Levocarnitine freeze-dried composition for injection and preparation method thereof
A composition and technology for injection, applied in the field of levocarnitine freeze-dried composition for injection and its preparation, and in the field of injections, can solve the problems of increasing the dextrorotatory product of levocarnitine, achieve simple preparation process and avoid degradation , Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
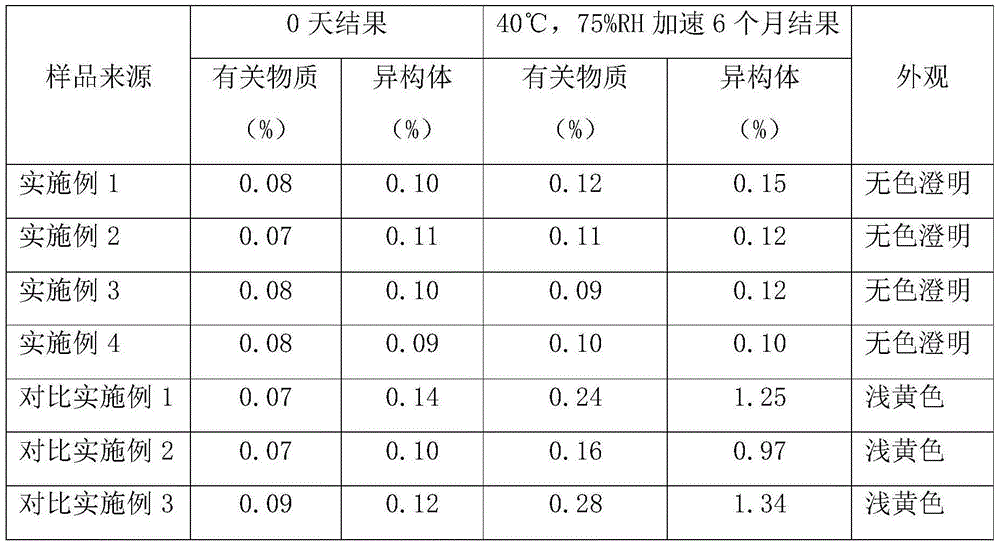
Examples
Embodiment 1
[0025] L-Carnitine 1000g
[0026] Fructose Bisphosphate 600g
[0027] Lactic acid amount
[0028] Add water for injection to 3000ml
[0029] Preparation Process:
[0030] Bottle washing, sterilizing and drying: the vials are first cleaned by an ultrasonic bottle washing machine, then sterilized and dried by a sterilizing dryer, and sent to the filling room for later use.
[0031] Butyl rubber stopper treatment: After the butyl rubber stopper is cleaned by a rubber stopper cleaning machine, it is sterilized by steam at 121°C for 30 minutes, and dried for later use.
[0032] Aluminum-plastic cover treatment: wash the aluminum-plastic cover with an aluminum cover washing machine, sterilize, dry, and take it out for use.
[0033] Preparation of medicinal solution: Weigh the prescribed amount of levocarnitine and sodium fructose diphosphate and add them to the water for injection, stir to dissolve, circulate and filter, adjust the pH to 5.0 with lactic acid, send samples to tes...
Embodiment 2
[0037] L-Carnitine 1000g
[0038] Fructose Bisphosphate 1800g
[0039] Lactic acid amount
[0040] Add water for injection to 3000ml
[0041] Preparation Process:
[0042] Bottle washing, sterilizing and drying: the vials are first cleaned by an ultrasonic bottle washing machine, then sterilized and dried by a sterilizing dryer, and sent to the filling room for later use.
[0043] Butyl rubber stopper treatment: After the butyl rubber stopper is cleaned by a rubber stopper cleaning machine, it is sterilized by steam at 121°C for 30 minutes, and dried for later use.
[0044] Aluminum-plastic cover treatment: wash the aluminum-plastic cover with an aluminum cover washing machine, sterilize, dry, and take it out for use.
[0045] Preparation of medicinal solution: Weigh the prescribed amount of levocarnitine and sodium fructose diphosphate and add them to the water for injection, stir to dissolve, circulate and filter, adjust the pH to 5.5 with lactic acid, send samples to te...
Embodiment 3
[0049] L-Carnitine 1000g
[0050] Fructose Bisphosphate 1000g
[0051] Lactic acid amount
[0052] Add water for injection to 3000ml
[0053] Preparation Process:
[0054] Bottle washing, sterilizing and drying: the vials are first cleaned by an ultrasonic bottle washing machine, then sterilized and dried by a sterilizing dryer, and sent to the filling room for later use.
[0055] Butyl rubber stopper treatment: After the butyl rubber stopper is cleaned by a rubber stopper cleaning machine, it is sterilized by steam at 121°C for 30 minutes, and dried for later use.
[0056] Aluminum-plastic cover treatment: wash the aluminum-plastic cover with an aluminum cover washing machine, sterilize, dry, and take it out for use.
[0057] Preparation of medicinal solution: Weigh the prescribed amount of levocarnitine and sodium fructose diphosphate and add them to the water for injection, stir to dissolve, circulate and filter, adjust the pH to 6.0 with lactic acid, send samples to te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com