Method for catalytically synthesizing asymmetric disulfide derivative by NFSI
A technology of disulfides and derivatives, which is applied in the field of NFSI catalytic synthesis of asymmetric disulfide derivatives, can solve the problems of use, limited application, and incompatible with green environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
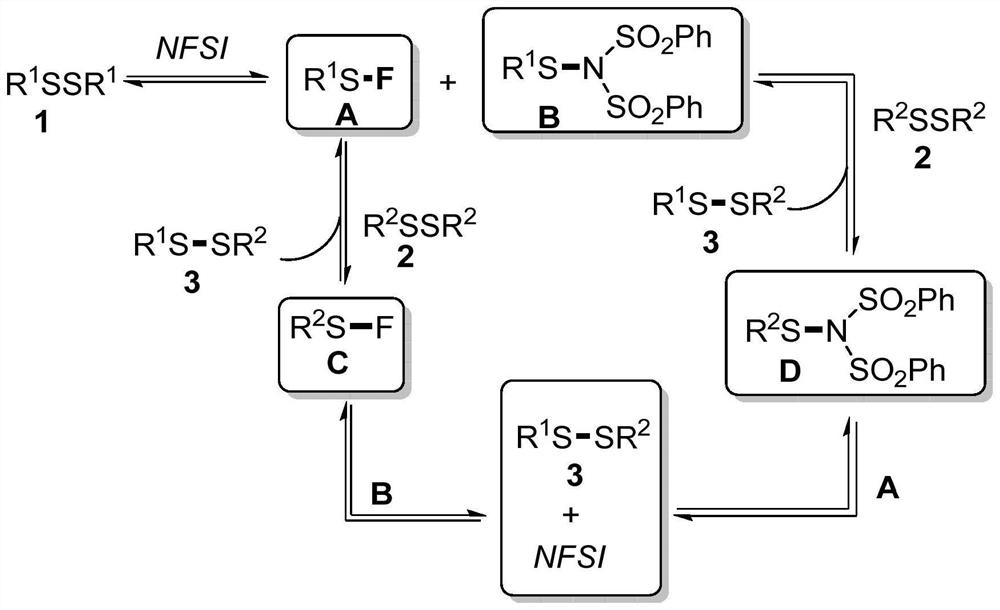
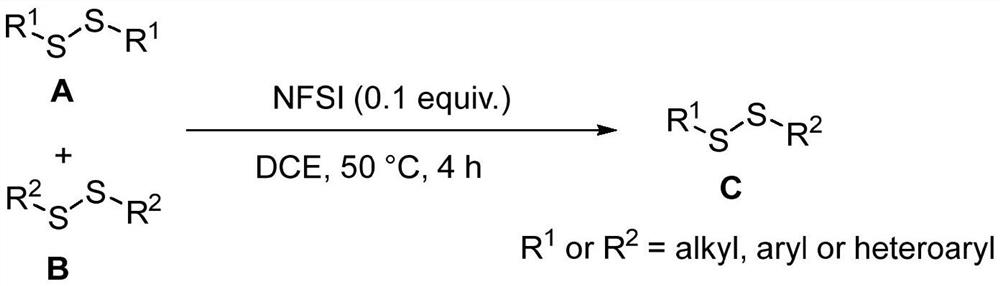
[0019] The reaction equation that embodiment 1 relates to is as follows:
[0020]
[0021] Synthesis of unsymmetrical disulfide derivatives: Add 1,2-dichloroethane (10 mL), symmetrical disulfide A (1.0 mmol), symmetrical disulfide B ( 1.0mmol) and NFSI (0.1mmol), the reaction temperature was controlled at 50 degrees Celsius, and the reaction was vigorously stirred for 4 hours. After the reaction is completed, the reaction solution is concentrated and separated by column chromatography in sequence to obtain the unsymmetrical disulfide derivative.
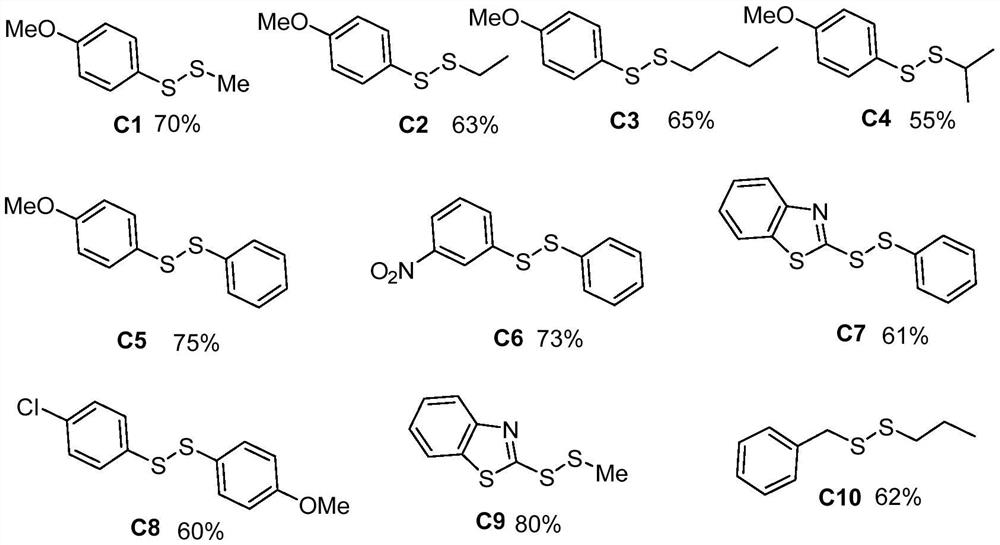
[0022] 1. According to the above experimental conditions, a series of unsymmetrical disulfides were synthesized, the structures and yields are as follows:
[0023]
[0024] 2. NMR and mass spectrometry data:
[0025] (1) Compound C1: 1 H NMR (300MHz, Chloroform-d) δ7.53–7.48(m,2H),6.92–6.87(m,2H),3.80(s,3H),2.45(s,3H). 13 C NMR (75MHz, Chloroform-d) δ159.76, 132.16, 127.81, 114.77, 55.44, 22.94. MS (EI): m / z = 185.9 [M + ]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com