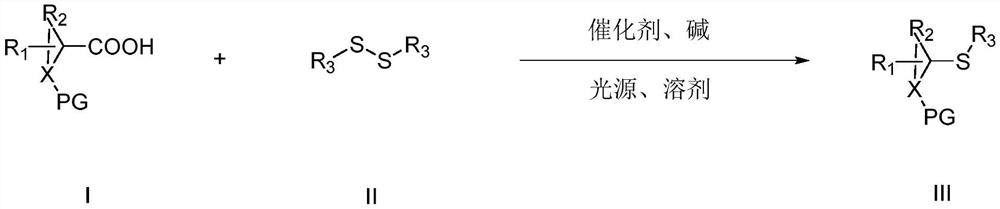
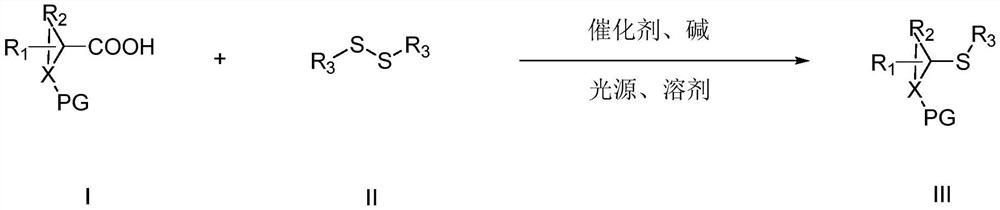
Photocatalytic synthesis method of thioether compound
A technology of thioether compound and synthesis method, which is applied in the fields of thioether preparation, organic chemistry, etc., can solve the problems of limited aryl and heteroaryl iodides of substrates, irritating odor, increased reaction time, etc., and achieves good functional groups. Compatibility, efficient reaction, mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Under nitrogen protection, 1a (43.0mg), 2a (87.0mg), 4CzTPN (3.2mg), Na 2 CO 3 (31.8mg), with a molar ratio of 1.0:2.0:0.02:1.5, was added to the Shrek reaction tube, and finally DMAc (2mL) was added, placed under a blue LED light, and irradiated at room temperature for 18h. After the reaction, Add 10mL water to the reaction solution, then extract with 3x15ml ethyl acetate, combine the organic layers, dry, precipitate, and separate by column chromatography (mobile phase is petroleum ether / ethyl acetate (vv: 40~5:1)) to obtain target product. The target product is a pale yellow solid with a yield of 94%. The post-processing steps in this embodiment are applicable to other embodiments.
[0031] The obtained product 1 H NMR data were characterized as follows: 1 H NMR (500MHz, Chloroform-d) δ 7.51(d,J=18.7Hz,2H),7.30(s,3H),5.45–5.24(m,1H),3.51–3.24(m,2H), 2.20–2.00 (m,3H),1.90(d,J=3.4Hz,1H),1.40(d,J=44.3Hz,9H).
Embodiment 2
[0033]
[0034] Under nitrogen protection, 1b (40.0mg), 2a (65.3mg), 4CzTPN (1.6mg), Et 3 N (30.4 mg), with a molar ratio of 1.0:1.5:0.01:1.5, was added to the Shrek reaction tube, and finally DMAc (2 mL) was added, placed under a CFL lamp, and irradiated at 10°C for 24 hours. After the reaction, 10 mL of water was added to the reaction liquid, and then extracted with 3×15 mL of ethyl acetate, the organic layers were combined, dried, precipitated, and separated by column chromatography to obtain the target product. The target product is a pale yellow solid with a yield of 43%.
[0035] The obtained product 1 H NMR data were characterized as follows: 1 H NMR (500MHz, Chloroform-d) δ 7.64–7.54(m,2H),7.38–7.32(m,3H),5.40(dd,J=8.3,5.2Hz,1H),3.75–3.37(m,2H) ,2.67–2.56(m,1H),2.17–2.04(m,1H),1.45(s,9H).
Embodiment 3
[0037]
[0038] Under nitrogen protection, 1c (27.0mg), 2a (43.5mg), 4CzPN (16.0mg), K 2 CO 3 (36.9mg), with a molar ratio of 1.0:1.0:0.1:1.0, was added to the Shrek reaction tube, and finally MeCN (2mL) was added, placed under an incandescent lamp, and irradiated at 25°C for 36h. Add 10mL of water to the liquid, and then extract with 3x15ml of ethyl acetate, combine the organic layers, dry, precipitate, and separate by column chromatography to obtain the target product. The target product is a pale yellow solid with a yield of 42%.
[0039] The obtained product 1 H NMR data were characterized as follows: 1 H NMR (600MHz, Chloroform-d) δ 7.31–7.22(m,5H),5.54(d,J=200.0Hz,1H),2.34(d,J=361.8Hz,1H),1.36–1.23(m,2H ),0.90(q,J=15.8,11.3Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com