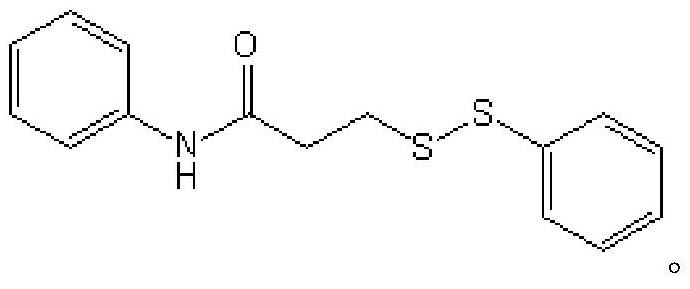
Method for preparing asymmetric disulfide
A disulfide, asymmetric technology, applied in the field of preparation of asymmetric disulfide N-phenyl-3-propionamide, can solve the problems of mercaptan odor, non-conforming to environmental protection, etc. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
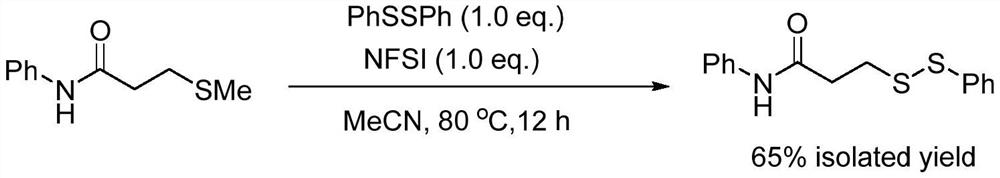
[0014] Specific Example 1: Synthetic method: Add acetonitrile (10mL), 3-methylthio-N-phenylpropanamide (1mmol, 0.195g), diphenyl disulfide (1mmol, 0.218g) successively in a sealed tube of 50mL ) and N-fluorobisbenzenesulfonamide (1mmol, 0.315g), the reaction temperature was controlled at 80 degrees Celsius, and the reaction was vigorously stirred for 12 hours. After the reaction was completed, the reaction solution was concentrated and separated by column chromatography in sequence to finally obtain N-phenyl-3-(phenyldisulfanyl)propanamide (0.187 g, 65%) as a pale yellow solid. The equations involved in the reaction are as follows:
[0015]
[0016] The NMR data and mass spectral data of the target product N-phenyl-3-(phenyldisulfanyl) propanamide are as follows:
[0017] 1 H NMR (300MHz, CDCl 3 )δ7.64–7.02(m,11H),3.06(t,J=6.8Hz,2H),2.71(t,J=6.9Hz,2H).
[0018] 13 C NMR (75MHz, CDCl 3 )δ169.08, 137.61, 137.16, 129.18, 129.02, 127.88, 127.18, 124.52, 120.07, 36.49, 34....
specific Embodiment 2
[0020] Specific example 2: comparative experiment: add acetonitrile (10mL), 3-methylthio-N-phenylpropanamide (1mmol, 0.195g) and diphenyl disulfide (1mmol, 0.218g) successively in a 50mL sealed tube ), the anti-temperature was controlled at 80 degrees centigrade, and the reaction was vigorously stirred for 12 hours. After the reaction was completed, the reaction solution was concentrated and separated by column chromatography, but the target product N-phenyl-3-(phenyldisulfanyl)propionamide could not be separated. The equations involved in the reaction are as follows:
[0021]
specific Embodiment 3
[0022] Specific example 3: comparative experiment: add acetonitrile (10mL), 3-methylthio-N-phenylpropanamide (1mmol, 0.195g), diphenyl disulfide (1mmol, 0.218g) successively in a sealed tube of 50mL ) and 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt [Selectfluor] (1mmol, 0.36g), and the reaction temperature was controlled at 80 degrees Celsius , and the reaction was vigorously stirred for 12 hours. After the reaction was completed, the reaction solution was concentrated and separated by column chromatography, but the target product N-phenyl-3-(phenyldisulfanyl)propionamide could not be separated. The equations involved in the reaction are as follows:
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com