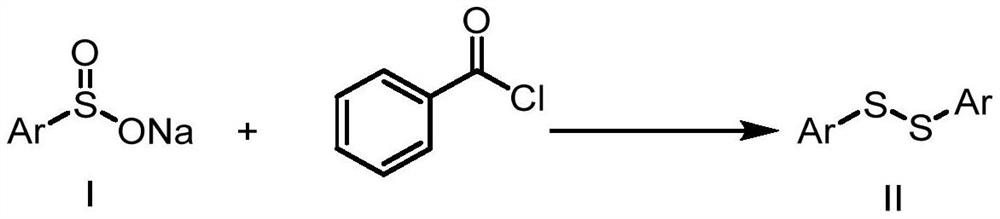
Method for synthesizing disulfide compound through concerted catalysis of visible light and titanocene complex
A synergistic catalysis, visible light technology, applied in the preparation of hydrogenated polysulfides/polysulfides, organic chemistry, etc., can solve the problems of high pollution and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
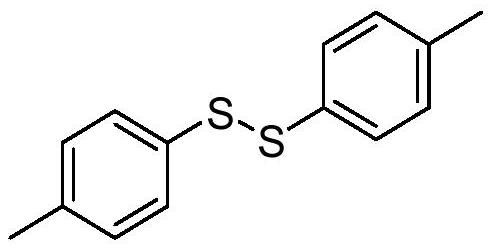
[0017] Synthesis of p-toluene disulfide with the following structural formula
[0018]
[0019] 0.036g (0.2mmol) sodium p-toluenesulfinate, 0.008g (0.02mmol) bis(pentamethylcyclopentadiene) titanium dichloride and (0.4mmol) 2,6-dimethyl-1,4 -Diethyl dihydro-3,5-pyridinedicarboxylate was added to the reaction tubes in sequence, gas replacement was carried out on the photocatalytic parallel reaction apparatus, and then the reaction tubes under the protection of argon atmosphere were placed in the glove box, and the reaction tubes were added in sequence 70 μL (0.6 mmol) of benzoyl chloride, 100 μL (0.6 mmol) of N,N-diisopropylethylamine, and 2 mL of redistilled 1,2-dichloroethane, the reaction mixture was stirred at room temperature and 10W 470nm blue The color LED light was irradiated for 6 hours, and the completion of the reaction was detected by TLC. The reaction mixture was concentrated in vacuo to remove 1,2-dichloroethane, and the residue was purified by flash column ch...
Embodiment 2
[0021] Synthetic structural formula following bis (4-methoxyphenyl) disulfide
[0022]
[0023] In this example, the sodium p-toluenesulfinate used in Example 1 is replaced with equimolar sodium p-methoxybenzenesulfinate, and the other steps are the same as in Example 1 to obtain bis(4-methoxyphenyl) Disulfide, its productive rate is 25%, and the spectral data of product is: 1 H NMR (600MHz, CDCl 3 )δ7.40(d, J=8.8Hz, 4H), 6.84(d, J=8.8Hz, 4H), 3.80(s, 6H); 13 C NMR (151MHz, CDCl 3 ) δ 161.59, 132.85, 124.77, 116.55, 55.15.
Embodiment 3
[0025] Synthetic structural formula following two (4-fluorophenyl) disulfides
[0026]
[0027] In this embodiment, the sodium p-toluenesulfinate used in Example 1 is replaced with equimolar sodium p-fluorobenzenesulfinate, and other steps are the same as in Example 1 to obtain bis(4-fluorophenyl) disulfide, Its yield is 32%, and the spectral data of product is: 1 HNMR (600MHz, CDCl 3 )δ7.37(dd,J=8.6,5.1Hz,4H),6.94(m,4H); 13 C NMR (151MHz, CDCl 3 ) δ 162.83 (d, J=249.2Hz), 132.39 (d, J=4.5Hz), 131.50 (d, J=9.1Hz), 116.48 (d, J=22.6Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com