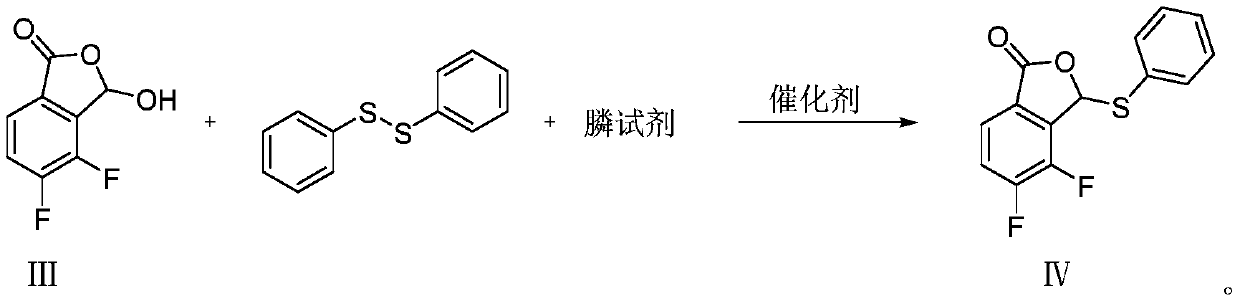
Synthesis method of baloxavir marboxil intermediate
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of few synthesis routes, difficult production control, purchase and use restrictions, etc., and achieve the effect of facilitating industrial production, avoiding potential dangers, and solving toxicity problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
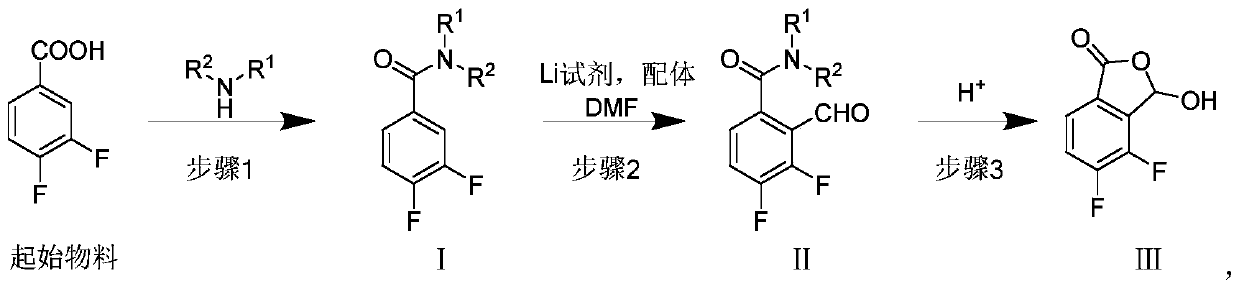
[0062] Embodiment 1: the preparation of compound I N, N-diethyl-3,4-difluorobenzamide
[0063] Put 31.62g of 3,4-difluorobenzoic acid, 500ml of dichloromethane, 5ml of N'N-dimethylformamide into the reaction bottle, stir at room temperature, drop in 48.22g of oxalyl chloride, stir at room temperature for 1 hour after dropping, evaporate under reduced pressure Dry solvent, add 200ml of dichloromethane to dissolve the residual oily matter and directly drop into diethylamine aqueous solution (dissolve 60.81g of potassium carbonate and 26.26g of diethylamine hydrochloride in 200ml of water), stir at room temperature after dropping, separate the organic layer, and use Extracted with dichloromethane, combined organic layers, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and evaporated to dryness under reduced pressure to obtain 39.23 g of an oily substance. Yield 92.1%, 1 H NMR (400MHz, CDCl 3 )δ7.13~7.25(m,3H),3.52(br m,2H),3.27(br m,2H),1.21(br m,3H)...
Embodiment 2
[0064] Example 2: Preparation of compound II N, N-diethyl-2-formyl-3,4-difluorobenzamide
[0065] Put 26.00g of N,N-diethyl-3,4-difluorobenzamide, 250ml of tetrahydrofuran, and 18.42g of tetramethylethylenediamine into the reaction flask, cool down to -80°C, and drop in 84ml of 1.6M n-butyl Lithium, stir for 1 hour after dropping, add 22.30g of N'N-dimethylformamide dropwise, remove the refrigerant after dropping for half an hour, quench the reaction with dilute hydrochloric acid, separate the organic layer, and extract the aqueous phase with ethyl acetate , combined the organic layers, and evaporated to dryness under reduced pressure to obtain 29.50 g of oily substance, with a yield of 100%. 1 H NMR (400MHz, CDCl 3 )δ10.33(s,1H),7.41~7.46(m,1H),7.03~7.07(m,1H), 3.56(q,J=7.2Hz,2H),3.06(q,J=7.2Hz,2H ),1.30(t,J=7.2Hz,2H),1.02(t,J=7.2Hz,2H); MS(ESI)m / z(M+H) + :242.1
Embodiment 3
[0066] Example 3: Preparation of compound III 4,5-difluoro-3-hydroxyisobenzofuran-1(3H)-one
[0067] Put 29.50g of N,N-diethyl-2-formyl-3,4-difluorobenzamide and 200ml of 6M hydrochloric acid into the reaction flask, heat up to 100°C, stir for 2.5h, extract with 100ml X4 dichloromethane, The organic layer was separated, washed with purified water and saturated brine, dried over anhydrous sodium sulfate, filtered and evaporated to dryness under reduced pressure to obtain 20.98 g of a brown solid with a yield of 92.4%. 1 H NMR (400MHz, CDCl 3 )δ7.66~7.70(m,1H),7.40~7.50(m,1H),6.78(s,1H),4.54(br s,1H); (MS-ESI)m / z(M-H) - :185.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com