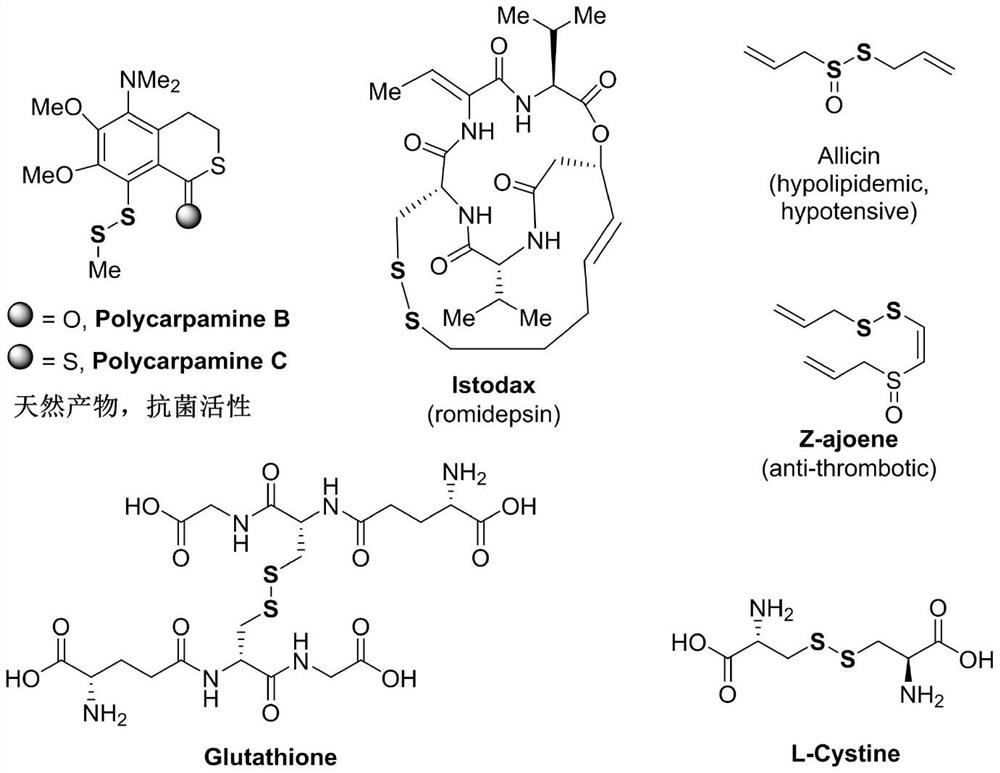
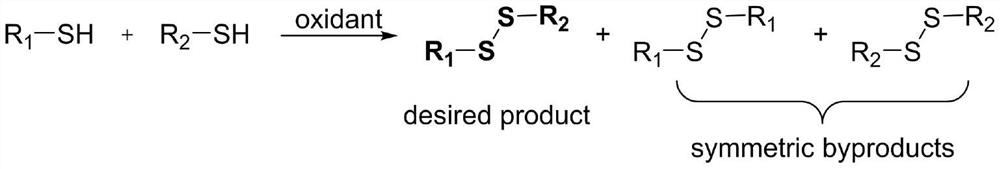
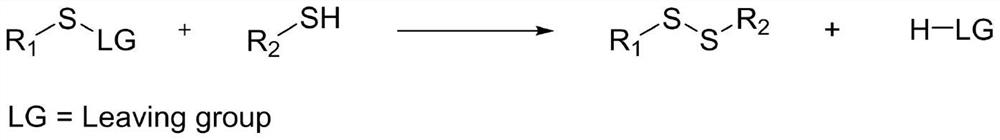
Preparation method of asymmetric disulfide compound
A disulfide and compound technology, which is applied in the field of preparation of asymmetric disulfide compounds, can solve the problems of complex reaction operation, limited substrate, and many by-products, and achieve simple catalytic system, easy-to-obtain raw materials, and easy operation. convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 2-(butyl disulfide group) benzo [d] thiazole
[0034]
[0035] A method for preparing the above-mentioned asymmetric disulfide compound is characterized in that the specific steps are as follows: under nitrogen protection, add 83.7mg 2-mercaptobenzothiazole and 178.4mg dibutyl disulfide and 4.5mg disulfide in a 25mL Schlenk tube Palladium chloride, 2.0mL DMSO was added under nitrogen gas, heated to 80°C, and reacted for 2h. After cooling, it was poured into 5 mL of water, the product was extracted with 30 mL of dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by column chromatography (PE:EA=100:1) to obtain 109.5 mg of a yellow oily liquid product with a yield of 86%. The characterization data of the resulting product are as follows: IR (KBr, cm -1 ):3426,2960,2916,1461,1454,1432,1002,752,719; 1 H NMR (500MHz, CD...
Embodiment 2
[0036] Example 2: 2-(methyl disulfide group)-pyridine
[0037]
[0038]A method for preparing the above-mentioned asymmetric disulfide compound is characterized in that the specific steps are as follows: under nitrogen protection, add 55.6mg 2-mercaptopyridine and 94.2mg dimethyl disulfide and 4.5mg disulfide in a 25mL Schlenk tube Palladium chloride, 2.0mL DMSO was added under nitrogen gas, heated to 80°C, and reacted for 2h. Pour into 5mL water after cooling, extract the product with 30mL dichloromethane, wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent with a rotary evaporator to obtain a crude product; the crude product is purified by column chromatography (PE:EA=50:1 ) was purified to obtain 65.9 mg of yellow oily liquid product with a yield of 84%. The characterization data of the resulting product are as follows: IR (KBr, cm -1 ):3425,3046,2982,2916,1569,1416,1118,814,760; 1 HNMR (500MHz, CDCl 3 ):δ8.47–8.45(m,1H),7.68–7.61(m,2H),7...
Embodiment 3
[0039] Example three: 2-(methyl disulfide group)-pyrimidine
[0040]
[0041] A method for preparing the above-mentioned asymmetric disulfide compound is characterized in that the specific steps are as follows: under nitrogen protection, add 56mg 2-mercaptopyrimidine and 94.2mg dimethyl disulfide and 4.5mg dichloropyrimidine in a 25mL Schlenk tube Palladium chloride, 2.0mL DMSO was added under nitrogen gas, heated to 80°C, and reacted for 2h. Pour into 5mL water after cooling, extract the product with 30mL dichloromethane, wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent with a rotary evaporator to obtain a crude product; the crude product is purified by column chromatography (PE:EA=50:1 ) was purified to obtain 56.7 mg of light yellow oily liquid product with a yield of 72%. The characterization data of the resulting product are as follows: IR (KBr, cm -1 ):3452,2916,2842,1553,1369,1186,767,627; 1 H NMR (500MHz, CDCl 3 ): δ8.62(d, J=5.45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com