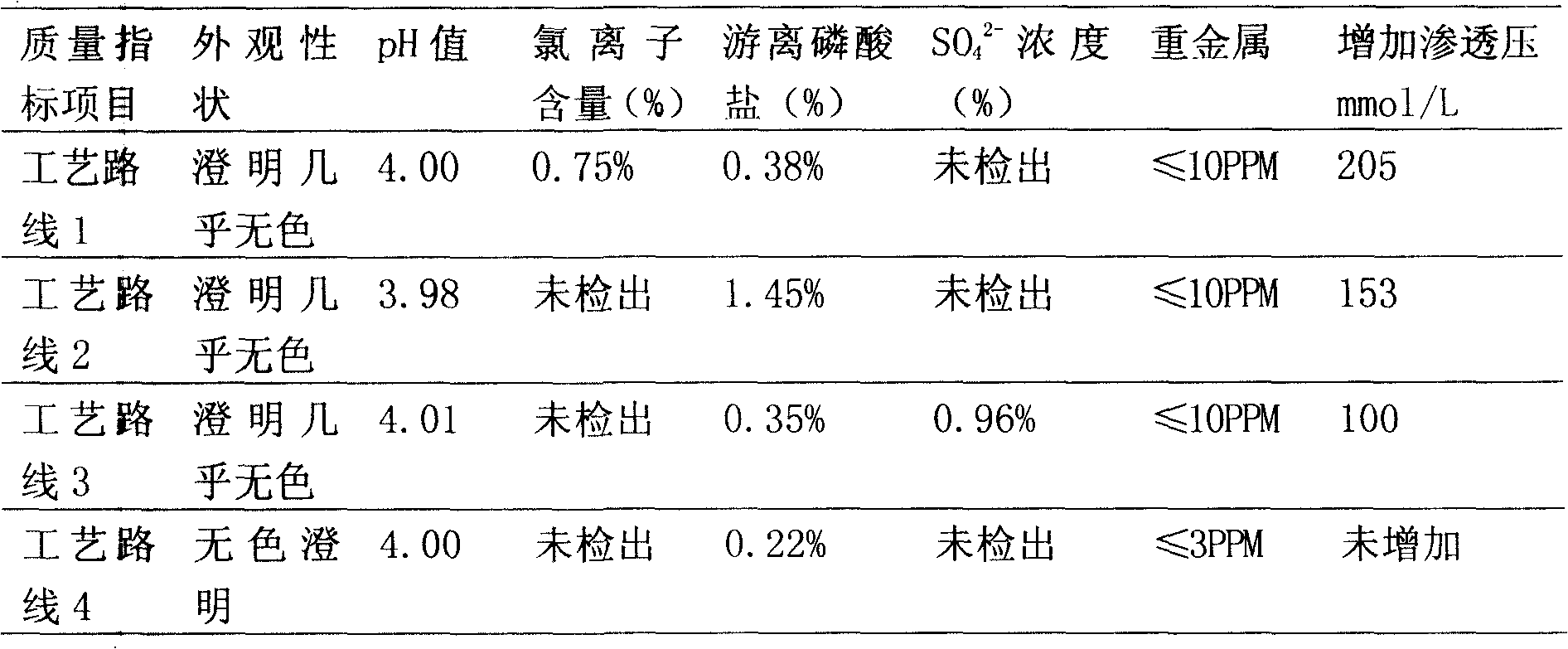
InactiveCN102579458ARelief the painRelieve and prevent phlebitisAntipyreticAnalgesicsFormularySodium phosphates
The invention discloses a medicament formula for relieving pain caused by infusion medicament and preventing phlebitis. The medicament formula can be used for relieving pain caused by intravenous supplementing potassium, infusion mannitol, azithromycin, fructose diphosphate sodium and chemotherapeutic medicaments, and can be used for effectively preventing and treating extravasation phlebitis caused by long-term use of a remaining needle, infusion of vein high-nutrition medicaments, infusion of chemotherapeutic medicaments, infusion extravasation and the like. According to the medicament formula, 20-35ng of anisodamine, 6-13ml of lidocaine, 15-26mg of dexamethasone and 4-6ml of glycerin are arranged in a sterile kidney basin, sterile gauze is coated above a puncture part after being uniformly wetted, and an external preservative film is wound on the gauze. The medicinal formula has the advantages of continually and efficiently relieving local pain caused by infusion medicaments, and effectively preventing and treating local inflammation, swelling pain and stripped cable change caused by infusion.
Owner:GENERAL HOSPITAL OF JIZHONG ENERGY FENGFENG GRPCO
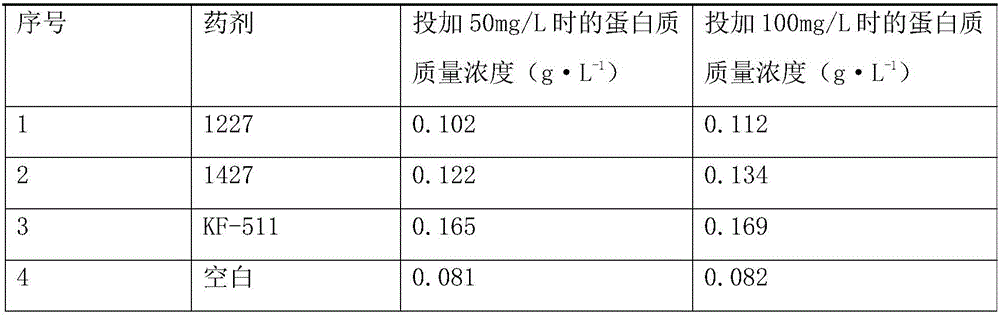
Industrial wastewater treating agent and preparation method thereof
InactiveCN106630181AWide variety of sourcesThorough treatmentWater treatment parameter controlWater contaminantsIron sulfateIndustrial waste water
The invention discloses an industrial wastewater treating agent which comprises the following components in parts by weight: 15-30 parts of polymeric aluminum silicate-sulfate, 15-30 parts of ferric sulfate, 1-5 parts of polyacrylamide, 1-5 parts of microorganisms, 15-30 parts of potato starch, 3-13 parts of chitosan, 7-17 parts of attapulgite powder, 5-10 parts of tannin, 4-11 parts of kaolin, 20-40 parts of modified vesuvianite, 10-20 parts of calcium hydroxide, 10-20 parts of activated carbon, 5-15 parts of sodium diphosphate, 1-5 parts of sodium hypochlorite and 5-20 parts of lignin. The raw materials of the industrial wastewater treating agent are wide in source, pollutants in the water can be effectively removed, the sewage treatment is thorough, the treatment cost is low, the treating agent does not has toxicity and does not cause pollution, and the treated wastewater meets the national sewage comprehensive emission standard requirements. The preparation method is simple and convenient to produce.
Owner:广西新六合环保有限责任公司
Paraffin-cleaning antiseptic corrosion inhibitor
ActiveCN101029222AMeet the requirements of wax removal and viscosity reductionMeet anti-corrosion and anti-fouling requirementsDrilling compositionPolymer scienceHexamethylenetetramine
A paraffin-cleaning antiseptic scale inhibitor for oil well consists of polyethylene-polyamine polyoxytrimethylene polyethenoxy ether 3.4-12wt%, lauryl-dimethylbenzyl ammonium chloride 13.5-17wt%, FC-NO1 fluorocarbon surface activator 0.02-0.10wt%, formaldehyde 4.5-9.5wt%, hexamine 3.2-7.4wt%, hydroxyl-ethidene-sodium nucleotide 15-25wt% and water 35-58wt%. It has excellent anticorrosive and anti-scaling effects.
Owner:PETROCHINA CO LTD
Sodium fructose diphosphate granule agent and its preparation method
InactiveCN1650871AImprove stabilityExtended storage timeCarbohydrate active ingredientsGranular deliveryHeart diseasePharmacology
Owner:FUKANGREN BIO PHARMA
Method for preparing sodium fructose diphosphate injection
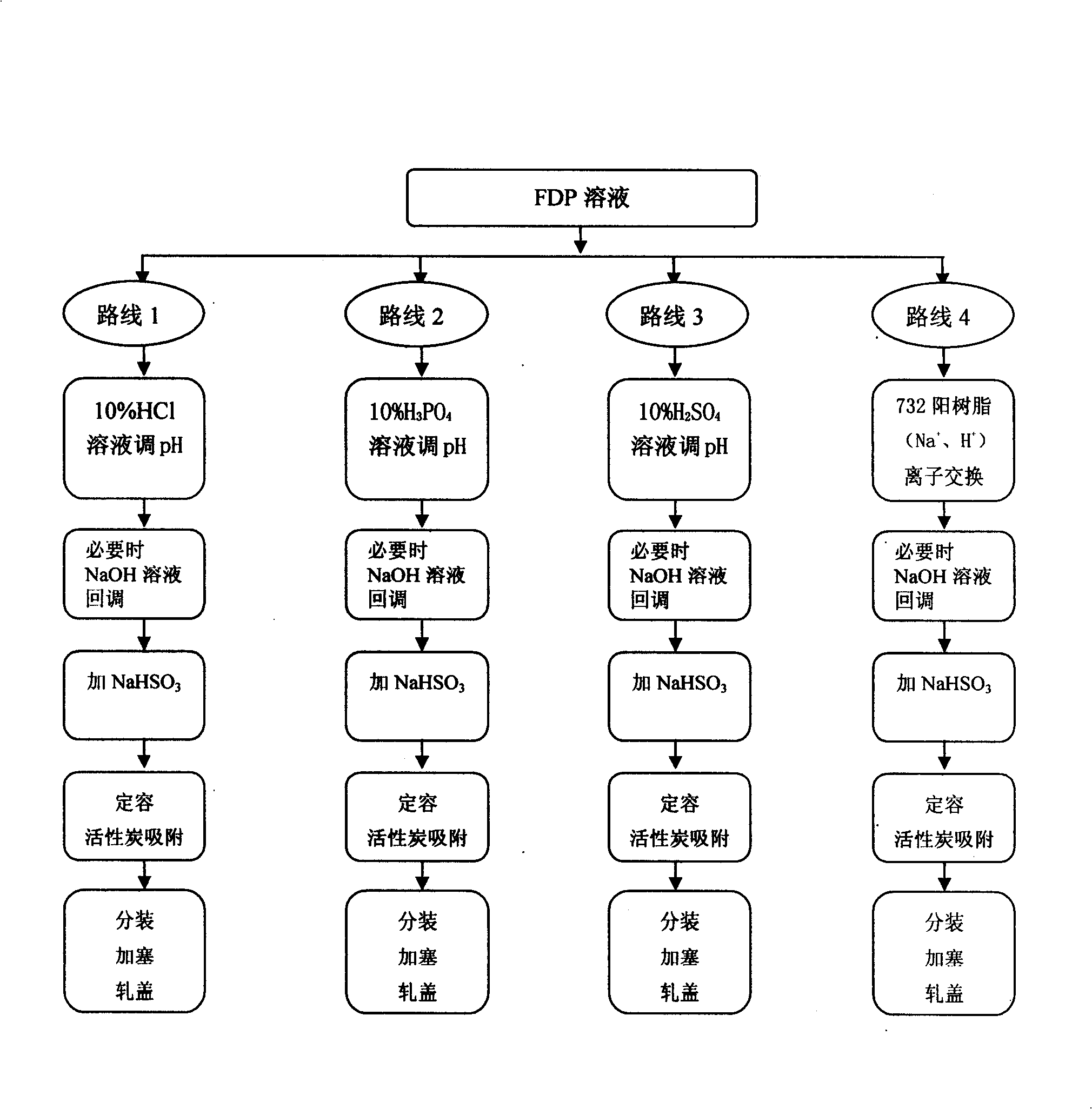
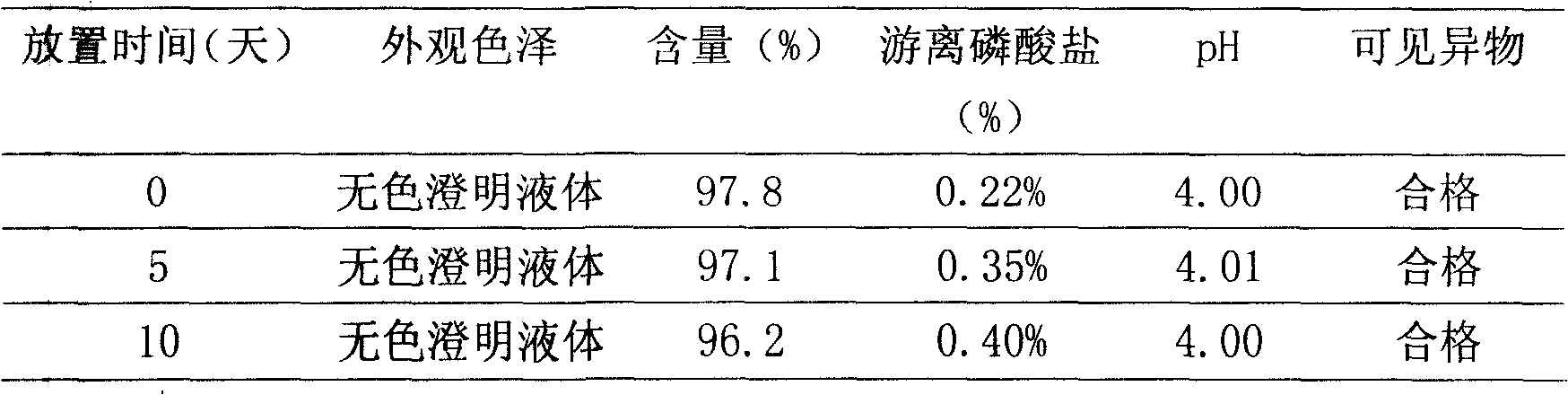
InactiveCN101244071ALow purityReduce osmotic pressurePharmaceutical delivery mechanismCarbohydrate active ingredientsCLARITYImpurity
The invention relates to a preparation method of fructose sodium diphosphate injection, belonging to the technical field of medicine. The preparation method is: medical solution is treated by cation exchange resin, which can reduce acidity, remove metal cation, absorb colorful impurities and pyrogens, increase the clarity of the medical solution, avoid the defects of decreasing the product purity, increase osmotic pressure, and influencing medication safety caused by acid radical ion when acid is used to regulate acidity, and provides a stable fructose sodium diphosphate injection with high purity. The fructose sodium diphosphate injection is applied to intravenous injection or intravenous drip in clinical practice, and is suitable for low phosphorus acidemia. The preparation method has the advantages: the preparation method is simple and controllable, and the prepared product quality is stable and safe for clinical use.
Owner:广东宏远集团药业有限公司
Medicine composition containing fructose sodium diphosphate compound
ActiveCN103054883ASimple prescriptionImprove stabilityOrganic active ingredientsInorganic non-active ingredientsHydrogenMANNITOL/SORBITOL
The invention provides a medicine composition containing a fructose sodium diphosphate compound, comprising fructose sodium diphosphate, sodium hydrogen sulfite, mannitol and water for injection. A preparation method for the medicine composition comprises the following steps of: sequentially dissolving fructose sodium diphosphate, sodium hydrogen sulfite and mannitol with the water for injection by stirring; adding active carbon to decolour; filtering and removing carbon; and filtering and removing bacteria. The fructose diphosphate medicine composition provided by the invention is simple in prescription, high in stability, capable of alleviating pain during injection, and conducive to application and popularization for fructose sodium diphosphate injection solution clinically. Additionally, high-temperature sterilization is avoided in the preparation method disclosed by the invention, the generation of related substances can be reduced, and the safety is improved.
Owner:罗诚
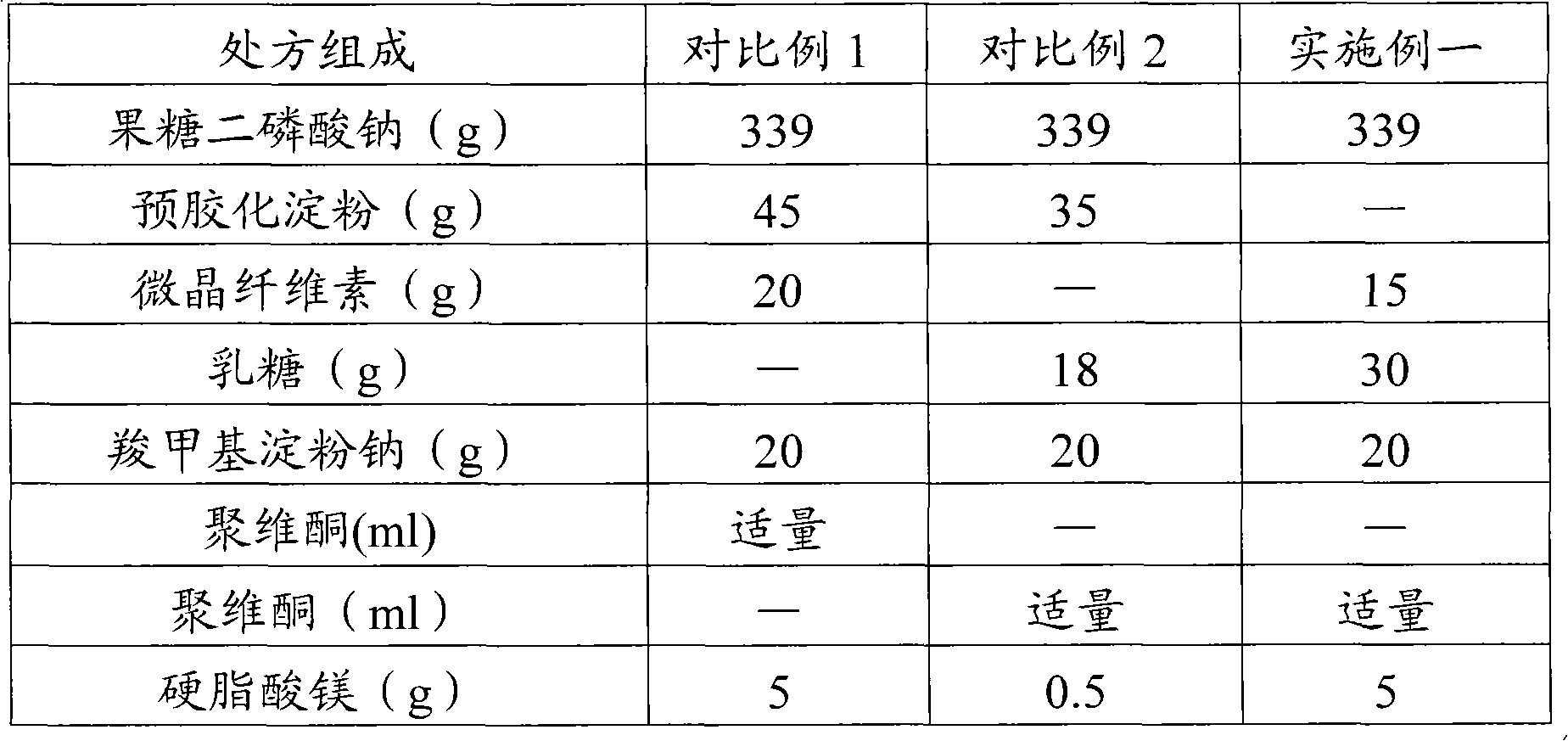
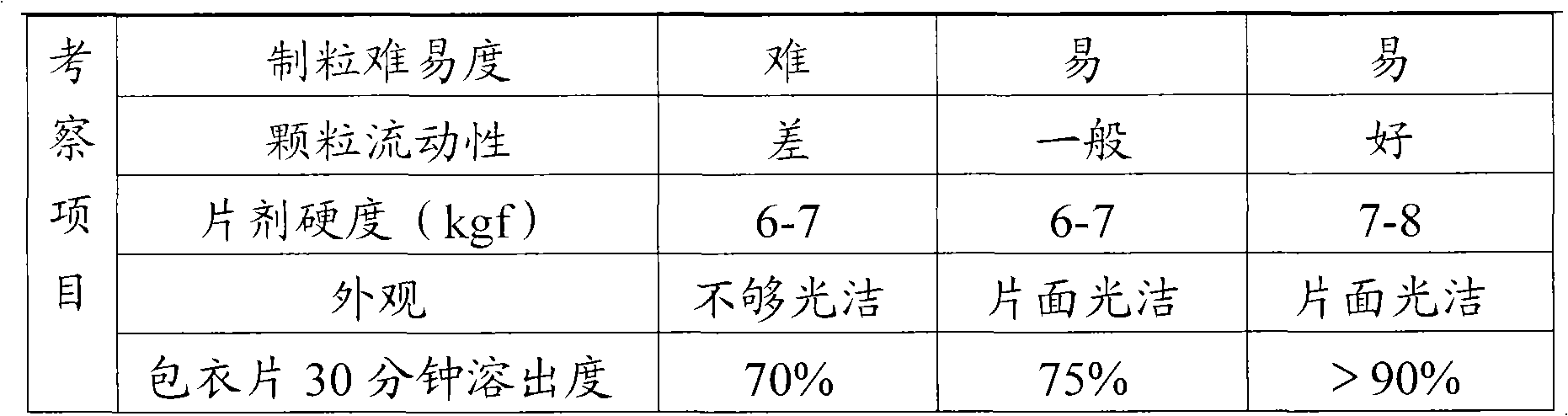
Fructose diphosphate sodium tablet
InactiveCN101829066AImprove hydrophilicityImprove water absorptionOrganic active ingredientsPill deliveryChemistrySodium carboxymethyl starch
The invention relates to a fructose diphosphate sodium tablet mainly comprising the following raw materials in a formula: 78-88% of fructose diphosphate sodium, 2-16% of lactose, 1-6% of microcrystalline cellulose, 1-7% of carboxymethyl starch sodium and 1-3% of povidone. The dissolution of the prepared fructose diphosphate sodium tablet is greater than 90% within 30 minutes.
Owner:张家港市华天药业有限公司
A kind of carnosine glycoside injection pharmaceutical composition and preparation method thereof
InactiveCN102266351AIncrease breathing vitalityStimulus index increasedPharmaceutical delivery mechanismUnknown materialsDiseaseSodium phosphates
The invention provides a muscular amino acids and nucleosides injection medicinal composition and discloses a preparation method of the medicinal composition. The medicinal composition is prepared from a muscular amino acids and nucleosides injection, pentoxifylline, fructose diphosphate sodium and polyethylene glycol. When the medicinal composition is applied in vitro, the liver homogenate breathing activity of a guinea pig can be increased, an activity stimulation index of breathing activity determined by using a Warburgs respirometer is about 4.2, which is far higher than the stimulation index of the liver homogenate breathing activity of the guinea pig during separate testing by using muscular amino acids and nucleosides, the pentoxifyllinum and the fructose diphosphate sodium, and thus, the treating effect on heart cerebrovascular disease is enhanced greatly.
Owner:长春白求恩制药有限公司
Method for yeast cell to biosynthesizing 1,6- fructose sodium diphosphate
InactiveCN101328489AEasy to trainHigh synthesis efficiencyMicroorganism based processesFermentationFructoseSodium phosphates
The invention discloses a method for biologically synthesizing 1, 6-di- (2- ethylhexyl ) phosphoric acid fructose sodium of a yeast cell, comprising the following steps that: by taking 100mL of sterilizing reaction fluid to count, 1 to 5 grams of thalli which is obtained by pre-culture process is added to react for 4 to 10 hours at a temperature of between 25 and 30 DEG C and at a speed of 100 to 200r / min, trichloroacetic acid is added to stop the reaction, the clear solution of the 1, 6-di- (2- ethylhexyl ) phosphoric acid fructose sodium is obtained by centrifugalization; the thalli is cells which are obtained by pre-culturing for 12 to 36 hours, freezing and centrifuging of Saccharomyces cerevisiae; the pH value of the reaction fluid is between 5.0 and 9.0, the compositions of the reaction fluid in weight percentage are: 2 to 12 percent of glucose, 2 to 15 percent of sodium phosphate dibasic and the balance being water. The 1, 6-di- (2- ethylhexyl ) phosphoric acid fructose sodium synthesized by the method has simple process for culturing the yeast cells, shortens the time, and has simple converting system, easy operation and high FDP sodium converting efficiency.
Owner:ZHEJIANG UNIV
Leech capsule
The invention relates to a leech capsule, and belongs to the technical field of leech deep processing. The preparation method comprises the following steps: removing excrements in leeches, soaking leeches in brine, after leeches die, fishing out leeches, blowing off impurities on the surface of leeches; grinding dried leeches, soaking grinded leeches in ethanol, carrying out sealed soaking; aftersealed soaking, concentrating and drying obtained substance to remove ethanol in the substance; mixing the substance with fructose sodium diphosphate and starch, sieving the mixture by a sieve with asize of 10-20 meshes, drying the mixture at a temperature of 30 to 45 DEG C, carrying out granulation and sterilization, and finally filling granules into capsules. At first, leeches are soaked in brine to change the tension force of the surface cells of leeches, then leeches are grinded, soaked in ethanol, concentrated, and dried so that hirudin is dissolved or to be dissolved, and the problem that the dissolution rate of hirudin in grinded leeches of a conventional oral leech capsule is low is solved.
Owner:广西鹿帅仁生物科技有限公司
Low-foam antirust cleaning agent of metal equipment
The invention discloses a low-foam antirust cleaning agent of metal equipment, which is prepared from the following raw materials in parts by weight: 3.2-4.2 parts of sodium diphosphate, 4.3-7.1 parts of coconut diethanolamide, 6.4-9.5 parts of lysine, 1.5-3.4 parts of corrosion inhibitor, 8.5-9.8 parts of 2-ethylhexyl sulfosuccinate, 1.5-2.9 parts of primary alcohol polyoxyethylene ether and 2.5-6.8 parts of adsorbent. The low-foam antirust cleaning agent disclosed by the invention has the beneficial effects that the antirust effect is good and pollution is avoided; the cleaning agent has the advantages of low foam, high efficiency, no corrosion to a metal surface, good stability and no pollution; the cleaning rate is over 90%; the cleaning agent can be widely applied to the rust prevention among the processes of steel, stainless steel, alloy steel product components and materials of all industries as well as the cleaning antirust process of various kinds of metal equipment.
Owner:QINGDAO HUIERTONG TRADING
Preparation method of fructose sodium diphosphate injection
ActiveCN109223709ALess impuritiesReduce usageOrganic active ingredientsMetabolism disorderDrugs solutionPhosphoric acid
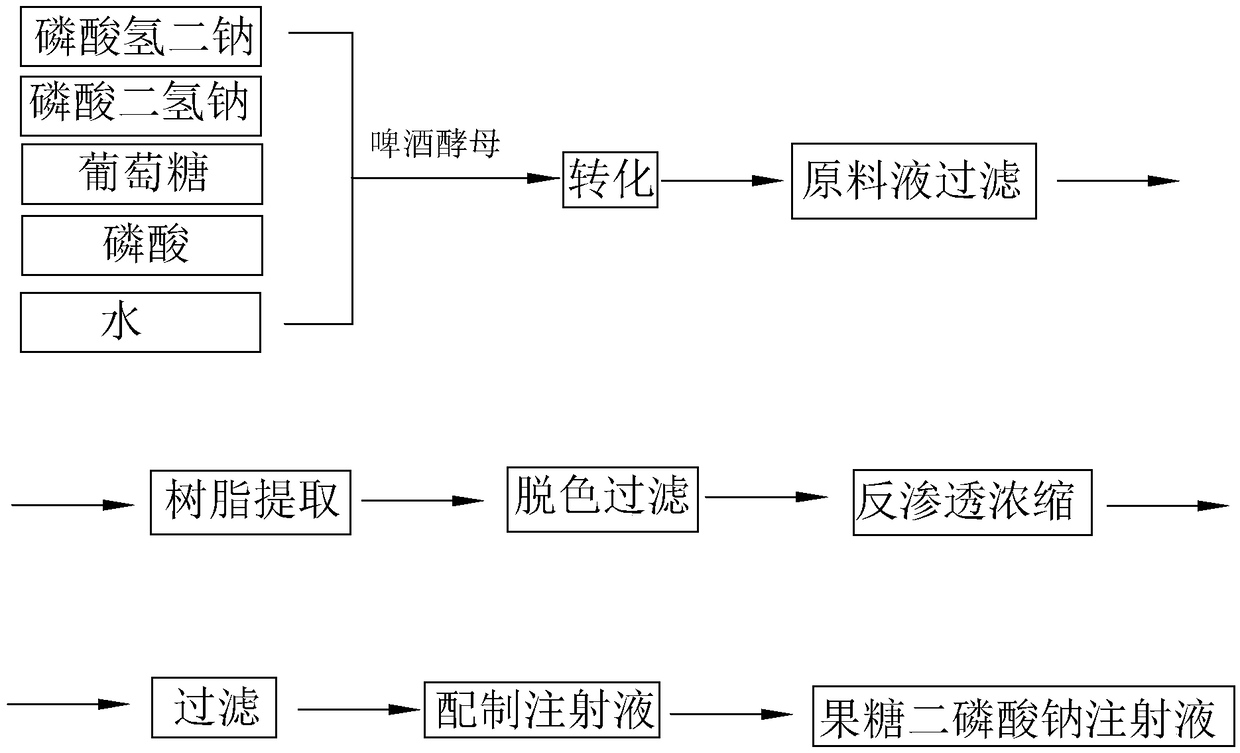
The invention discloses a preparation method of fructose sodium diphosphate injection, which comprises the following steps: S1, dissolving disodium hydrogen phosphate, sodium dihydrogen phosphate, glucose and phosphoric acid in water to prepare a reaction liquid, mixing with beer yeast, stirring and reacting to obtain a raw material liquid containing fructose sodium diphosphate; 2, filter that rawmaterial liquid plate frame; S3, adsorbing and purifying the solid-liquid separated raw liquid in S2 by anionic resin to obtain resin extract containing fructose sodium diphosphate; 4, decolorizing and filtering, decolorize that resin extract solution by activated carbon, and filtering and decarburizing; 5, carrying out initial concentration on that decolorization filtrate in S4, washing and desalinate the decolorization filtrate with water, and finally pressurizing the decolorization filtrate to concentrate the specific gravity of the decolorization filtrate to more than 1.05 g / ml; 6, filterthat concentrated drug solution to obtain RO solution; 7, filter that RO solution in S6, diluting, filter, and filling to obtain sterile fructose sodium diphosphate injection. The invention has the advantages of greatly reducing the use amount of the organic solvent in the preparation process and reducing the production cost.
Owner:BEIJING HUAJIN PHARM CO LTD
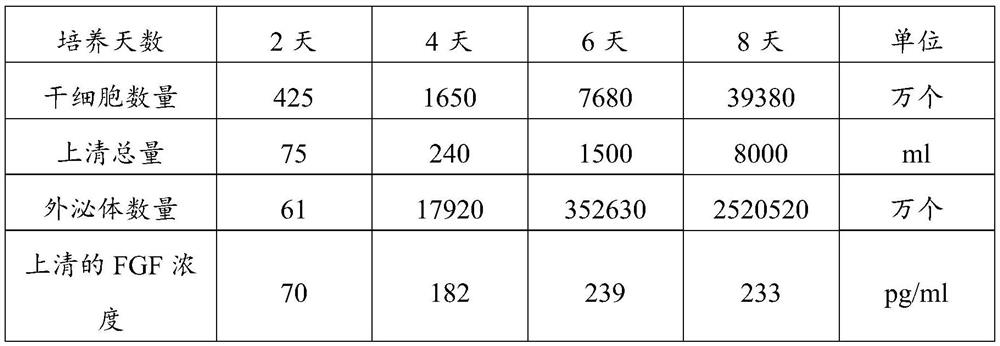
Exosome secretion culture medium and culture and separation method of umbilical cord mesenchymal stem cell exosomes
ActiveCN112920996AEase of mass productionImprove biological activityCell dissociation methodsCulture processSodium phosphatesCell culture media
The invention discloses an exosome secretion culture medium and a culture and separation method of umbilical cord mesenchymal stem cell exosomes, and relates to the technical field of exosome enrichment and separation. A serum-free stem cell culture medium is used as a basic culture medium; and the following components are added: EGF with the concentration of 100 to 300ng / ml, TGF with the concentration of 50 to 150ng / ml, coenzyme Q10 with the concentration of 10 to 30mu g / ml, potassium salt with the concentration of 300 to 600mu g / ml, fructose sodium diphosphate with the concentration of 0.3 to 0.9 mu g / ml and histamine dihydrochloride with the concentration of 100 to 300 mu g / ml. By adopting the exosome secretion culture medium provided by the invention, more exosomes can be obtained under the condition of the same number of culture cells. The exosome secretion culture medium is beneficial to large-scale production of umbilical cord mesenchymal stem cell exosomes with good biological activity, further promotes wide application of the exosomes in medical treatment, anti-aging, beauty treatment and the like, and has good economic benefits.
Owner:广州研华生物科技有限公司
Effervescent tablet containing fructose sodium diphosphate
InactiveCN102077948AEasy to storeImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismEffervescent tabletSugar
The invention relates to an effervescent tablet containing fructose sodium diphosphate, comprising the fructose sodium diphosphate, citric acid, baking soda, oligosaccharide or other edible sugar in a weight ratio of (10-20):30:7:200. In the invention, the effervescent tablet is prepared with the fructose sodium diphosphate as a functional component, and compared with the other dosage forms, the effervescent tablet is easy to preserve, dissolve and absorb, has good stability and taste, and can be directly drunk after being soaked in water without stir.
Owner:张家港市华菱化工机械有限公司
Facial mask containing fructose sodium diphosphate and preparation method thereof
InactiveCN106667787AAnti agingAvoid damageCosmetic preparationsToilet preparationsFruit juiceWheat germ
The invention relates to facial masks, and discloses a facial mask containing fructose sodium diphosphate. The facial mask is prepared from t he following components in parts by weight: 25 to 30 parts of fructose sodium diphosphate, 10 to 15 parts of wheat germ extract, 10 to 15 parts of corn extract, 15 to 20 parts of cherry tomato juice, 8 to 10 parts of honeysuckle flower leaf extract, 12 to 20 parts of dried potato powder and 20 to 50 parts of deionized water. According to the facial mask disclosed by the invention, by taking the fructose sodium diphosphate as the main material and matching a certain proportion of wheat germ extract rich in vitamin E and a certain proportion of cherry tomato juice rich in glutathione, the obvious effects of resisting cellular oxidation, whitening and rapidly repairing cells can be achieved. The invention also discloses a preparation method of the facial mask containing the fructose sodium diphosphate. The preparation method disclosed by the invention is simple in operation, is environment-friendly, economic, efficient and non-toxic, and has wide application prospect. The facial mask prepared by the invention is capable of repairing, whitening, moisturizing and nourishing skin and delaying skin aging.
Owner:HANGZHOU ETK INTELLIGENT TECH CO LTD
A kind of preparation method of sodium fructose diphosphate injection
ActiveCN103735498BSimple processImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSodium phosphatesPharmaceutical Aids
Owner:CISEN PHARMA
Food astringent removing agent
ActiveCN103519031AGood effect of removing astringencySimple preparation processFood ingredient as taste affecting agentFood preparationAcetic acidEthyl acetate
The invention discloses a food astringent removing agent. The food astringent removing agent is prepared from the following components in parts by weight: 2-6 parts of fructose sodium diphosphate, 1-3 parts of potassium citrate, 3-8 parts of xylitol, 10-20 parts of ethyl acetate, 0.5-0.8 part of edible essence and 800-1000 parts of water. Compared with the prior art, the food astringent removing agent has the following beneficial effects: (1) the food astringent removing agent has wide application range, can be widely suitable for various fruits, foods, wines and the like, has good astringent removing effect and is non-toxic and harmless to a human body; (2) the preparation process is simple and the cost is low.
Owner:启东鹏隆投资管理有限公司
Preparation method of levocarnitine composition for injection and liposome injection thereof
ActiveCN110731944BMicrostructural stabilityGood resolubilityOrganic active ingredientsPowder deliverySodium phosphatesFreeze-drying
The invention relates to a preparation method of levocarnitine composition for injection and liposome injection thereof. The preparation method comprises the following steps: using sodium dihydrogen phosphate and sodium hydroxide with a volume ratio of 10:1-12:1 solution, preparing an acid-base buffer solution, wherein the molar concentration of the acid-base buffer solution is between 0.010-0.012mol / L; fructose diphosphate sodium and levocarnitine injection are added to the acid-base buffer solution, wherein the fructose diphosphate The mass ratio of sodium phosphate to levocarnitine is between 0.8:1-1.1:1, and the mass concentration of levocarnitine is between 18-22g / L; add a needle with a mass concentration of 0.1g / L and stir well with activated carbon, Stirring and adsorption for 30 minutes; ultrasonic filtration of activated carbon; vacuum freeze-drying to obtain the finished levocarnitine composition. The levocarnitine composition for injection prepared by the invention has a stable microstructure of the finished product, good resolubility, good safety, and better drug properties than existing drugs.
Owner:HAINAN GENERAL & KANGLI PHARMA
Food astringent removing agent
ActiveCN103519031BGood effect of removing astringencySimple preparation processFood ingredient as taste affecting agentFood preparationAcetic acidEthyl acetate
Owner:启东鹏隆投资管理有限公司
Compositions and methods for inhibition of cathepsins
InactiveUS20190010173A1Organic active ingredientsGroup 4/14 element organic compoundsCathepsin LCathepsin B
This present disclosure is directed to compound of Formula I and methods of using these compounds in the treatment of conditions in which modulation of a cathepsin, particularly cathepsin L, cathepsin K, and / or cathepsin B, will be therapeutically useful. Formula I: or a solvate or pharmaceutically acceptable salt thereof. Each of R1-R10 are independently selected from the group consisting of: hydrogen, alkoxy, halo, hydroxy, phosphate, phosphate salts, disodium phosphate, diphosphate dimer, diphosphate dimer salt, and sodium diphosphate dimer with at least one of R1-R10 is a phosphate or diphosphate dimer group.
Owner:BAYLOR UNIVERSITY +1
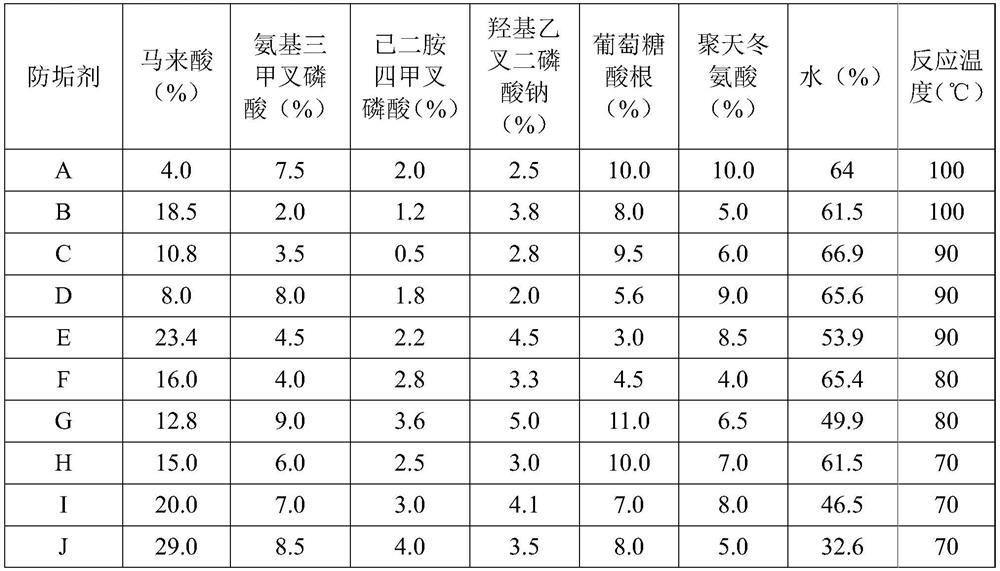
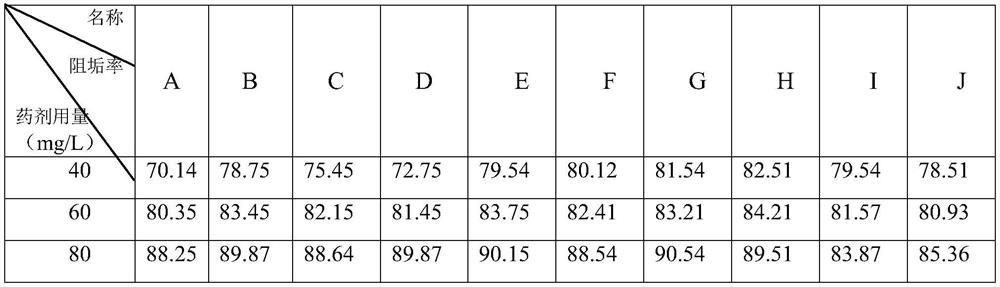
Anti-scale agent suitable for high-salt and high-chlorine working conditions and preparation method thereof
PendingCN114656045AGood recombinationImprove antifouling performanceSpecific water treatment objectivesWater contaminantsO-Phosphoric AcidSodium phosphates
The invention discloses an anti-scale agent suitable for high-salt and high-chlorine working conditions, which comprises the following components in percentage by weight: 4.0 to 29.0 percent of maleic acid, 2.0 to 9.0 percent of amino trimethylene phosphoric acid, 0.5 to 4.0 percent of ethylenediamine tetramethylene phosphoric acid, 2.0 to 5.0 percent of hydroxy ethylidene sodium diphosphate, 3.0 to 15.0 percent of gluconate radical and the balance of water. The content of polyaspartic acid or polyepoxysuccinic acid is 4.0%-10.0%, and the balance is water. By compounding maleic acid with other substances and by virtue of the synergistic effect of the components, the scale inhibitor improves the scale inhibition effect, is especially suitable for scale inhibition under high-salt and high-chlorine working conditions, reduces the influence on the environment, and has better scale inhibition performance due to the good synergistic effect, thereby obtaining greater social and economic benefits.
Owner:CHINA PETROLEUM & CHEM CORP +1
Muscular amino acids and nucleosides injection medicinal composition and preparation method thereof
InactiveCN102266351BIncrease breathing vitalityStimulus index increasedPharmaceutical delivery mechanismUnknown materialsDiseaseSodium phosphates
The invention provides a muscular amino acids and nucleosides injection medicinal composition and discloses a preparation method of the medicinal composition. The medicinal composition is prepared from a muscular amino acids and nucleosides injection, pentoxifylline, fructose diphosphate sodium and polyethylene glycol. When the medicinal composition is applied in vitro, the liver homogenate breathing activity of a guinea pig can be increased, an activity stimulation index of breathing activity determined by using a Warburgs respirometer is about 4.2, which is far higher than the stimulation index of the liver homogenate breathing activity of the guinea pig during separate testing by using muscular amino acids and nucleosides, the pentoxifyllinum and the fructose diphosphate sodium, and thus, the treating effect on heart cerebrovascular disease is enhanced greatly.
Owner:长春白求恩制药有限公司
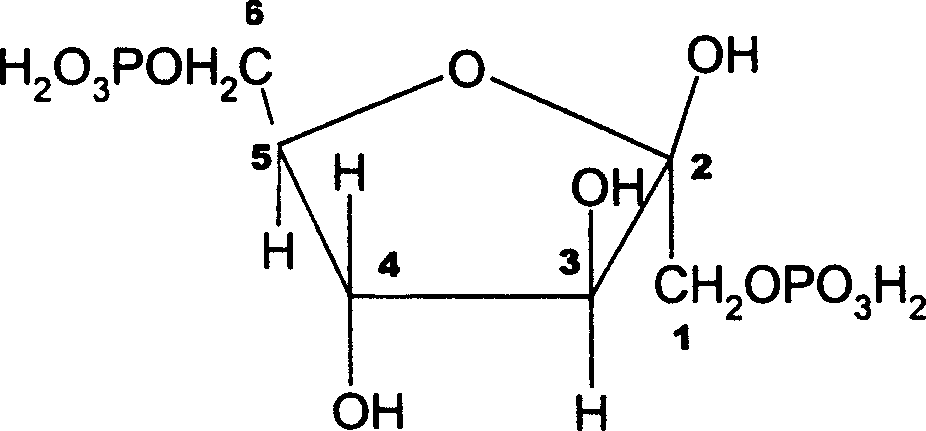
Zinc fructose-diphosphate and its preparing process and application
InactiveCN1176932CPromote absorptionImprove utilizationEsterified saccharide compoundsOrganic active ingredientsDiseaseFructose
A compound fructose-1,6-zinc diphosphate is prepared from fructose-1,6-sodium diphosphate through ion column exchange, reaction on zinc oxide until pH=3-3,5, and post-treating. Said compound has stable property and can be used to prepare the medicine to treat cerebral ischemia (including cerebral disfunction), myocardial ischeme and zinc-deficiency diseases. Its preparing process is simple.
Owner:JINAN UNIVERSITY
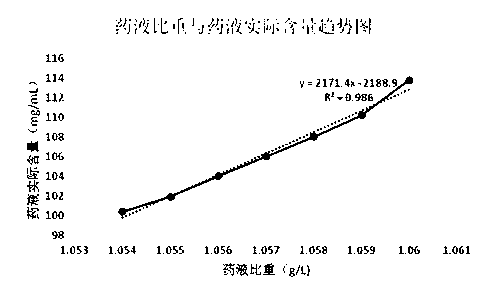
Preparation method of fructose sodium diphosphate powder injection
InactiveCN112110964AHigh yieldImprove quality stabilityEsterified saccharide compoundsSugar derivativesFructoseSodium phosphates
The invention discloses a preparation method of a fructose sodium diphosphate powder injection. According to the method, the yield and quality stability of fructose sodium diphosphate are effectivelyimproved by adopting the modes such as accurately proportioning a ratio of a solute and a solvent, adjusting an addition amount of ethanol, and controlling crystallization temperature and stirring speed in the preparation process. According to the preparation method, seed crystals do not need to be added in the recrystallization process, and the preparation method has the advantages of simple andconvenient operations and simplified production steps. The purity of fructose sodium diphosphate produced by the preparation method is also improved, and the sensitization of fructose sodium diphosphate is reduced.
Owner:HAINAN HUALON PHARM
A kind of preparation method of sodium fructose diphosphate injection
ActiveCN109223709BLess impuritiesReduce usageOrganic active ingredientsMetabolism disorderSodium phosphatesReverse osmosis
Provided are a preparation method for a solution containing fructose sodium diphosphate, use thereof, and a preparation method for a fructose sodium diphosphate injection. The preparation method for the solution containing fructose sodium diphosphate comprises performing solid-liquid separation on a yeast reaction solution containing fructose sodium diphosphate, anion exchange resin extraction, decolorization, reverse osmosis treatment and sterilization treatment. The yeast reaction solution is obtained by contacting a sugar-containing material containing glucose and phosphoric acid with yeast. Other dosage forms such as fructose sodium diphosphate injections or fructose sodium diphosphate oral solutions can be further prepared using said method. The preparation method has advantages of greatly reducing the dosage of an organic solvent and reducing the production cost.
Owner:BEIJING HUAJIN PHARM CO LTD
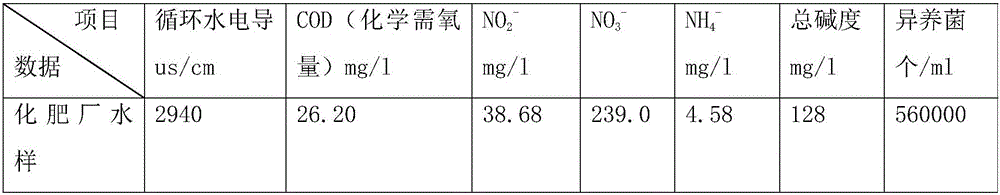
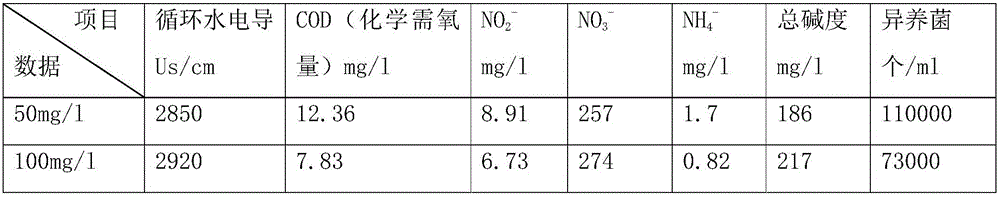
A kind of sterilizing and stripping agent for slime in industrial circulating water
InactiveCN104430332BEfficient killingEasy to degradeBiocideFungicidesBiodispersanDidodecyldimethylammonium chloride
Owner:URUMQI KEFAZHAN FINE CHEM
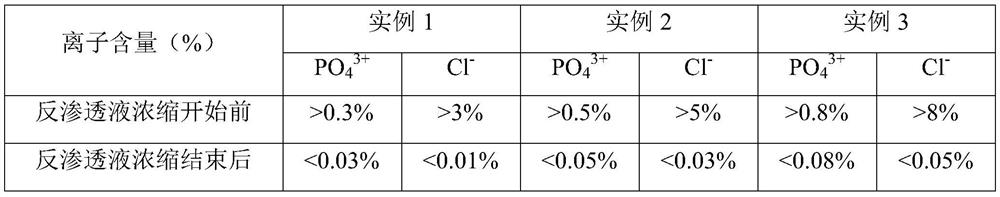
Fructose diphosphate sodium reverse osmosis concentrated solution and preparation method thereof
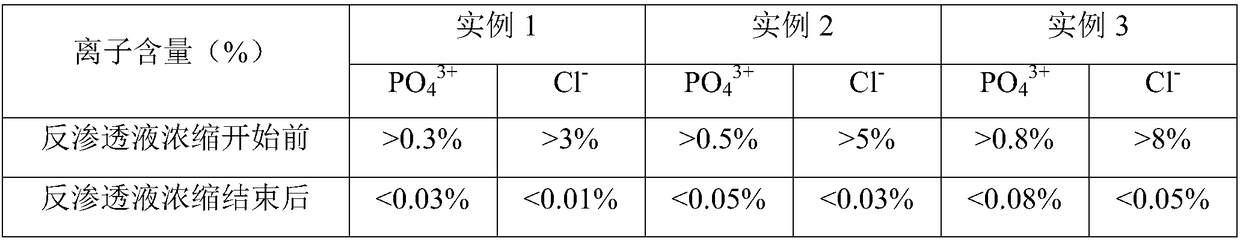
ActiveCN112245442AAvoid problems such as lossRaise the pHOrganic active ingredientsMetabolism disorderFructoseSodium phosphates
The invention provides a method for preparing a fructose diphosphate sodium reverse osmosis concentrated solution. The method comprises the following steps of: dissolving fructose diphosphate sodium in water to obtain a fructose diphosphate sodium aqueous solution, wherein the pH value of the fructose diphosphate sodium aqueous solution is 5-6; adding the fructose diphosphate sodium aqueous solution into a strong acid cation exchange resin column, and collecting effluent, wherein the pH value of the effluent is 3.2-3.5, and the strong acid cation exchange resin is selected from strong acid cation exchange resin; and performing reverse osmosis treatment on the effluent to obtain the fructose diphosphate sodium reverse osmosis concentrated solution. According to the method provided by the invention, the pH value is adjusted by adopting the strong acid cation exchange resin, the introduction of ions is reduced, and meanwhile, phosphate radicals and other impurities can be removed, so thatthe fructose diphosphate sodium reverse osmosis concentrated solution is high in stability and high in purity. Moreover, the method is simple, convenient and rapid to operate and suitable for wide popularization and application.
Owner:BEIJING HUAJIN PHARM CO LTD
Fructose or medicine composition of fructose, preparation of the medicine composition of the fructose and application of fructose in preparing antiepileptic drugs
InactiveCN105168231AImprove stabilityQuality improvementOrganic active ingredientsNervous disorderSodium phosphatesAntiepileptic drug
The invention provides fructose or a medicine composition thereof, preparation of the medicine composition of the fructose and an application of the fructose in preparing antiepileptic drugs. The medicine composition of the fructose is composed of the fructose serving as an active ingredient and pharmaceutic adjuvant. The content of the fructose in the medicine composition of the fructose ranges from 70%-75%, and the medicine composition of the fructose is fructose chewable tablets or fructose granules or fructose tablets. The application of the fructose in preparing the antiepileptic drugs is provided. A blind method pharmacodynamic test is carried out on the fructose, fructose diphosphate, sodium valproate sustained-release tablets, fructose diphosphonic acid+fructose (1:1) and fructose diphosphonic acid+fructose (2:1) through a LiCl-Pilo epileptogenic rat model. A result indicates that the anti-epileptic function of the fructose is obviously superior to that of other drugs. Preparation of the medicine composition of the fructose is provided.
Owner:HUNAN DONGTING PHARMA +1
Low-foam cleaning solution
A low-foam cleaning solution comprises, by weight, 7-9 parts of fatty alcohol-polyoxyethylene ether, 1-3 parts of fire retardant, 4-8 parts of dyhydroxy acetic ether, 3.5-8 parts of sodium silicate, 5-9 parts of layered sodium disilicate, 1.5-3 parts of talcum powder, 3-6 parts of organic silicon defoamer, 1.2-3.6 parts of ethylene glycol butyl aldehyde, 2.2-5.6 parts of tetraphosphorus hesasulfide perborate, 4.5-8.7 parts of sulfonation succinic acid 2-ethyl caproic ester salt and 1.3-2.9 parts of adsorbent. The low-foam cleaning solution has the advantages that the corrosion to a cleaning device can be reduced, the corrosion phenomenon of the device can be delayed, the defoamer is added, the foam can be greatly reduced, cleaning is easier to conduct, and the pollution to the environment is small.
Owner:QINGDAO QIYUAN ZHENDONG ELECTRIC
Sodium fructose diphosphate capsule and preparation method thereof
ActiveCN108078950BGuaranteed drug efficacyStrong targetingOrganic active ingredientsPharmaceutical non-active ingredientsSilicon dioxideFructosediphosphates
The invention discloses a fructose sodium diphosphate capsule and a preparation method thereof. The fructose sodium diphosphate capsule is prepared from the following components in parts by weight: 100-300 parts of fructose sodium diphosphate and 1-5 parts of gel method silicon dioxide, the average particle size of the fructose diphosphate is 300-600 microns, and the average particle size of the silicon dioxide is 2-14 microns. The capsule disclosed by the invention has the advantages that the capsule can maximally preserve the pesticide effect of the medicine in use, the stability is improved, and the content is uniform, and the like.
Owner:HAINAN JINXING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com