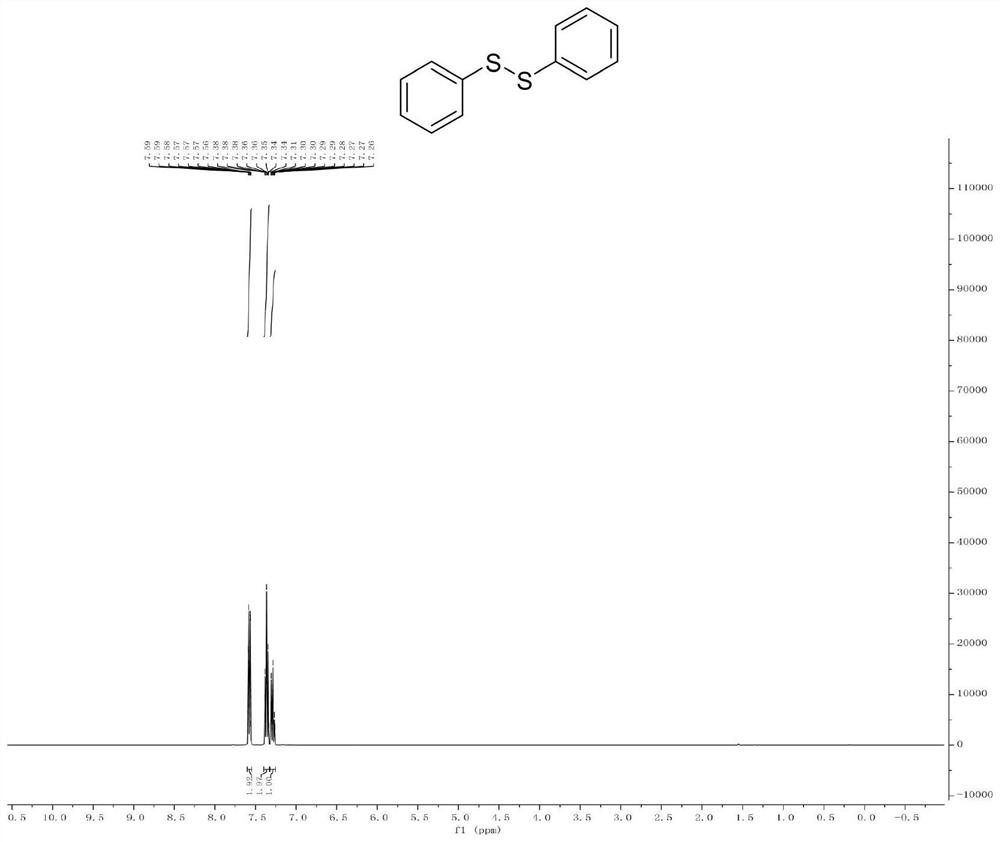
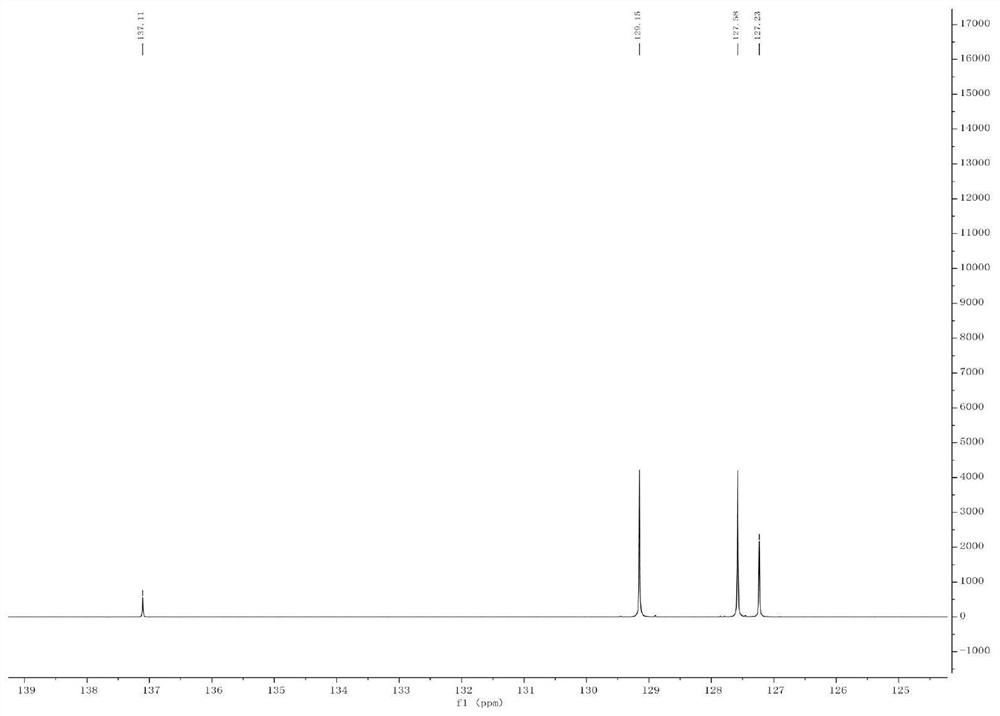
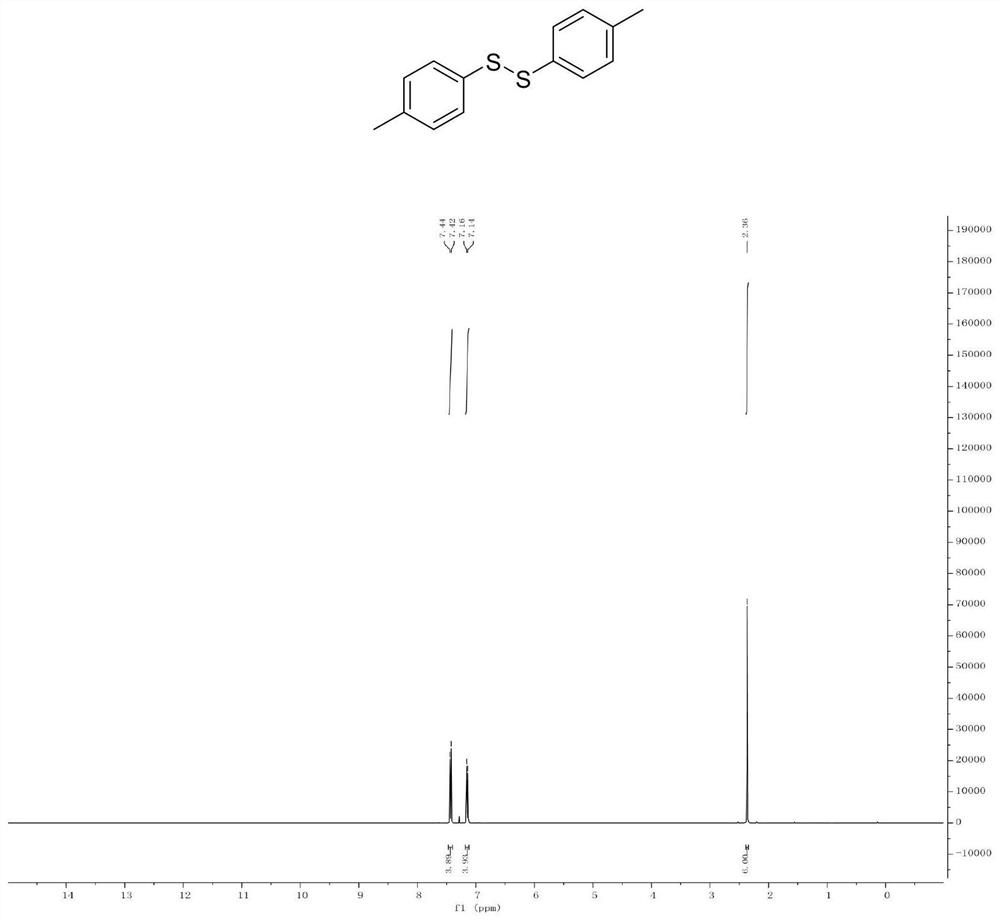
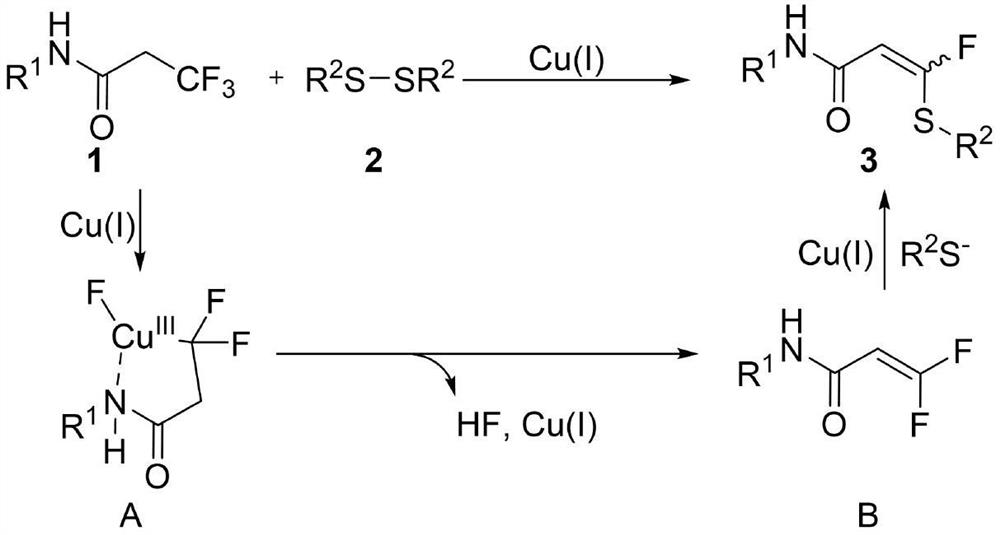
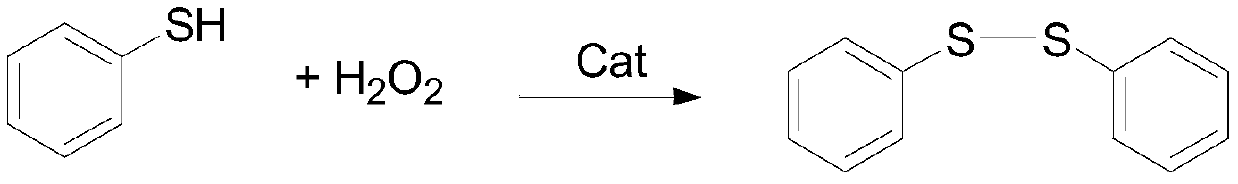
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Dithiete" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp²-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated.
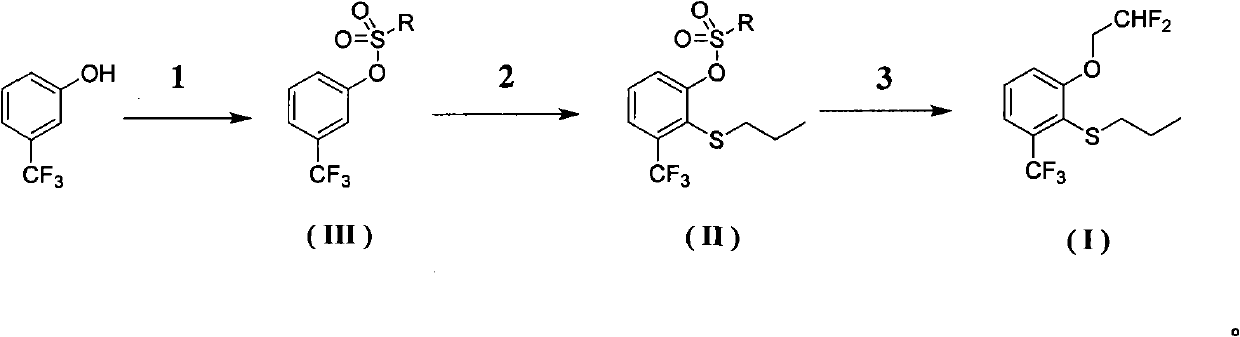
Preparation method of 2-(2', 2'-difluoroethoxyl)-6-trifluoromethyl phenyl propyl sulfide
InactiveCN102001979ASimple reaction conditionsOptimize the synthesis processSulfide preparationButyl lithiumDimethpyrindene
The invention provides a preparation method of 2-(2', 2'-difluoroethoxyl)-6-trifluoromethyl phenyl propyl sulfide, having the following processes: reacting m-trifluoromethyl phenol with p-toluenesulfonyl chloride, trifluoromethanesulfonic anhydride or 3, 5-dinitrobenzol sulfonyl chloride in a solvent containing organic base or a solvent containing triethylamine hydrochloride and organic base, andthen, collecting a compound having a formula (III) from reaction products, dissolving the compound in butylene oxide, adding tetramethyl ethylene diamine and diisopropylamine, at a temperature range from -25 DEG C to -100 DEG C, adding butyl lithium cyclohexane solution for reaction, and then, adding dipropyldisulfide, and collecting a compound having a formula (II); reacting the compound having the formula (II) with 4-nitrogen, nitrogen-dimethyl pyridine and difluoroethanol for catalytic reaction in the solvent, and then, collecting a compound having a formula (I) from the reaction products.The invention simplifies reaction conditions, optimizes the technology, reduces production cost, and improves synthesis effects. The general formula of the reaction is as follows.
Owner:SHANGHAI UNIV OF ENG SCI
Fine desulfurization method for liquid hydrocarbon
ActiveCN105885937AHigh regeneration rateTreatment with plural serial refining stagesFiberCarbonyl sulfide
The invention discloses a fine desulfurization method for liquid hydrocarbon. The method includes steps: a) removing hydrogen sulfide; b) removing carbonyl sulfide; c) removing mercaptan; d) water washing for alkali removal; e) removing disulfides; f) removing residual sulfurs. The method has advantages that 1) aiming at liquid hydrocarbons including liquefied gas, carbon 3, carbon 4, carbon 5 and the like containing different forms of sulfurs, MDEA (methyldiethanolamine) liquid and a fiber film contactor are adopted for extraction to realize hydrogen sulfide removal, and the hydrogen sulfide removal rate is larger than 99.9%; 2) the fiber film contactor and a carbonyl sulfide removing agent which is capable of hydrolyzing carbonyl sulfide and absorbing hydrogen sulfide generated in hydrolysis are adopted for extraction to realize carbonyl sulfide removal, and the carbonyl sulfide removal rate is larger than 99%; 3) by adoption of the complete desulfurization process, total sulfurs in liquid hydrocarbons can be reduced below 1ppm.
Owner:NINGBO ZHANGFU ENERGY TECH CO LTD
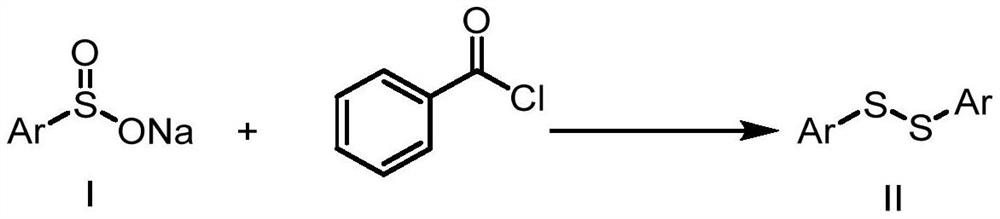
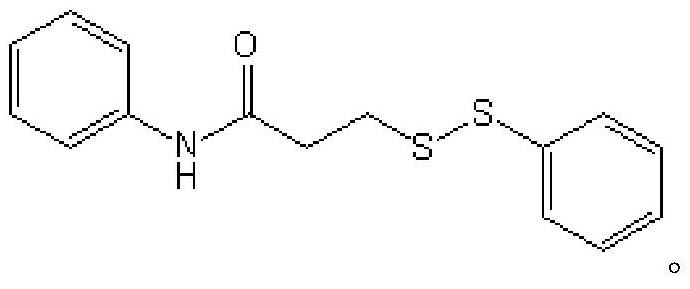
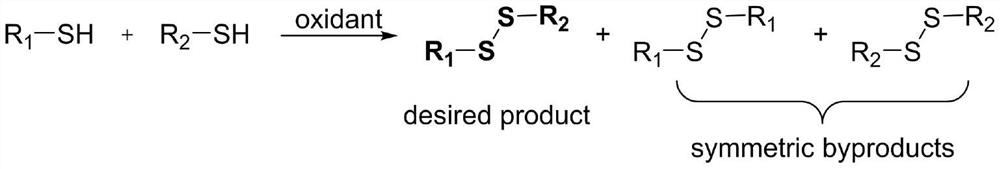
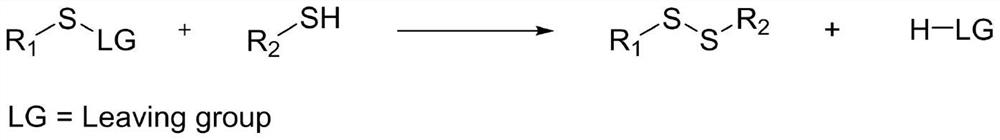
Method for preparing asymmetric disulfide
ActiveCN111777536AEfficient synthesisSimple and fast operationHydropoly/poly sulfide preparationPhenylsulfonamidePtru catalyst
The invention relates to the technical field of fine chemical engineering, and discloses a method for preparing asymmetric disulfide. The preparation method comprises the following specific steps: taking 3-methylthio-N-phenylpropionamide and diphenyl disulfide as raw materials, taking N-fluorobisbenzenesulfonamide as an additive, and preferably carrying out a reaction in an acetonitrile solvent under a heating condition so as to obtain a target product, namely the symmetric disulfide N-phenyl-3-(phenyldithioalkyl)propionamide. The method is simple and convenient to operate and mild in reaction, 3-methylthio-N-phenylpropionamide and symmetric diphenyl disulfide are used as reaction raw materials, the asymmetric disulfide product is efficiently synthesized in one step, the use of a thiol rawmaterial with unpleasant smell or a transition metal catalyst is avoided, and the method has a potential application value.
Owner:CHANGZHOU UNIV
Preparation method of asymmetric disulfide compound
ActiveCN112047902AImprove compatibilityEasy to operateHydropoly/poly sulfide preparationDisulfide bondingCombinatorial chemistry
The invention relates to a synthetic method of an asymmetric disulfide compound. According to the method, mercaptan which is convenient and easy to obtain and symmetrical disulfide compounds are usedas raw materials, and the asymmetric disulfide compounds are efficiently synthesized under the catalytic action of palladium salt or copper salt. The method has the advantages of accessible raw materials, simple catalytic system, high functional group compatibility, high yield and the like, and is convenient to operate. The method is particularly suitable for selectively introducing disulfide bonds into complex substrates, and can be widely applied to the industries of medicines, foods and the like.
Owner:SHANGHAI UNIV
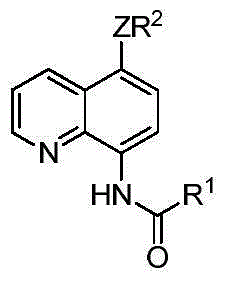
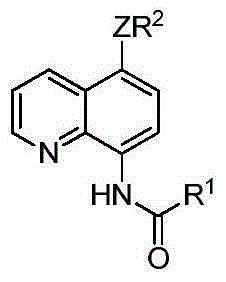
5-substituted sulfur etherified/selenium etherified/tellurium etherified quinoline compound and preparation method therefor
The invention relates to a 5-substituted sulfur etherified / selenium etherified / tellurium etherified quinoline compound and a preparation method therefor. The preparation method is characterized by comprising: carrying out a reaction at a certain temperature for a certain time to obtain the 5-substituted sulfur etherified / selenium etherified / tellurium etherified quinoline compound with a high yield and high selectivity by taking quinoline, diaryl(alkyl) disulfide (R2SSR2) or diaryl(alkyl) diselenide (R2SeSeR2) or diaryl(alkyl) ditelluride (R2TeTeR2) as raw materials, copper salt that is low in price and is easily available as a catalyst and a common oxidant as an oxidant and a common organic solvent as a reaction solvent. The method has the advantages of being relatively low in cost, high in yield, simple to operate, less in pollution and the like, and has certain feasibility for realizing industrial production.
Owner:HUNAN UNIV
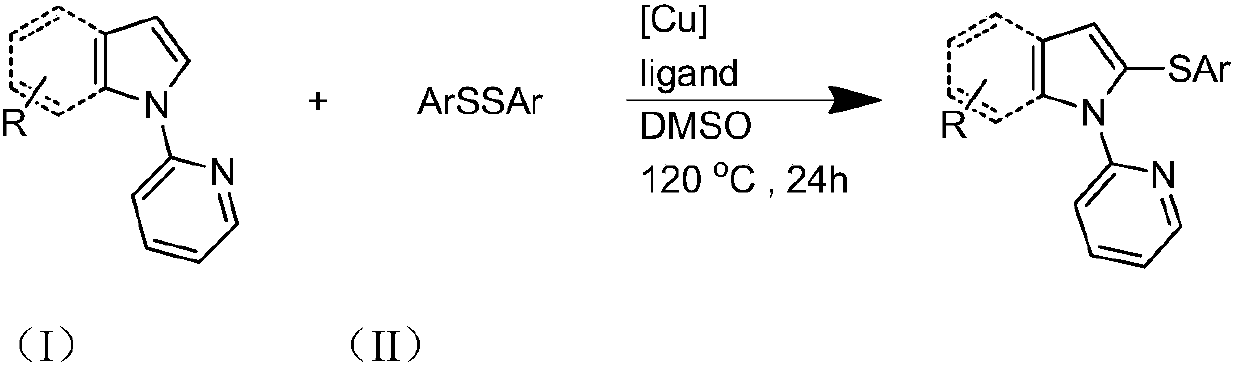
Preparation method and purification method of heterocyclic nitrogen sulfide
The invention discloses a preparation method and a purification method of heterocyclic nitrogen sulfides. The preparation method includes the steps: taking N-pyridyl pyrrole or pyridyl indole and disulfide ether as substrates, taking dimethyl sulfoxide as solvents, performing heating reaction on the N-pyridyl pyrrole or pyridyl indole, the disulfide ether and the dimethyl sulfoxide under catalysisof transition metal copper salt and the action of a ligand in the air environment to prepare crude products, and performing filtration and solvent removal on the crude products to obtain remainders;performing silica column chromatography on the remainders, spraying and washing the remainders by the aid of eluting liquid, collecting effluents, combining the effluents containing products, performing concentration and solvent removal on the combined effluents, and performing vacuum drying to obtain a target product. The preparation method has the advantages of simple technological process, lowcost and high yield.
Owner:WENZHOU UNIVERSITY
Moisture curable polydisulfides
InactiveCN102498157AGroup 4/14 element organic compoundsOther chemical processesEndcappingPolymer science
Provided are polydisulfides that are useful in moisture curable sealants. The polydisulfides have an S-S link in the backbone and are end-capped with at least one alkoxysilane functional group. Also provided are methods of making the polydisulfides, including methods that do not require the presence of a catalyst.
Owner:HENKEL CORP +1
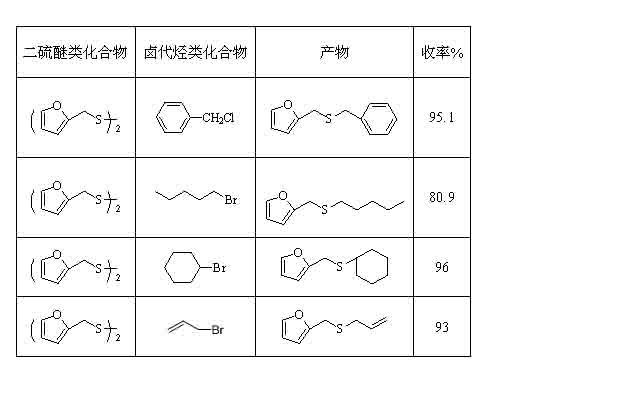
Method for synthetizing mono-thioether compound
InactiveCN102127038ALess investmentSimple and safe operationSulfide preparationHalohydrocarbonPtru catalyst
The invention relates to a method for synthetizing mono-thioether compound. The technical scheme adopted by the invention is as follows: in the presence of carbon monoxide and water, disulfide compound and halogenated hydrocarbon compound are used as raw materials, selenium is used as catalyst, organic base or inorganic base is used as cocatalyst or any cocatalyst is not added, the raw materials react in organic solvent at 20-100 DEG C under atmospheric pressure for 1-24 hours, the product is cooled to the room temperature, then carbon monoxide is displaced by air, stirring is performed for 0.2-2 hours, filtration is performed, distilled water and cyclohexane are used to extract the filtrate, and the solvent in the extract liquor is distilled out through reduced pressure distillation to obtain the target product. The method is convenient and safe to operate, adopts one-pot reaction, has common raw materials, no pollution, high selectivity and high yield; and the catalyst can be separated and recycled after the reaction.
Owner:LIAONING UNIVERSITY
Method for preparing baloxavir intermediate and intermediate obtained by method
ActiveCN112062750AReduce usageSuitable for industrial productionOrganic chemistryChemical synthesisGrignard reagent
The invention belongs to the technical field of chemical synthesis, and provides a method for preparing a balosavir intermediate. The method comprises the following steps: (1) methylating 3,4-difluorobromobenzene to obtain a compound A-1; (2) reacting the compound A-1 with a Grignard reagent, then introducing carbon dioxide gas for reaction, and adding acid for acidification to obtain a compound A-2; (3) brominating the compound A-2 to obtain a compound A-3; (4) adding diphenyl disulfide and a reducing agent 1 into THF, dropwise adding methanol, dropwise adding the compound A-3, and reacting to obtain a compound A-4; (5) carrying out ring closing reaction on the compound A-4 in polyphosphoric acid to obtain a compound A-5; and (6) reducing the compound A-5 into alcohol by adopting a reducing agent 2 to obtain a compound A-6. According to the method, highly toxic and foul thiophenol is prevented from being used while expensive reagents are prevented from being used, so that the processcost is reduced. The invention also provides an intermediate compound prepared by the method.
Owner:HEADING NANJING PHARMTECH CO LTD
Composite metal photocatalysis system and preparation method and application thereof
ActiveCN110975940AFast transferEfficient transferOrganic-compounds/hydrides/coordination-complexes catalystsOrganic free radical generationPtru catalystCycloaddition
The invention discloses a composite metal photocatalysis system which is represented by Ni-M-X-Y-Z, wherein M is selected from the group consisting of Al, Ag, Bi, Cd, Ge, Sb and Sn; x is selected fromthe group consisting of triphenylphosphine, triethylphosphine, tributylphosphine, tri-tert-butylphosphine and tricyclohexylphosphine; Y is selected from the group consisting of carbon nanorods, molecular sieves, ordered mesoporous carbon and silicon dioxide; Z is selected from the group consisting of diphenyl disulfide, dicumyl peroxide, hydrazine and 2,4-dinitrophenylhydrazine; the mass fractionof Ni is 10-20%; the mass fraction of M is 20-30%; the mass fraction of X is 20-40%; the mass fraction of Y is 15-40%,; and the mass fraction of Z is 10-30%. The catalytic system is used for preparing 1,1,2,3,3-pentamethylindane through free radical cycloaddition of alpha-methylstyrene and 2-methyl-2-butene, an conversion rate is larger than 90%, and selectivity is larger than 85%; and a catalystis stable and not prone to loss in the free radical cycloaddition reaction. A method provided by the invention is easy to operate and good in economic benefit.
Owner:WANHUA CHEM GRP CO LTD
Method for generating C-S bond by decarboxylating active ester compound
ActiveCN113402430AWide range of optionsSimple structureChemical recyclingSulfide preparationOrganic solventOrganic base
One aspect of the invention provides a method for generating a C-S bond by decarboxylating an active ester compound. The method comprises the following steps: under an illumination condition, active ester with a general formula of Ra-COONPhth and disulfide with a general formula of Rb-S-S-Rc are subjected to the following reaction: Ra-COONPhth + Rb-S-S-Rc -> Ra-S-Rb in a liquid environment provided by an organic solvent containing organic alkali under the catalysis of a photocatalyst, wherein in Ra-S-Rb, Ra and S atoms are bonded through C-S, the photocatalyst is Ru(bpy)2Cl2.6H2O, and Rb and Rc are independently selected from one of alkyl, optionally substituted alkyl, polyheterocycle or optionally substituted polyheterocycle, and both Rb and Rc are aromatic groups. The selectable range of reactants in the reaction is wide, and alkyl sulfides with various structures can be prepared through the reaction.
Owner:HAINAN NORMAL UNIV
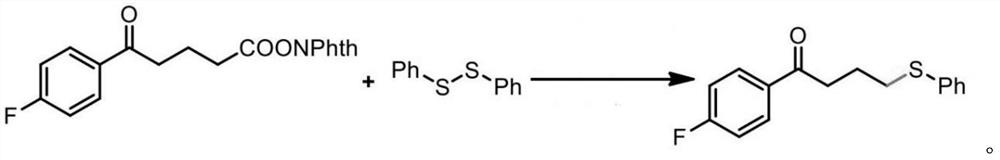
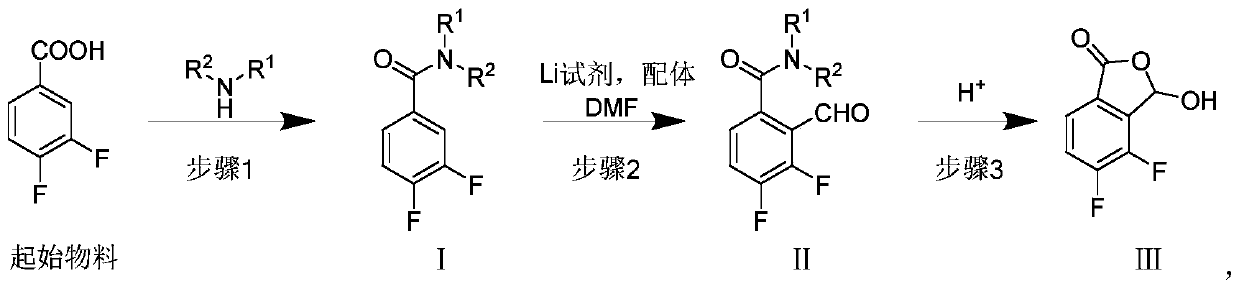
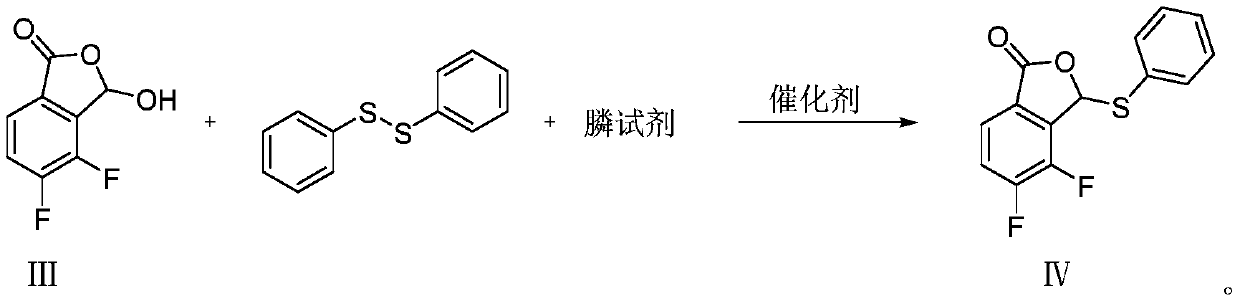
Synthesis method of baloxavir marboxil intermediate
ActiveCN110143941AHigh yieldReduce manufacturing costOrganic chemistrySynthesis methodsCombinatorial chemistry
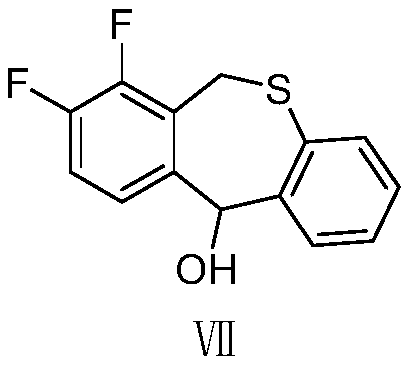
The invention provides a synthesis method of a baloxavir marboxil intermediate. The invention provides a synthesis method of a compound (III), which is prepared through three steps. The yield is improved, and the production is easy to control. At the same time, a synthesis method of a compound (IV) is provided. According to the synthesis method, the compound (III) and disulfide diphenyl carry outone-pot self-catalytic circulation reactions to generate the compound (IV). The invention provides a method for preparing a baloxavir marboxil intermediate (VIII) through the reactions mentioned above. Through the one-pot self-catalytic circulation reactions, thiophenol, which is strongly toxic and is strictly controlled, is not used, a safe baloxavir marboxil synthesis method is provided, and thesynthesis method is suitable for industrial production.
Owner:BEIJING SIHUAN PHARMA +2
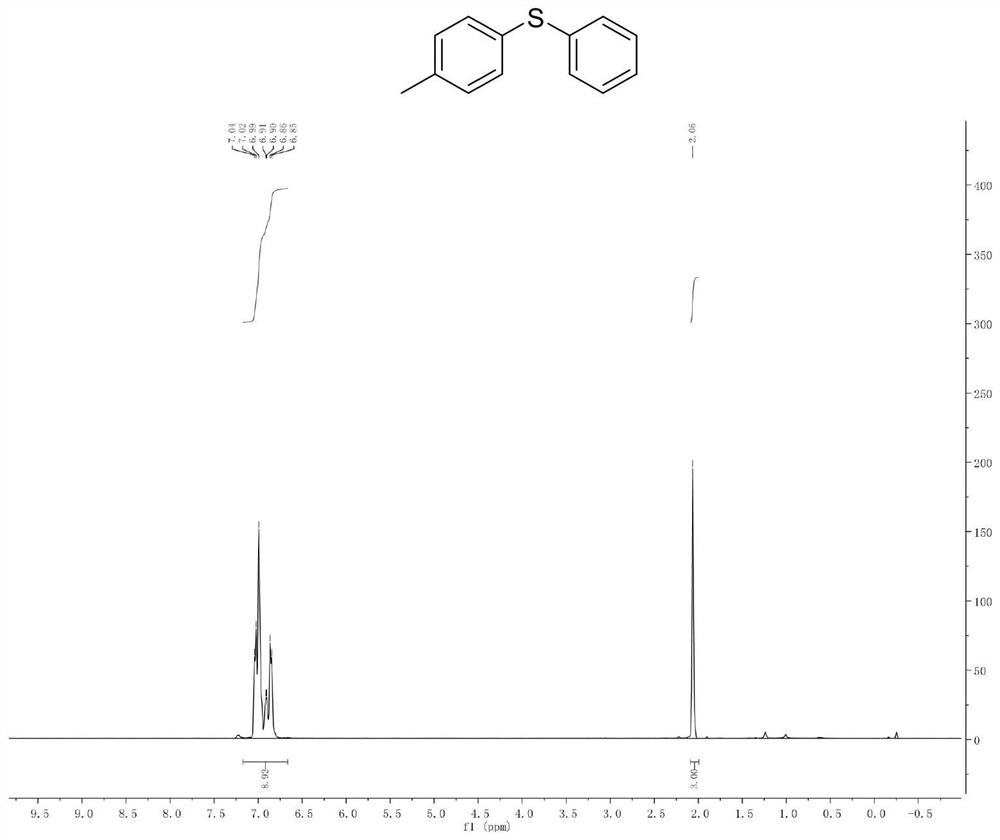
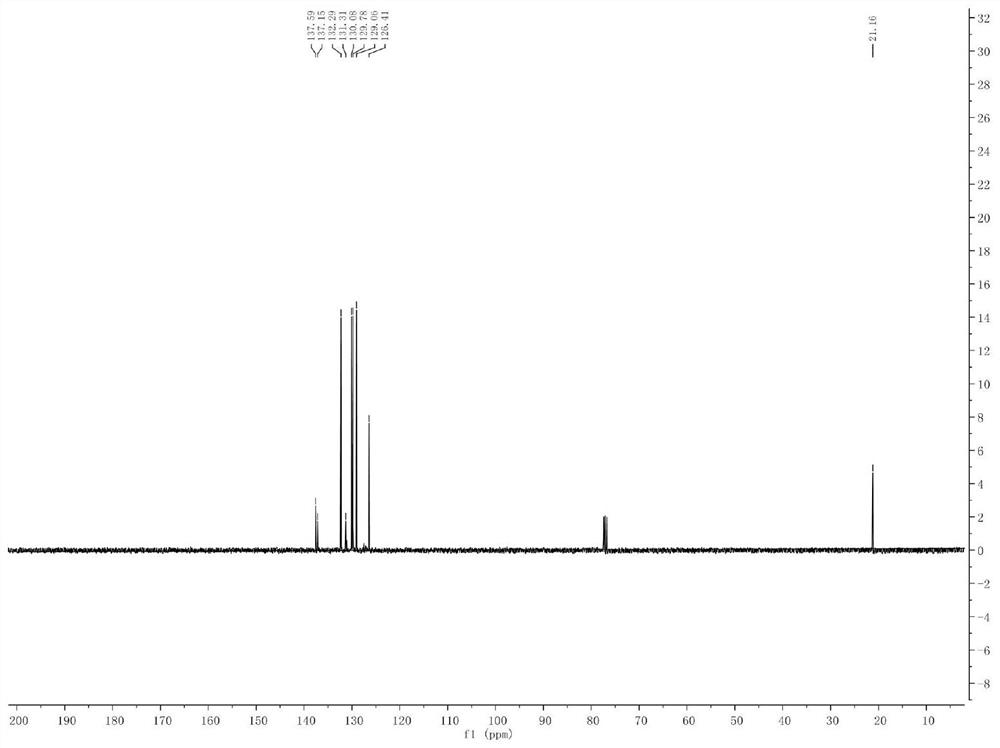
Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound
ActiveCN112442002AHigh industrial practical valueSimple and fast operationOrganic chemistryThiosulfonic AcidsThio-
The invention discloses a method for synthesizing a 11-sulfenylnaphtho[2,3-b]benzofuran compound. The method is characterized in that 2-ethyleneoxyphenylacetylene and thiosulfonate are used as substrates, disulfide is used as an additive, Na2-Eosin Y is used as a photosensitizer, and light-irradiation stirring is performed under inert gas and heating conditions to obtain the product 11-sulfenylnaphtho[2,3-b]benzofuran. The method has the advantages of no need for transition metal addition in reactions, simple operation, considerable yield, environmental protection performance and good application prospects.
Owner:GUILIN MEDICAL UNIVERSITY
Preparation method of diphenyl disulfide compounds
ActiveCN111763163AImprove conversion rateLow costOrganic compound preparationMagnesium organic compoundsGrignard reagentDiphenyl disulfide
The invention discloses a preparation method of diphenyl disulfide compounds. The preparation method comprises the following steps: stirring an isopropyl magnesium halide Grignard reagent and a substituted halogen benzene compound in an organic solvent at -78 DEG C to -20 DEG C for 30-90 minutes to obtain a thoroughly halogen-magnesium exchanged substituted phenyl Grignard reagent; and adding dichlorodisulfide into the reaction system, slowly heating to room temperature after the reaction is finished, quenching the reaction by using a saturated ammonium chloride aqueous solution, extracting byusing ethyl acetate or diethyl ether, drying by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the diphenyl disulfide compounds. According to the method, the diphenyl disulfide compounds are prepared by taking the phenyl Grignard reagent as a raw material through a one-pot method, and has the following advantages: the synthetic route is short, the preparation process is simple, the cost is low, the operation is easy, the yield is excellent, and the industrial production is easy.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for synthesizing alpha-fluorinated thioacrylamide derivative
ActiveCN112341368ASimple processSimple and fast operationSulfide preparationRotary evaporatorPtru catalyst
The invention relates to a method for synthesizing an alpha-fluorinated thioacrylamide derivative. The method comprises the following steps that: with trifluoroacrylamide taken as a reaction substrate, disulfide as a sulfur source, cuprous iodide as a catalyst, potassium carbonate as alkali, triphenylphosphine and water as additives and tetrahydrofuran as a solvent, the components are stirred andreact with one another for 12 hours at 80 DEG C; and after the reaction is finished, a reaction liquid is filtered, a solvent is removed from filtrate by using a rotary evaporator, so that residues can be obtained, column chromatography separation is carried out on the residues by using a silica gel column, leaching is performed with eluent, effluent containing a target product is collected, the effluent is merged, a solvent is removed through vacuum concentration, so that the target product is obtained. The method has the advantages of simple and easily available raw materials, relatively mild reaction conditions, novel and simple preparation process, less pollution and low energy consumption.
Owner:WENZHOU UNIVERSITY +1
Etching fluid, replenishing fluid, and method for forming copper wiring
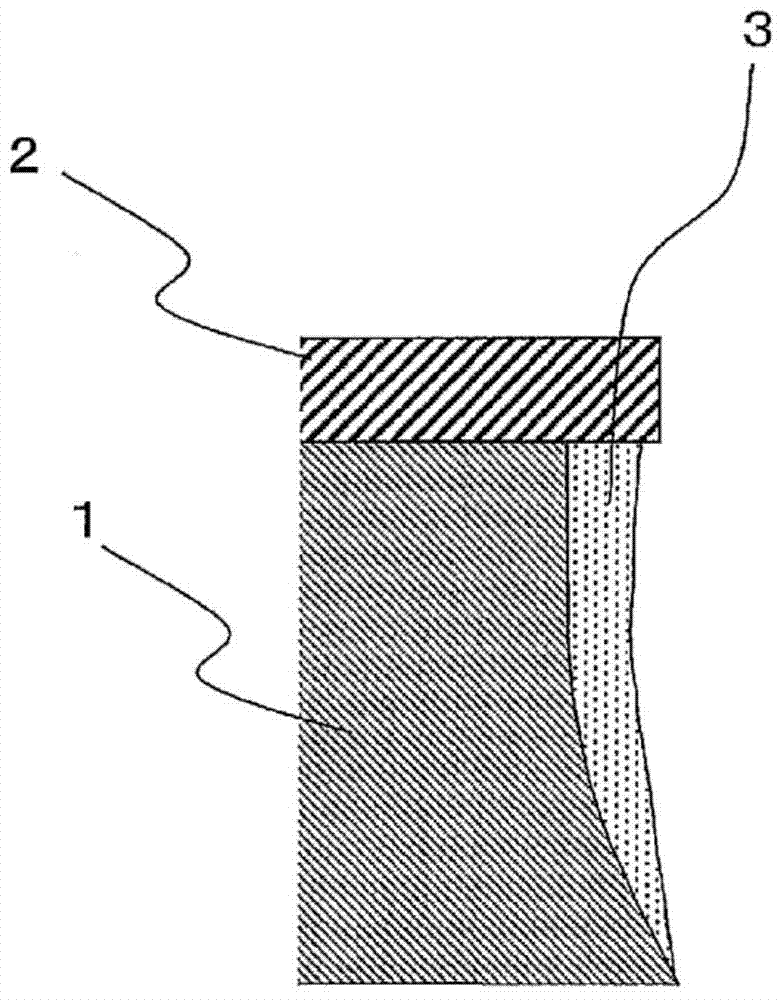
InactiveCN104769159ASuppression of side erosionWithout compromising linearityConductive material chemical/electrolytical removalHeteroatomCopper-wiring
Provided are an etching fluid that is capable of suppressing side etching while not impairing the linearity of copper wiring, a replenishing fluid for the same, and a method for forming copper wiring. This etching fluid is a copper etching fluid characterized in being an aqueous solution containing acid, oxidizing metal ions, and a compound (A), the compound (A) having in molecules thereof an amino group and at least one sulfur-containing functional group selected from the group consisting of thiole groups, sulfide groups, and disulfide groups (where the sulfide and disulfide groups are groups in which the sulfur atoms and the heteroatoms linked thereto are linked by a single bond, and which do not form π conjugates).
Owner:MEC CO LTD
NBR composition for rubber laminated metal
ActiveCN111386307AImprove the extrusion effectVibration damping characteristics maintainedEngine sealsNoise/vibration controlPolymer scienceThio-
An NBR composition for rubber laminated metal, having mixed therein, 1-3 parts by weight sulfur and 1-15 parts by weight of a disulfide compound such as a 4,4'-dithiodimorpholine, dithiocaprolactum indicated by general formula RN-S-S-NR (RN being a cyclic linking group formed together with an N atom bonded to an S atom), relative to 100 parts by weight NBR. This NBR composition for rubber laminated metals is capable of improving rubber layer protrusion properties, when used as a rubber laminated metal plate, e.g., a brake shim being a laminated composite metal that has been laminated on to a gasket or another metal plate.[0]
Owner:NOK CORP
Method for synthesizing disulfide compound through concerted catalysis of visible light and titanocene complex
ActiveCN113387855AWide applicabilityInhibits the cross-coupling processHydropoly/poly sulfide preparationPhoto catalyticIsopropyl
The invention discloses a method for synthesizing a disulfide compound through concerted catalysis of visible light and a titanocene complex. According to the method, visible light and a reduction quenching agent N, N-diisopropylethylamine or triethylamine are used for reducing a titanocene (IV) complex into a titanocene (III) complex, the cross coupling process of sulfur free radicals and acyl free radicals is inhibited, and self-coupling of sulfur free radicals is promoted to generate the disulfide compound. The method disclosed by the invention is green and environment-friendly, has certain substrate applicability, is also beneficial to further exploring the selective coupling process of free radicals, and expands the application of titanocene in the field of photocatalysis.
Owner:SHAANXI NORMAL UNIV
Resist underlayer film formation composition having disulfide structure
PendingCN111670410AHigh dry etching speedMicrofabricationSemiconductor/solid-state device manufacturingPhotomechanical coating apparatusPolymer scienceDevice material
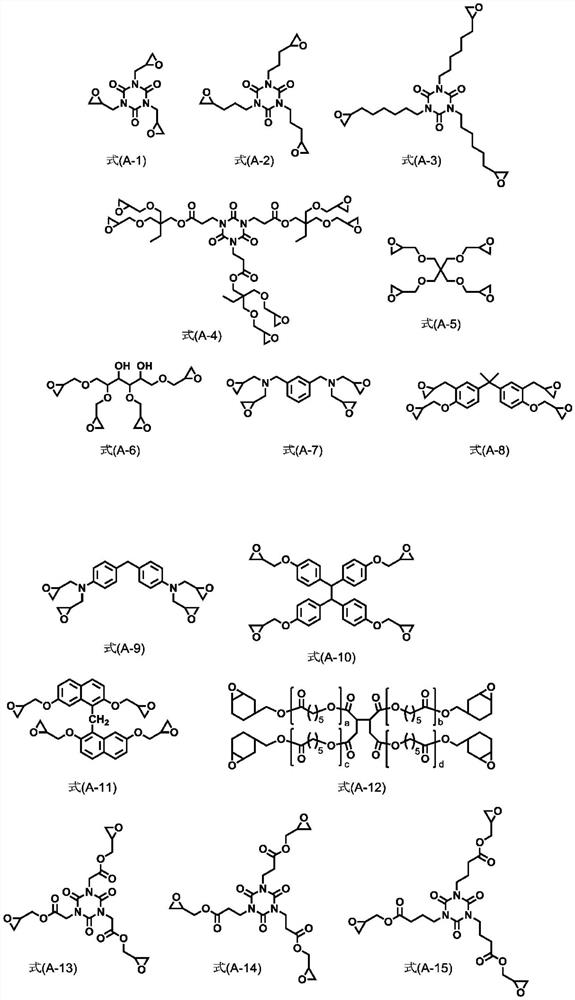
Provided are: a resist underlayer film having, in particular, a high dry etching speed; the resist underlayer film formation composition; a resist pattern formation method, and a method for manufacturing a semiconductor device. The resist underlay film formation composition contains: a bifunctional or higher compound having one or more disulfide bonds; a trifunctional or higher compound and / or a reaction product; and a solvent. The bifunctional or higher compound is preferably a dicarboxylic acid containing a disulfide bond. The trifunctional or higher compound is preferably a compound containing three or more epoxy groups.
Owner:NISSAN CHEM CORP
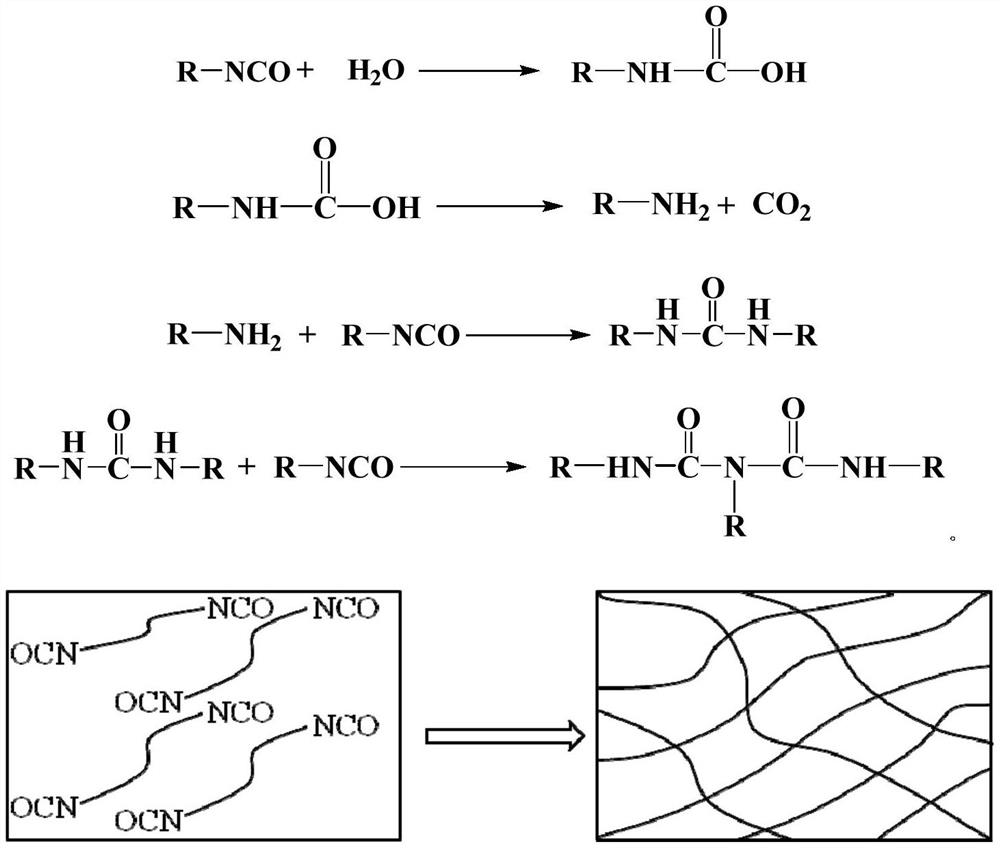
Heat-detachable polyurethane hot melt adhesive, preparation raw materials, and preparation method and bonding method of heat-detachable polyurethane hot melt adhesive
PendingCN114044868AReduce bond strengthWill not automatically disengagePolyureas/polyurethane adhesivesAdhesive processes with adhesive heatingAdhesive cementPolymer science
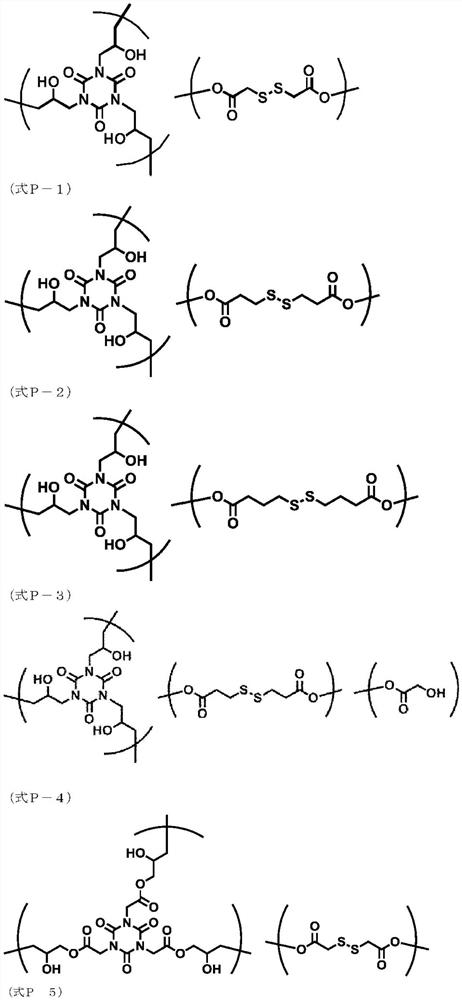
The invention belongs to the field of polyurethane adhesives, and relates to a heat-detachable polyurethane hot melt adhesive, preparation raw materials, and a preparation method and a bonding method of the heat-detachable polyurethane hot melt adhesive. The preparation raw materials for the detachable polyurethane hot melt adhesive comprise a polyol compound, a polyisocyanate compound, a sulfur-containing compound and a catalyst in a mass ratio of 100: (35-120): (2.5-150): (0.1-2), wherein the sulfur-containing compound is at least one selected from 2,2-dithiodiethanol, 4,4-dihydroxy diphenyl disulfide, 4,4-diaminodiphenyl disulfide and liquid polysulfide resin. The polyurethane hot melt adhesive prepared from the raw materials can be completely detached at a high temperature and cannot be automatically separated at the high temperature, so the aim that a bonded part can be detached under a heating condition is perfectly fulfilled.
Owner:XIAMEN WELDTONE TECH CO LTD
Preparation method of aryl monothioether compound
ActiveCN111635343AReduce manufacturing costQuick responseMercapto/sulfide group formation/introductionSulfide preparationHalohydrocarbonGrignard reagent
The invention discloses a preparation method of an aryl monothioether compound. The method comprises the following steps: reacting halogenated hydrocarbon with an isopropyl magnesium halide Grignard reagent at -20 DEG C for 30 minutes until a halogen-magnesium exchange reaction is completely completed; cooling a reaction solution to -78 DEG C, slowly dropwise adding a tetrahydrofuran solution of asubstituted diphenyl disulfide compound into the newly prepared Grignard reagent, maintaining the concentration of the reactants to be 0.5-1 mmol / mL, carrying out a stirring reaction for 1 hour in anorganic solvent at -78 DEG C, and then slowly heating the reaction solution to room temperature; and carrying out a quenching reaction by using a saturated ammonium chloride solution, extracting an organic phase by using ethyl acetate or diethyl ether, drying the organic phase by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the aryl thioether compound. The method is simple in preparation process, low in cost, high in speed, easy to operate and small in environmental pollution, groups sensitive to Grignard reagents can also be tolerated, and high yield is obtained.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
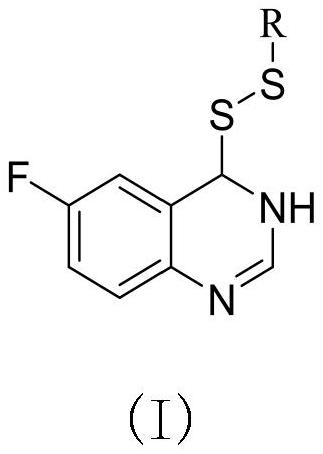
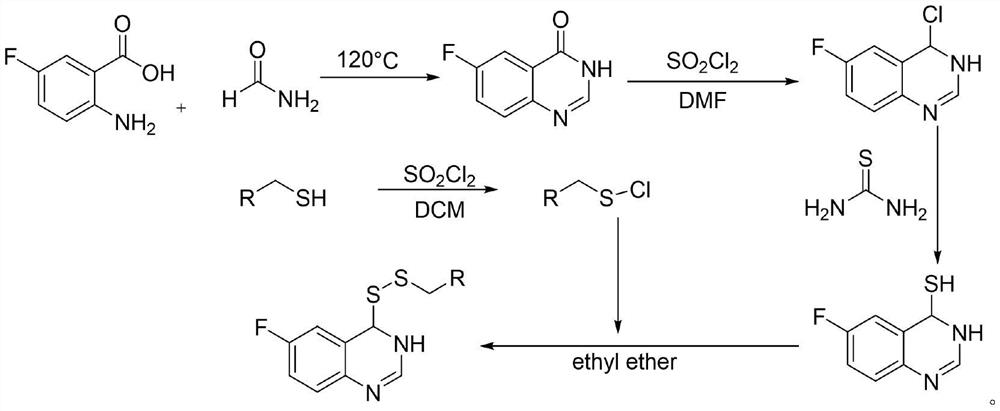
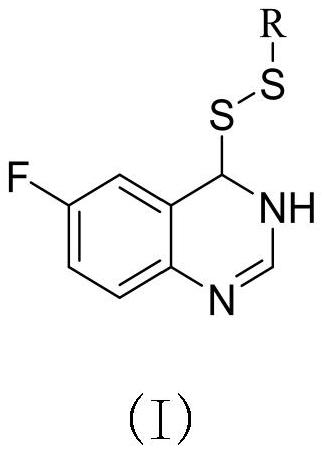
Preparation method and application of 6-fluoroquinazoline derivatives containing disulfide structures
PendingCN113754595AEnhanced inhibitory effectBiocideOrganic chemistryXanthomonas axonopodisActinidia
The invention relates to a preparation method and application of 6-fluoroquinazoline derivatives containing disulfide structures. The compound has a structure as shown in a general formula (I) which is described in the specification. According to the invention, 6-fluoroquinazoline is taken as a parent structure, disulfanyl (heterocyclic) structures are introduced into a system, so a series of 6-fluoroquinazoline derivatives containing the disulfide structures are synthesized; and the series of compounds have a very good inhibition effect on plant pathogenic bacteria such as rice bacterial leaf blight, ralstonia solanacearum, Pseudomonas syringae pv. actinidiae, Xanthomonas axonopodis pv. citri and the like.
Owner:GUIZHOU UNIV
Preparation method of disulfide compound 3,3'-dithiodipropionic acid
InactiveCN105085343AHigh yieldReduce pollutionHydropoly/poly sulfide preparationIodine solutionsSodium hydrosulfide
A preparation method of a disulfide compound 3,3'-dithiodipropionic acid comprises the steps of: subjecting the raw materials of sodium hydrosulfide, acrylonitrile, iodine solution and acid to Michael addition, acidification, oxidation and hydrolysis; and recrystallizing and purifying to obtain the disulfide compound 3,3'-dithiodipropionic acid. Compared with the prior art, the method has the beneficial effects of safety, simpleness, green, environment-friendliness, simple technology, high yield and low production costs.
Owner:SHANXI QIYOU BUILDING MATERIALS TECH CO LTD
Dynamic crosslinking degradable epoxy resin and preparation method and high-temperature leaking stoppage application thereof
PendingCN114573792AAchieve self-blockingPlastic recyclingDrilling compositionDisulfide bondingPolymer science
The invention discloses dynamic crosslinking degradable epoxy resin and a preparation method and high-temperature leaking stoppage application thereof. The preparation method of the dynamic crosslinking degradable epoxy resin comprises the following steps that an epoxy monomer, a curing agent and an auxiliary are heated, mixed, heated and cured, a solid product is obtained, the epoxy monomer is a monomer containing 2-4 epoxy groups, the curing agent is anhydride, polyamine and / or a curing agent containing disulfide bonds, and the auxiliary is a solvent. The auxiliary agent is at least one of zinc acetate, acetylacetone, 1, 8-diazabicyclo [5.4. 0] undec-7-ene, diphenyl disulfide, phenol, 2-amino-2-methyl-1-propanol and ethylene glycol amine. According to the invention, the dynamically cross-linked (dynamic sulfur-sulfur bond and dynamic ester exchange bond cross-linked) epoxy resin is prepared to prepare the high-temperature leaking stoppage material capable of being used at 150-210 DEG C, and the obtained material can be gradually degraded at high temperature and lose pressure-bearing leaking stoppage capability, so that the purposes of self-plugging removal and leakage layer protection are achieved.
Owner:CNOOC TIANJIN BRANCH +1
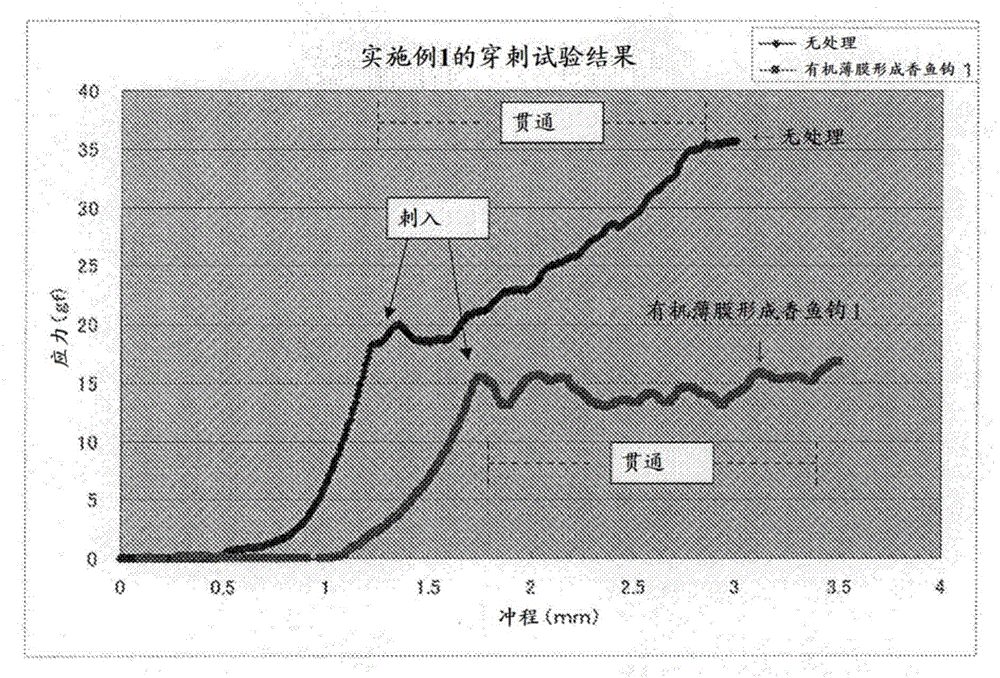
Fishhook
InactiveCN106714553AReduce stressGood fishing effectFish-hooksMetallic material coating processesThiolActive agent
The purpose of the present invention is to provide a fishhook for fishing etc. that has excellent durability and with which it is easy to impale fish etc. The invention involves treating the surface of a fishhook with a compound selected from the group consisting of metal-based surfactants including at least one hydroxyl group or hydrolyzable group, fluorine-based surface-treating agents, thiol compounds, and disulfide compounds.
Owner:NIPPON SODA CO LTD +1
Photocatalytic synthesis method of thioether compound
PendingCN114292220AEasy to operateMild conditionsSulfide preparationBulk chemical productionOrganic synthesisCarboxylic acid
The invention belongs to the field of organic synthesis, and particularly relates to a photocatalytic synthesis method of a thioether compound. The method comprises the following steps: adding a carboxylic acid compound, a non-toxic and odorless vulcanizing reagent disulfide compound, a photocatalyst and alkali into a solvent, reacting under inert gas protection and illumination conditions, and after the reaction is finished, performing post-treatment to obtain the thioether compound as shown in the formula. According to the method, reagents and substrates are simple, economical, cheap and easy to obtain, thiophenol reagents with pungent odor in a traditional process are abandoned, and a coupling reaction of C-S bonds is completed through decarboxylation by utilizing a metal-free visible light catalysis system. The method is simple in reaction operation, mild in condition, efficient in reaction and good in substrate adaptability, coupling of various substrates with different groups can be achieved, the C-S bond compound is selectively and efficiently constructed in one step, and the compound has great application prospects in medicine synthesis intermediates and has very important significance in construction of complex sulfur-containing compounds.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of diaryl disulfide
ActiveCN110776446AImprove conversion rateHigh yieldMagnesium organic compoundsHydropoly/poly sulfide preparationArylGrignard reagent
The invention relates to a preparation method of diaryl disulfide. The method comprises the following steps: reacting halogenated aromatic hydrocarbon with magnesium powder to generate a Grignard reagent, adding sulfur powder, and carrying out a reaction under the action of an acid and an oxidant to obtain the diaryl disulfide. The aromatic disulfide is synthesized by using the cheap and easily available halogenated aromatic hydrocarbon as a synthesis raw material, and then reacts with the magnesium powder to generate the Grignard reagent, and the Grignard reagent reacts with the sulfur powderunder the action of the acid and the oxidant to obtain the diaryl disulfide. The whole method has the advantages of realization of direct synthesis without heavy metal catalysis or complex ligands inthe process and midway discharging, environmental friendliness, high conversion rate, high yield, high purity, simple process, convenience in operation, and suitableness for large-scale production.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
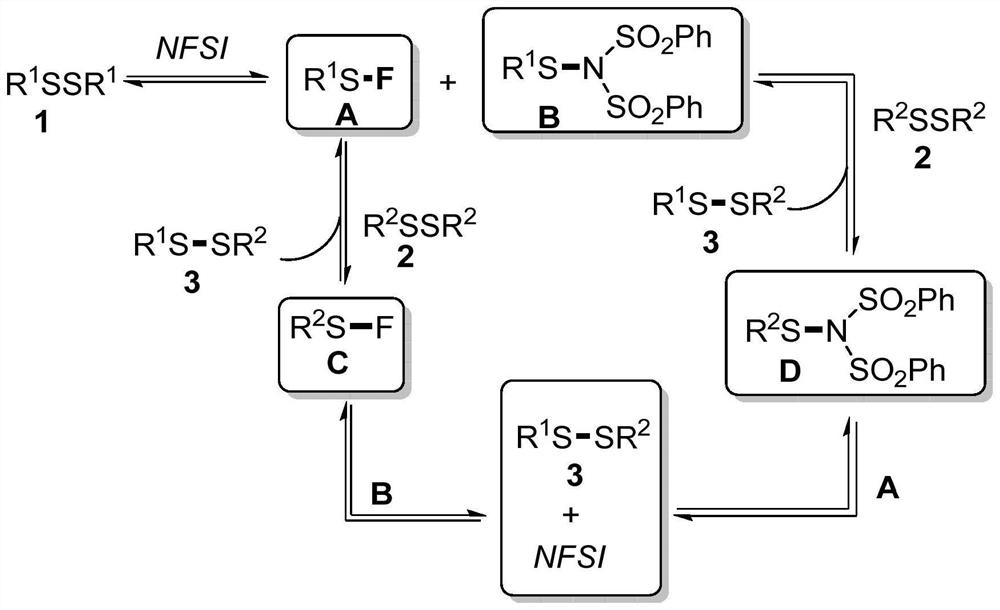
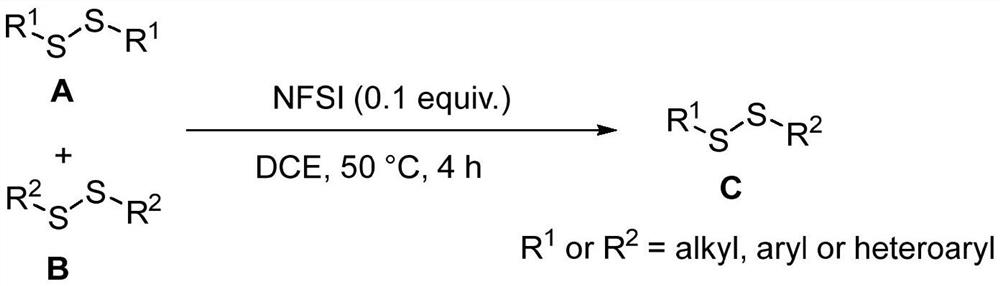
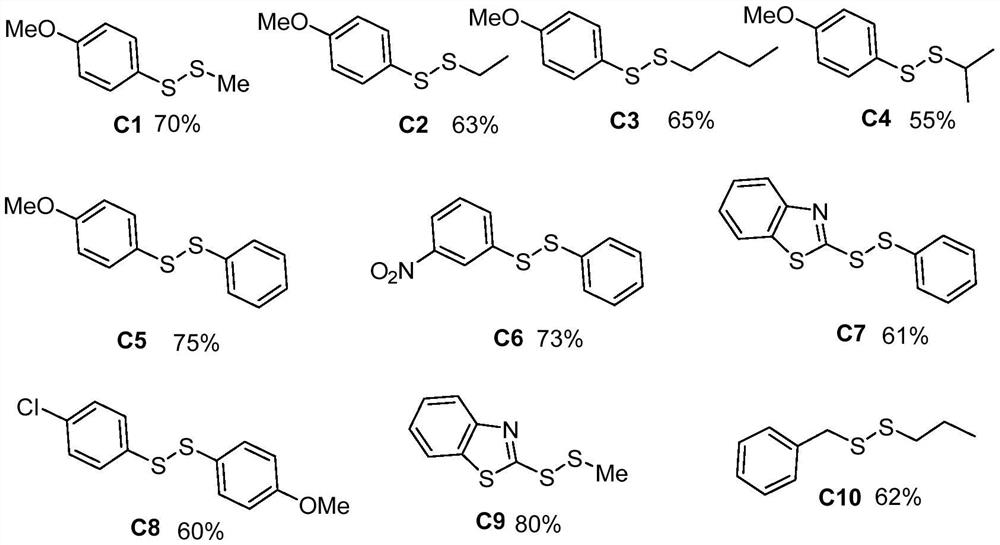
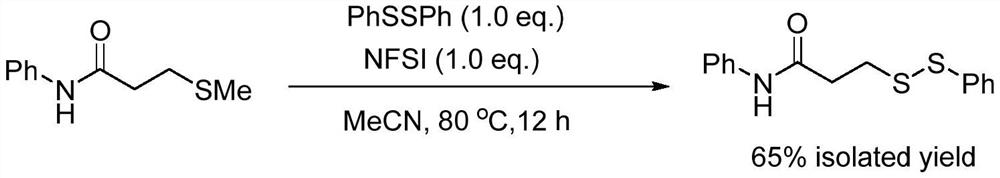
Method for catalytically synthesizing asymmetric disulfide derivative by NFSI
ActiveCN113563241AEfficient synthesisHydropoly/poly sulfide preparationPtru catalystCombinatorial chemistry
The invention belongs to the field of fine chemical engineering, and relates to a method for catalytically synthesizing an asymmetric disulfide derivative by NFSI. The method comprises the steps of adding NFSI, symmetric disulfide A and symmetric disulfide B into a sealed tube containing a reaction solvent 1, 2-dichloroethane, reacting at the temperature of 25-80 DEG C for 1-8 hours, ending the reaction, and separating and purifying to obtain the asymmetric disulfide derivative, wherein the molar ratio of the NFSI to the symmetric disulfide A to the symmetric disulfide B is 0.1: 1.0: 1.0. According to the method, the asymmetric disulfide derivative can be efficiently synthesized without using an expensive rhodium metal catalyst and odorous mercaptan.
Owner:CHANGZHOU UNIV
Three-dimensional and large-steric-hindrance disulfide compound as well as synthesis method and application thereof
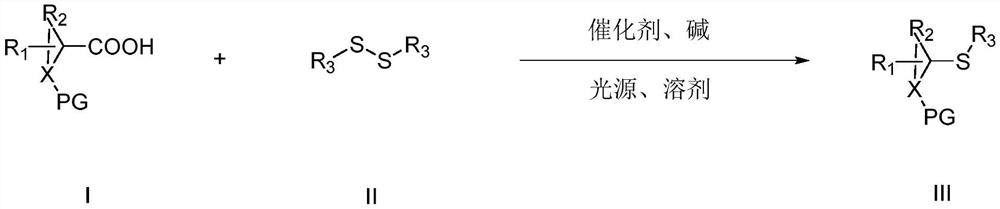
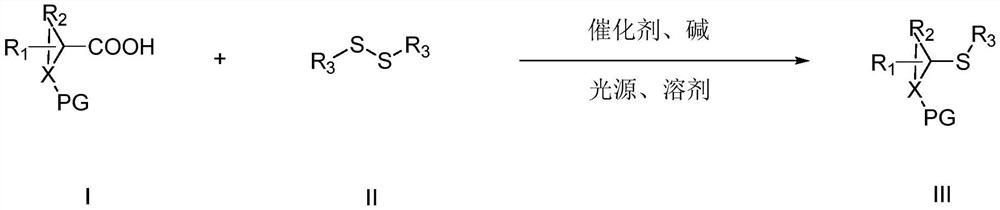
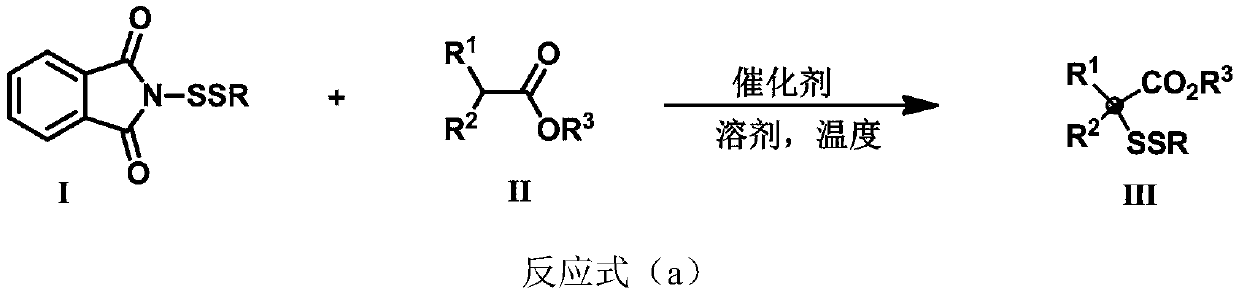
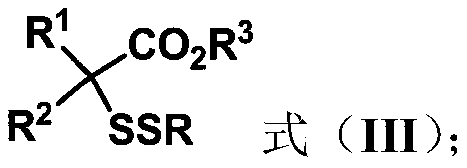
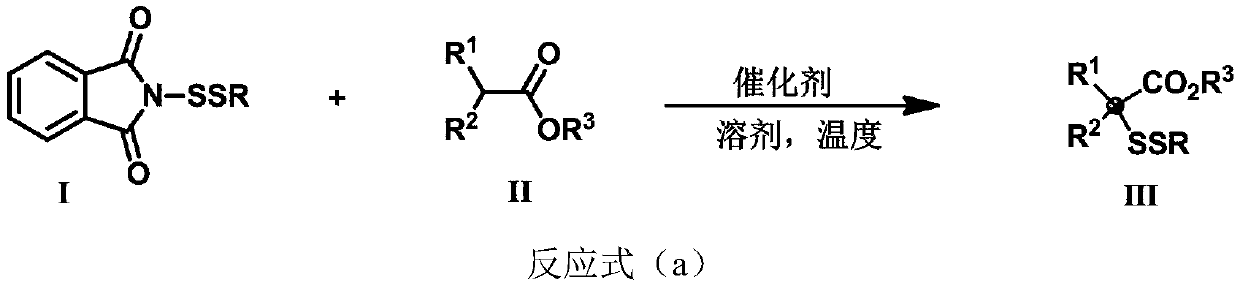
ActiveCN111302991AStrong toleranceEasy to operateEsterified saccharide compoundsSugar derivativesPtru catalystDithiete
The invention belongs to the technical field of organic compound process application. The invention discloses a synthesis method for constructing a three-dimensional and large-steric-hindrance disulfide compound (III). In a reaction solvent, N-dithiophthalimide (I) and ester (II) with alpha hydrogen are used as reaction raw materials, lewis base or cinchona base is used as a catalyst, and variousthree-dimensional and large-steric-hindrance disulfide compounds (III) with quaternary carbon centers are obtained at a high yield under a specific temperature condition. The synthesis method providedby the invention has the advantages of easily available and cheap raw materials, simple reaction operation, mild reaction conditions, high yield and good functional group tolerance. According to theinvention, disulfide connection and modification of some drug molecules, biological small molecules and fluorescent molecules are realized, some chiral disulfide compounds are obtained, a rapid and stable connection mode is provided for connection of pharmaceutical chemistry, especially ADC drugs, and the compounds are expected to be widely applied in the field of pharmaceutical chemistry.
Owner:EAST CHINA NORMAL UNIV
Method for catalytic synthesis of disulfide compounds by using alkaline zeolite molecular sieve
PendingCN114213294AImplement responseImprove toleranceHydropoly/poly sulfide preparationMolecular sieveThiol
The invention discloses a method for catalytic synthesis of disulfide compounds by using an alkaline zeolite molecular sieve, and belongs to the field of green organic fine catalytic synthesis. According to the method for synthesizing the disulfide-containing compound by using a thiol compound as a substrate, aromatic thiol, aliphatic thiol and heterocyclic thiol are subjected to self-coupling reaction in a nitrogen atmosphere under the condition of no oxidant and metal through ETS-10 zeolite molecular sieve solid base catalysis and sulfur hydrogen bond activation. Finally, a series of disulfide compounds are obtained. The method is simple in step, high in product yield and easy to separate and purify.
Owner:CHANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



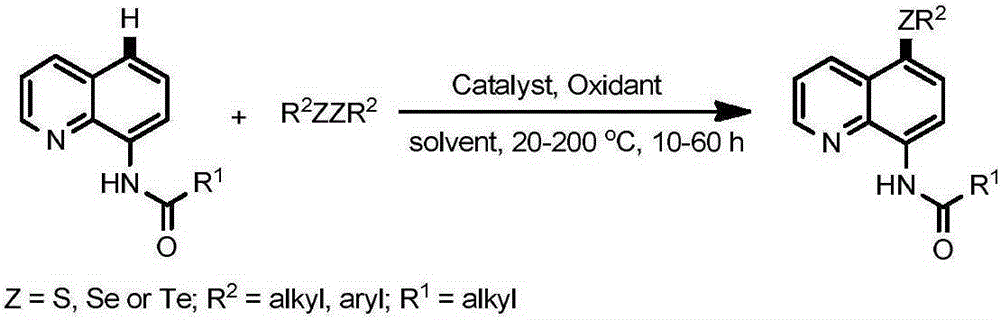



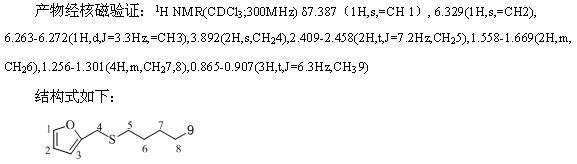
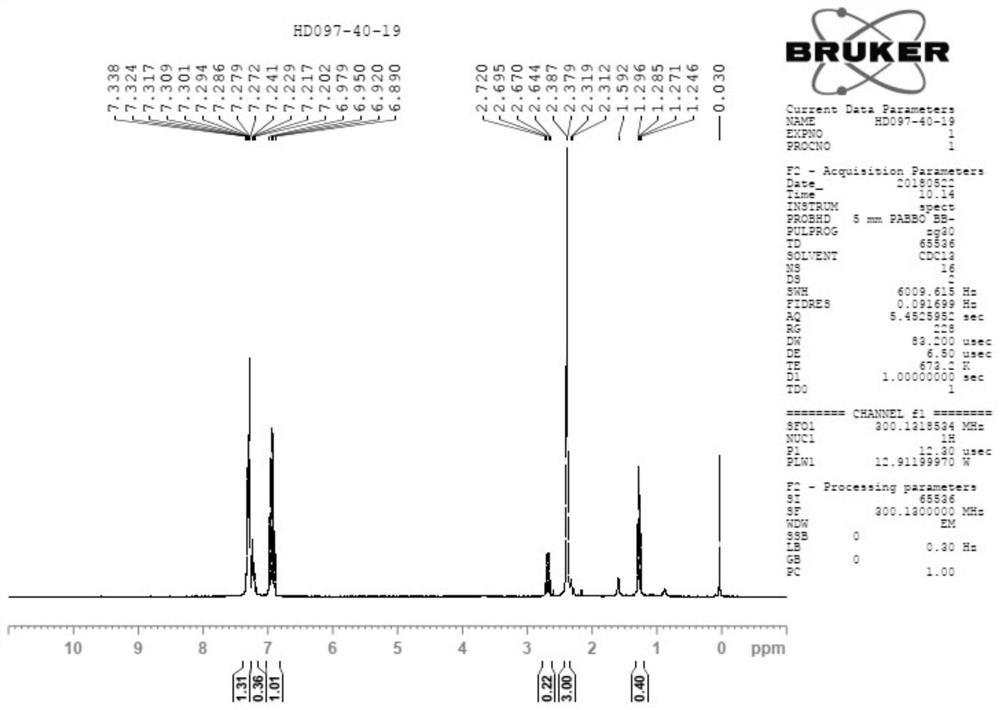
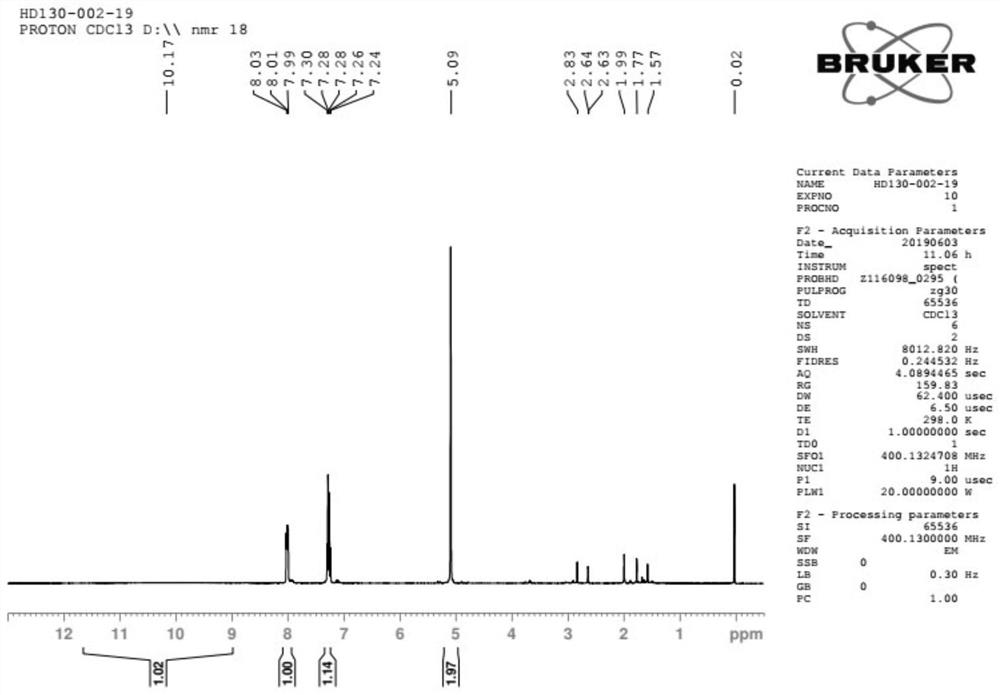



![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a559868-4712-4fad-a191-fda874e50aad/FDA0002823925220000011.png)
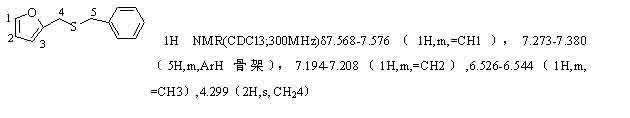
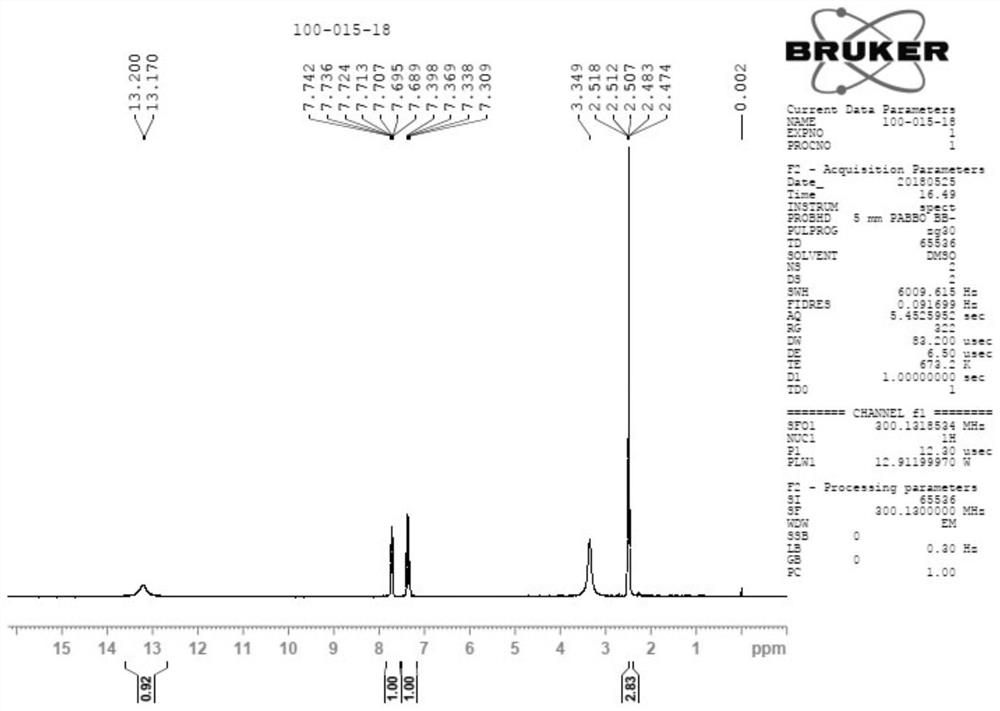
![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a559868-4712-4fad-a191-fda874e50aad/BDA0002823925230000011.png)
![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a559868-4712-4fad-a191-fda874e50aad/BDA0002823925230000021.png)