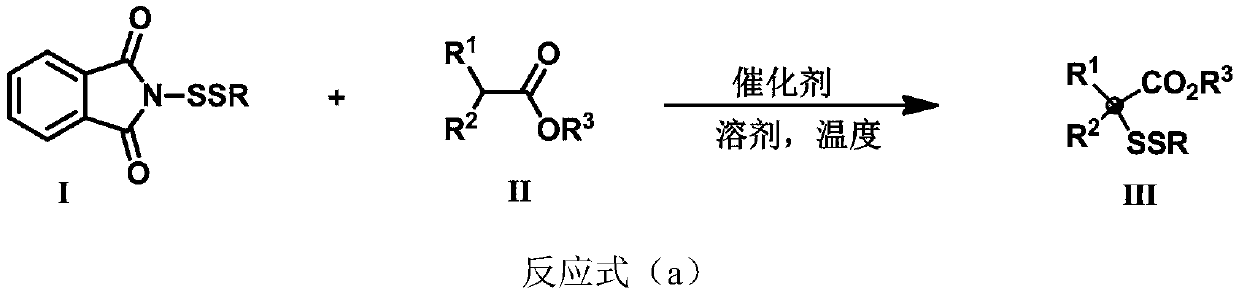
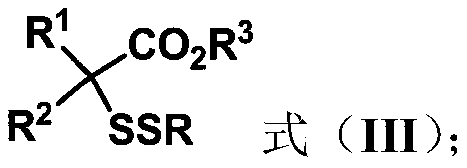
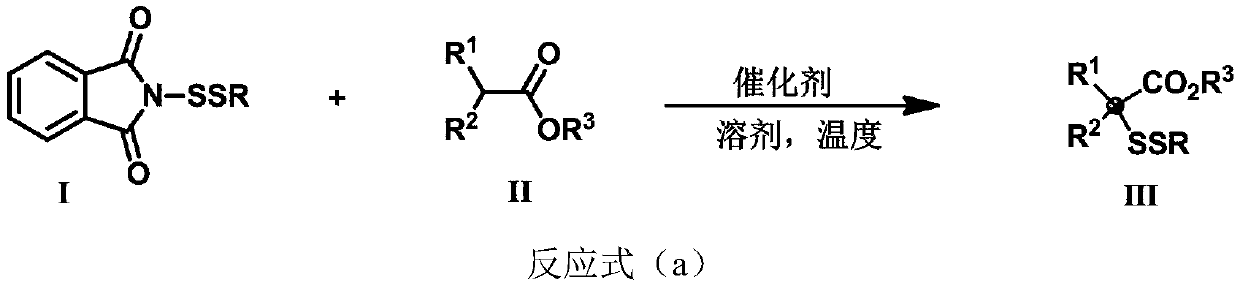
Three-dimensional and large-steric-hindrance disulfide compound as well as synthesis method and application thereof
A synthesis method and a large sterically hindered technology, which can be applied in the fields of organic chemistry methods, chemical instruments and methods, hydrogenated polysulfide/polysulfide preparation, etc. Problems such as the abundance of hindered disulfides and the inability to achieve structural changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Synthesis of compound 3a
[0075] 1 HNMR (400MHz; CDCl 3 ):δ7.65-7.61(m,2H),7.43(d,J=8.0Hz,1H),7.36(t,J=7.6Hz,1H),7.21(d,J=8.4Hz,2H),7.02 (d, J=8.0Hz, 2H), 3.98(d, J=18.0Hz, 1H), 3.61(s, 3H), 3.54(d, J=18.0Hz, 1H), 2.31(s, 3H); 13 CNMR (100MHz; CDCl 3 ): δ196.1, 168.3, 151.4, 138.0, 135.6, 134.5, 132.3, 129.6, 129.5, 128.0, 126.0, 125.2, 64.7, 53.3, 38.4, 21.1; 1294,1213,962,804,735cm -1 ;HRMS(ESI)C 18 h 17 o 3 S 2 [M+H] + :calcd345.0614,found345.0626.
Embodiment 2
[0077] Synthesis of compound 3b
[0078] mg, 97%), 1 HNMR (400MHz; CDCl 3 ): δ7.61-7.57(m,2H),7.41(d,J=7.6Hz,1H),7.34(t,J=7.2Hz,1H),7.13(d,J=8.0Hz,2H),6.98 (d, J=8.0Hz, 2H), 5.04-4.97(m, 1H), 3.90(d, J=16.0Hz, 1H), 3.51(d, J=16.0Hz, 1H), 2.29(s, 3H) ,1.21-1.18(m,6H); 13 CNMR (100MHz; CDCl 3 ):δ196.7,167.4,151.6,137.8,135.5,134.7,132.6,129.5,129.4,127.8,125.9,125.0,70.7,65.1,38.3,21.5,21.3,21.0; IR(KBr)2361,1713,16405, 1269,1101,920,806,746cm -1 ;HRMS(ESI)C 20 h 20 NaO 3 S 2 [M+Na] + :calcd395.0746,found395.0765.
Embodiment 3
[0080] Synthesis of compound 3c
[0081] mg, 87%), 1 HNMR (400MHz; CDCl 3 ): δ7.60(t, J=8.0Hz, 1H), 7.56(d, J=7.6Hz, 1H), 7.40(d, J=7.6Hz, 1H), 7.33(t, J=7.6Hz, 1H ), 7.11(d, J=8.0Hz, 2H), 6.97(d, J=8.0Hz, 2H), 3.84(d, J=16.0Hz, 1H), 3.50(d, J=16.0Hz, 1H), 2.29(s,3H),1.42(s,9H); 13 CNMR (100MHz; CDCl 3 ):δ197.3,166.9,151.8,137.7,135.3,134.9,132.8,129.4,129.3,127.7,125.8,124.9,83.9,65.9,38.5,27.8,21.0; IR(KBr)2924,1701,14211,13794, 1251,1143,991,810,727,694cm -1 ;HRMS(ESI)C 21 h 23 o 3 S 2 [M+H] + :calcd387.1083,found387.1061.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com