Method for synthetizing mono-thioether compound
A compound, monosulfide technology, applied in the field of synthesizing monosulfide compounds, can solve problems such as complex operation methods, achieve good economy, low reaction process difficulty, and be conducive to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
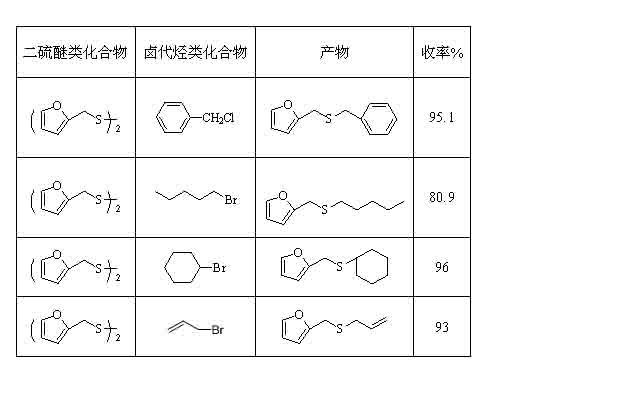
[0020] Add furfuryl disulfide (2mmol), bromo-n-pentane (4.2mmol), selenium powder (0.2mmol), H 2 O (50 mmol), NaOAC (1.25 mmol) and solvent DMF (20 ml), continue to feed carbon monoxide, under normal pressure, heat to 90oC and stir for 5 hours, after cooling to room temperature, switch carbon monoxide to air, and stir for 0.5-1 After one hour, the selenium powder was filtered out, the filtrate was extracted with distilled water and cyclohexane respectively, and the solvent in the extract was distilled off under reduced pressure to obtain the target product with a yield of 80.9%.
[0021]
Embodiment 2
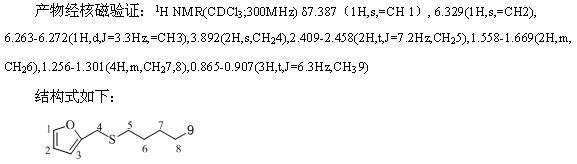
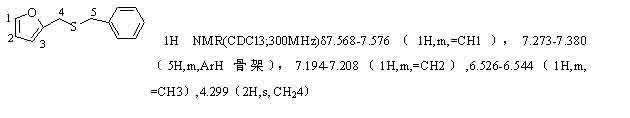
[0023] The method is the same as in Example 1, the yields for different substrates are as follows, and the product is verified by NMR, and the structural formula is as follows
[0024]
[0025]
[0026]
[0027]
[0028]
Embodiment 3
[0030] Selenium dosage (mmol)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com