Method for catalytic synthesis of disulfide compounds by using alkaline zeolite molecular sieve
A technology of thiol compounds and compounds, which is applied in the field of green organic fine catalytic synthesis, can solve the problems of increased processing costs, unfriendly environment, and difficult separation, and achieve the effect of simple synthesis method, low toxicity environment, and high tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
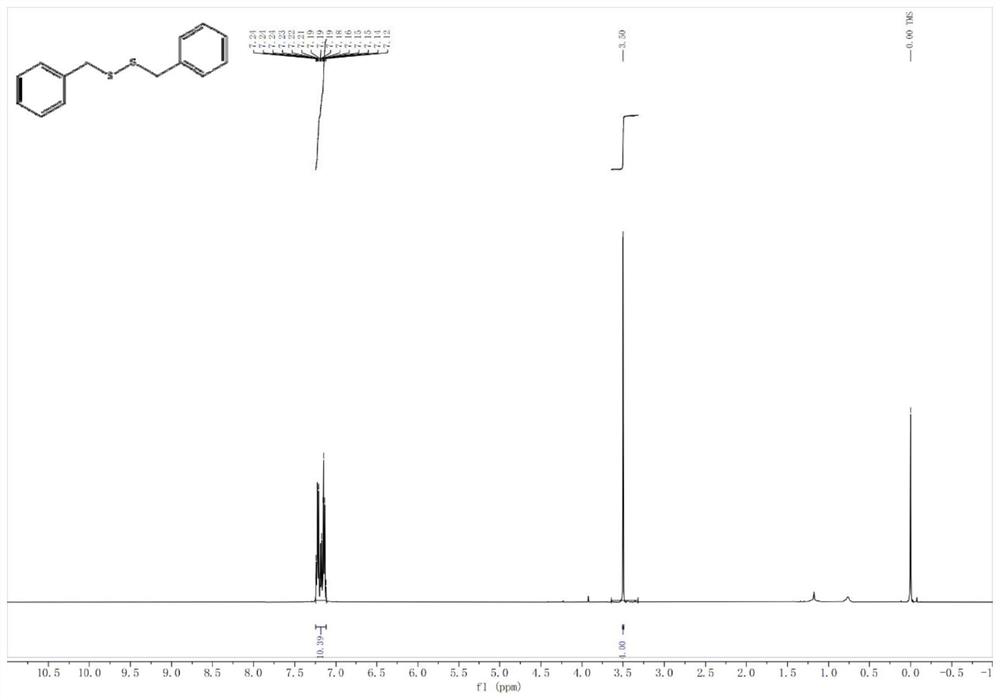
[0031] Weigh 30 mg of ETS-10 catalyst into the reaction tube, then add 0.2 mmol of benzyl mercaptan and 1 mL of cyclohexane. Under a nitrogen atmosphere, react in a heater at 120° C. for 5 h, and centrifuge after the end of the experiment. After rotary evaporation, the obtained liquid phase product was separated by flash column chromatography (the volume ratio of eluent petroleum ether and ethyl acetate was 10:1), and a yellow oily substance was obtained. The product yield can reach 95%, and the characterization data of the product are as follows: 1 H NMR (500 MHz, Chloroform-d) δ 7.25–7.12 (m, 10H), 3.50 (s, 4H).
Embodiment 2
[0033]
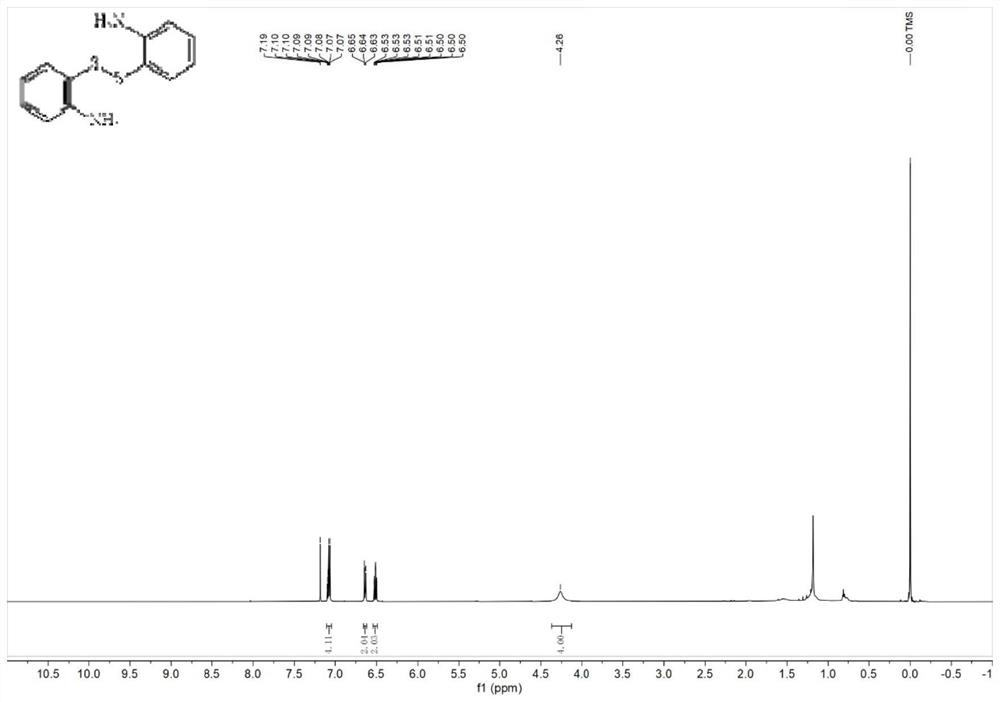
[0034] Weigh 30 mg of ETS-10 catalyst into the reaction tube, then add 0.2 mmol of o-aminothiophenol and 1 mL of cyclohexane. Under a nitrogen atmosphere, react in a heater at 120° C. for 5 h, and centrifuge after the end of the experiment. The obtained solid-phase product was subjected to flash column chromatography (the volume ratio of eluent petroleum ether and ethyl acetate was 4:1) to separate the obtained solid phase product by rotary evaporation to obtain a yellow solid. The product yield can reach 90%, and the characterization data of the product are as follows: 1 H NMR (500MHz, Chloroform-d) δ7.09(ddd, J=7.6,6.1,1.8Hz,4H),6.66–6.62(m,2H),6.54–6.49(m,2H),4.26(s,4H ).
Embodiment 3
[0036]
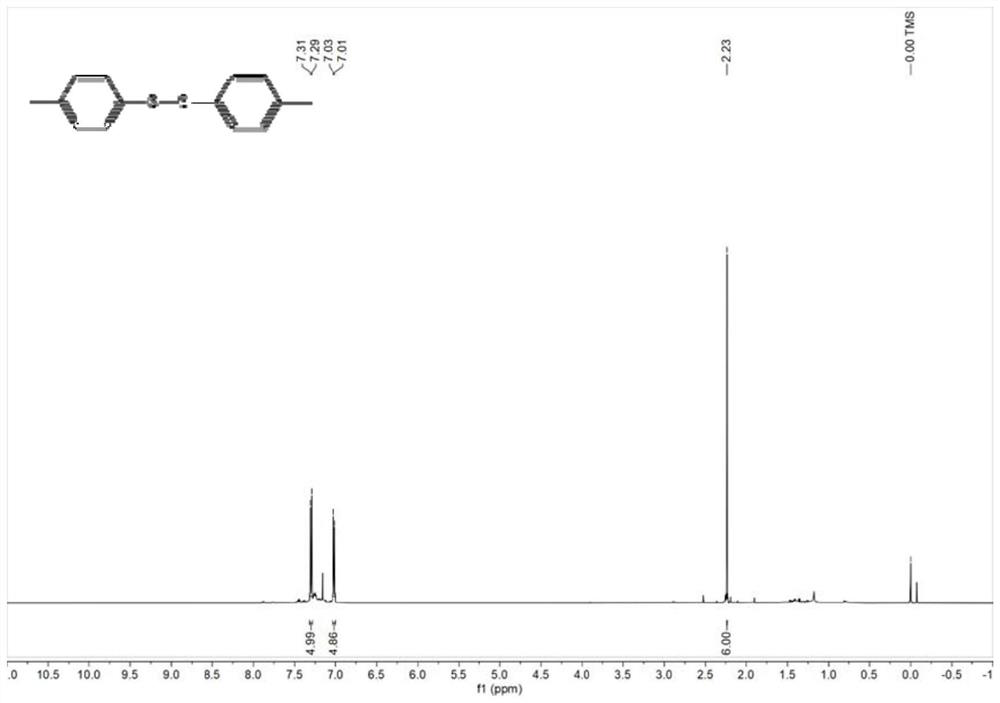
[0037] Weigh 30 mg of ETS-10 catalyst into the reaction tube, then add 0.2 mmol of p-methylthiophenol and 1 mL of cyclohexane. Under a nitrogen atmosphere, react in a heater at 120° C. for 5 h, and centrifuge after the end of the experiment. The obtained solid-phase product was subjected to flash column chromatography (the volume ratio of eluent petroleum ether and ethyl acetate was 50:1) for separation by rotary evaporation to obtain a white solid. Product yield can reach 84%, and the characterization data of product are as follows: 1 H NMR (500MHz, Chloroform-d) δ 7.30 (d, J = 8.2Hz, 5H), 7.02 (d, J = 7.9Hz, 5H), 2.23 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com