Method for generating C-S bond by decarboxylating active ester compound
An ester compound, C-S technology, applied in the direction of organic chemistry, sulfide preparation, etc., can solve the problem of limited sulfur source, and achieve the effect of high reactivity and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
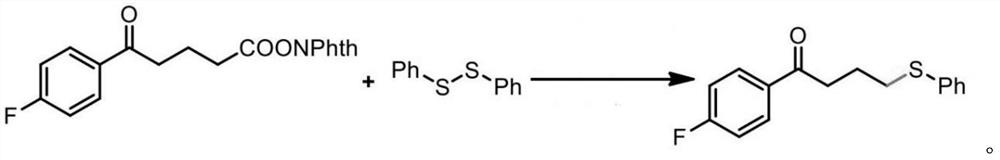
[0020] In this embodiment, several treatment groups are set based on the following comparison treatment methods. Except for the description of special variables, the treatment methods of each treatment group are strictly consistent with the comparison treatment methods. The reaction formula is as follows:
[0021]
[0022] In the reactants used in the control treatment, as active ester, with as a sulfur source.
[0023] Control treatment method: 0.1 mmol of 0.2mmol 0.2 mmol of N,N-diisopropylethylamine was added to 1 mL of dimethylacetamide (DMAc), and the photocatalyst Ru(bpy) was added to the reactant in an amount of 1 mol%. 2 Cl 2 ·6H 2 O; at room temperature, provide blue light illumination, and react for 20 hours.
[0024] The variable settings of each treatment group and the yield of the target product are shown in Table 1.
[0025] Table 1 Influence of the setting mode of each treatment group in the present embodiment on the yield of the target product
...
Embodiment 2
[0030] In this embodiment, a variety of general formulas are selected as R a -The active ester of COONPhth and the general formula are As a reactant (in the present embodiment, X is a C atom or a N atom), the decarboxylation coupling reaction is carried out according to the following steps:
[0031] Put 0.2 mmol of R a -COONPhth, 0.4 mmol 0.4 mmol of DIPEA was added to 1 mL of DMAc, and the photocatalyst Ru(bpy) was added to the reactant according to the addition amount of 1 mol% 2 Cl 2 ·6H 2 O; at room temperature, provide blue light illumination, and react for 20 hours. The reaction formula is as follows:
[0032]
[0033] Each group of reactants used in this example and their corresponding target products are shown in Table 2.
[0034] Table 2 Reactants and their corresponding target products
[0035]
[0036]
[0037] After the reaction, the product yields of various target products were counted, as shown in Table 3.
[0038] According to Table 2 and Ta...
Embodiment 3
[0043] In this embodiment, the general formula is amino acid active esters and As a reactant, the decarboxylation coupling reaction was carried out according to the following steps:
[0044] will be 0.2 mmol 0.4mmol 0.4 mmol of DIPEA was added to 1 mL of DMAc, and the photocatalyst Ru(bpy)2Cl2·6H2O was added to the reactant according to the addition amount of 1 mol%; at room temperature, the reaction was performed for 20 hours under blue light illumination. The reaction formula is as follows:
[0045]
[0046] Each group of reactants used in this example and their corresponding target products and product yields are shown in Table 4. All the tested amino acid active esters in this example can obtain the target product by performing the above reaction, and the yield of the target product can reach a moderate to excellent grade. As shown in Table 4, phenylalanine (corresponding product 15), leucine (corresponding product 16), valine (corresponding product 17), alanin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com