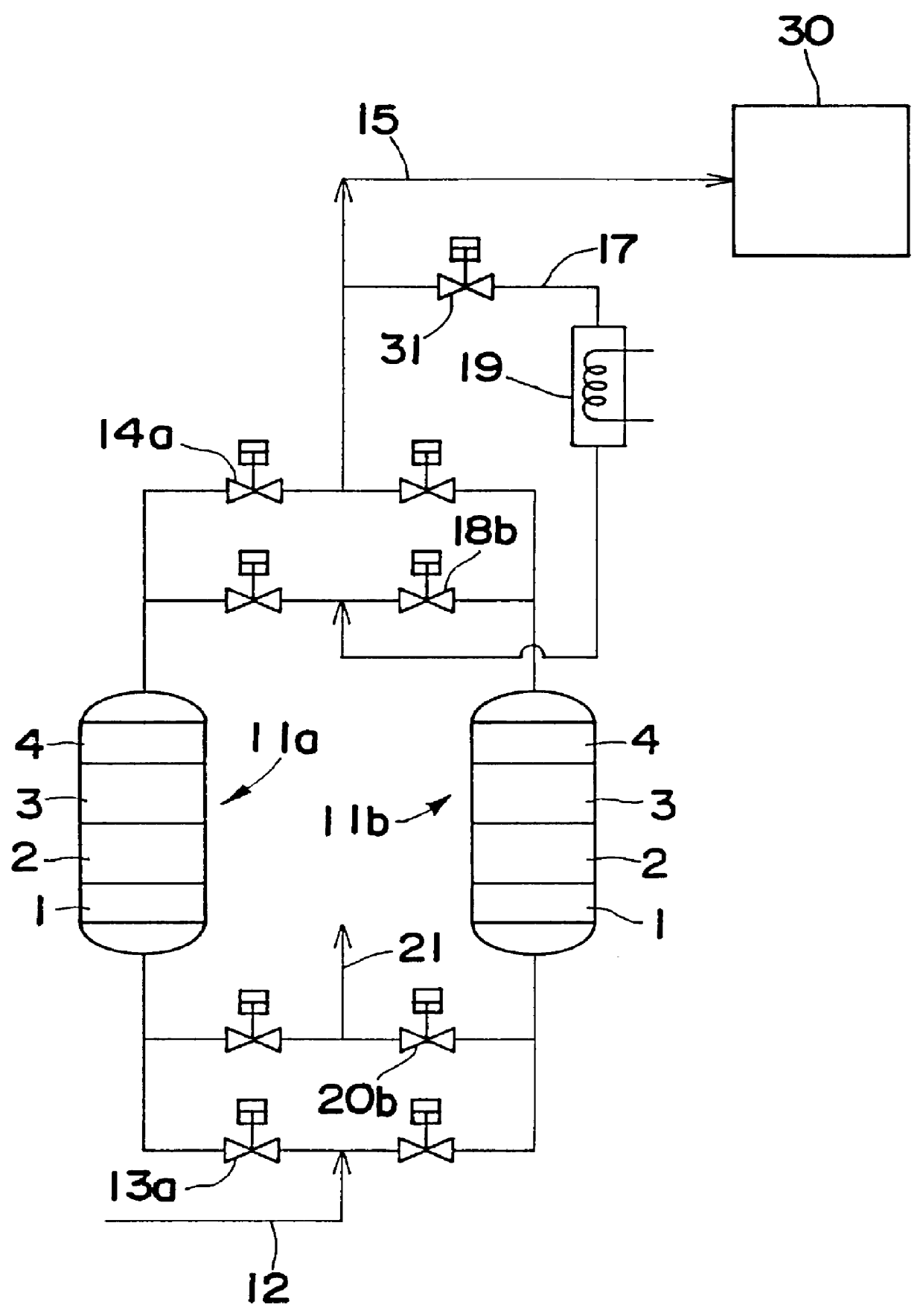
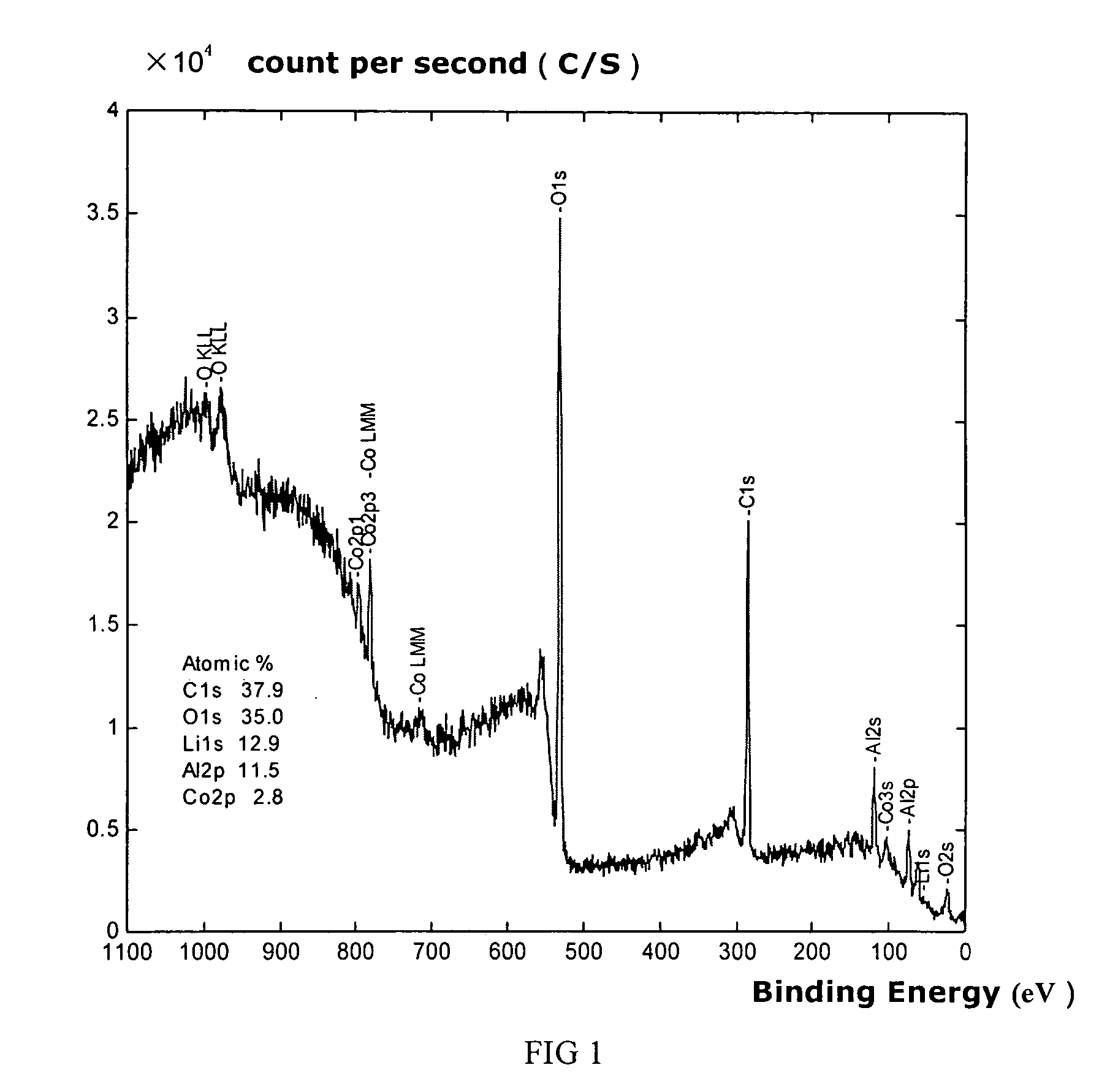
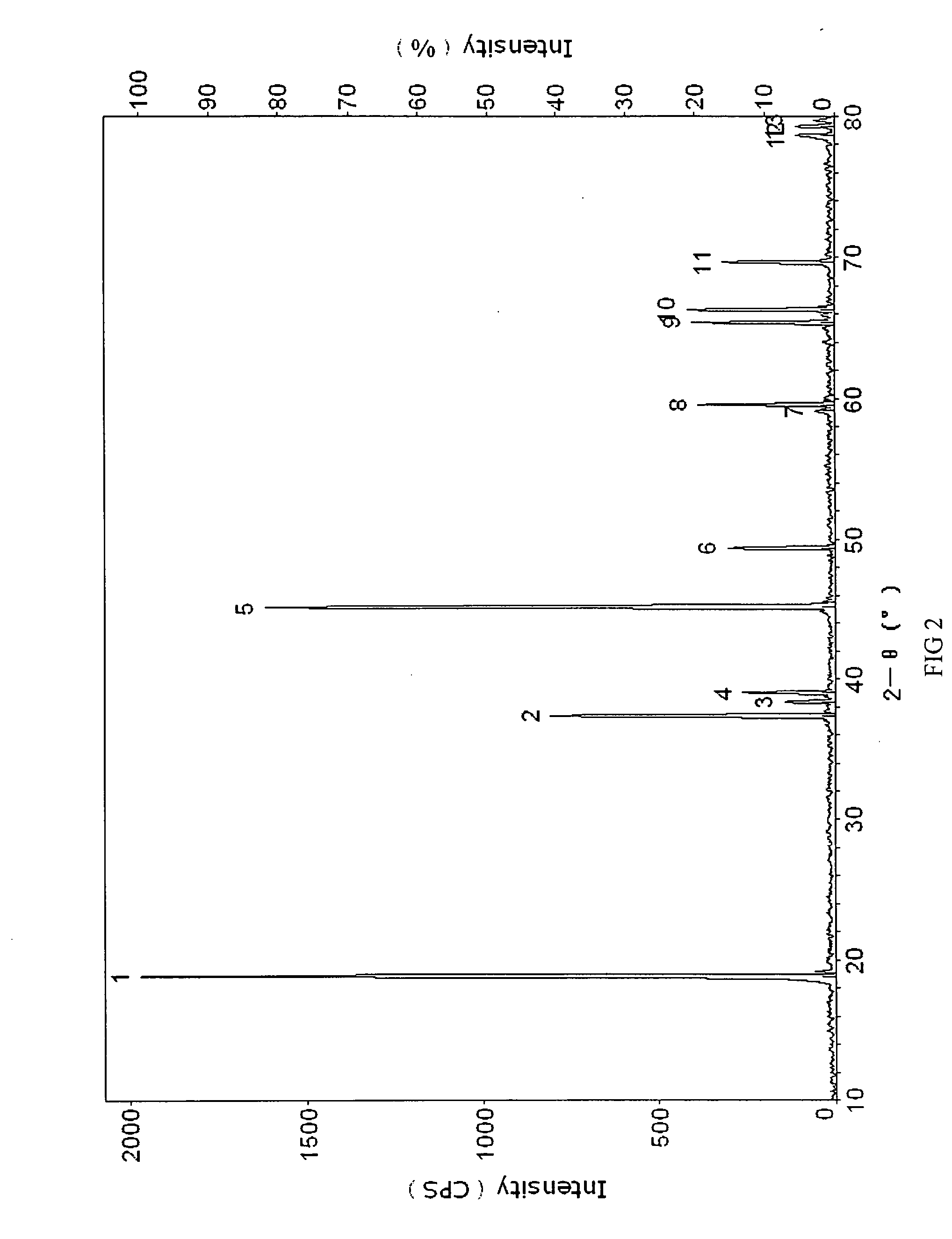
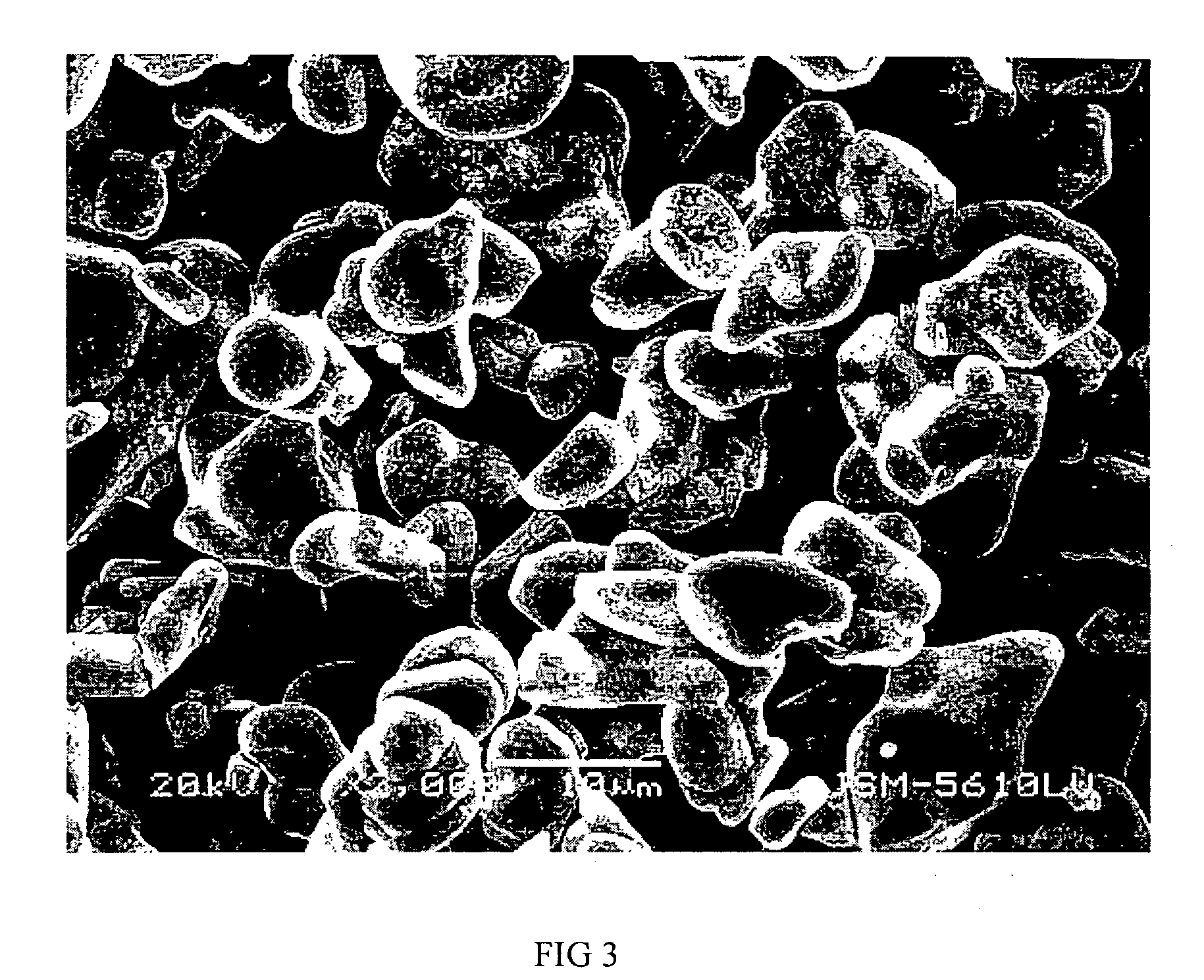
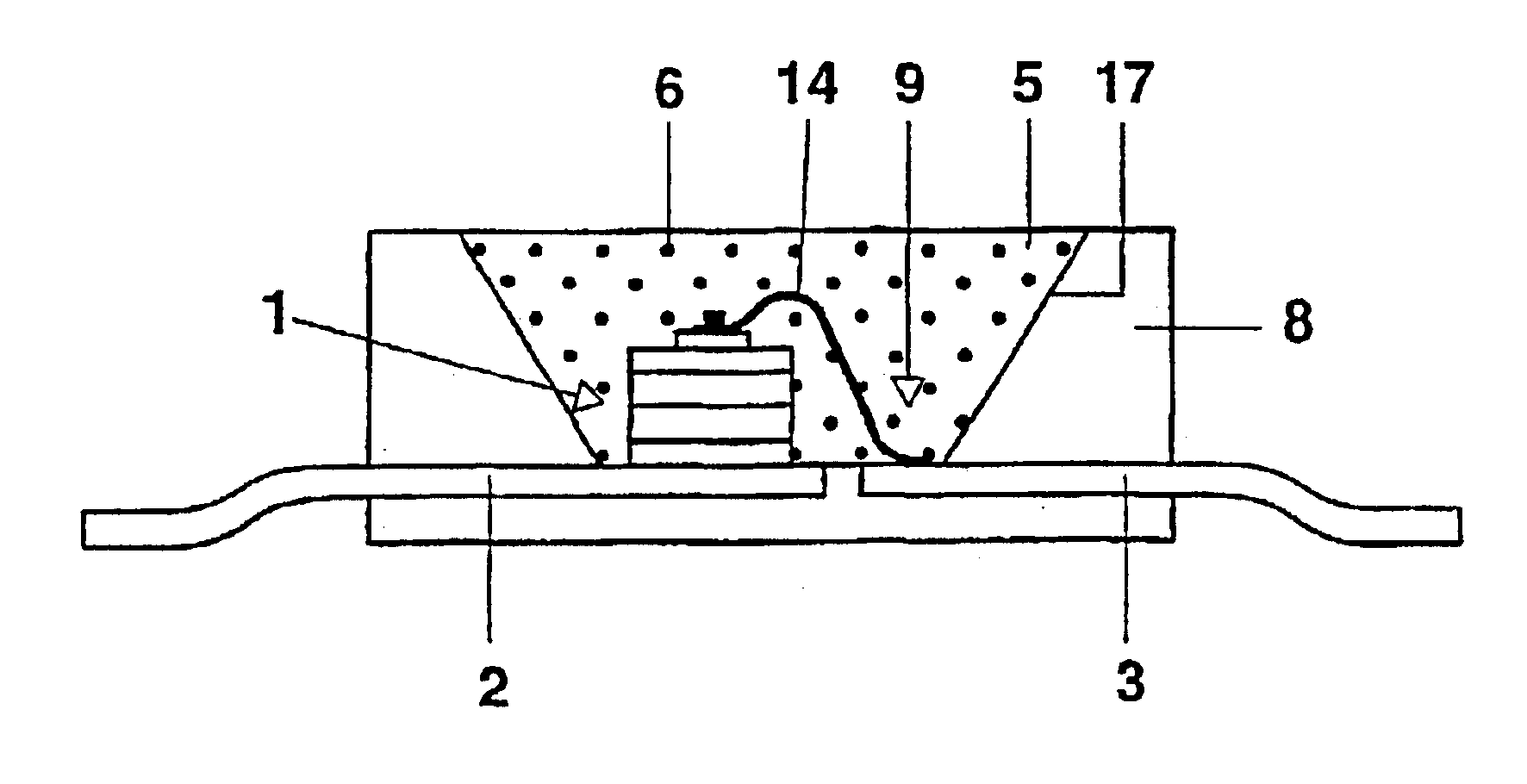
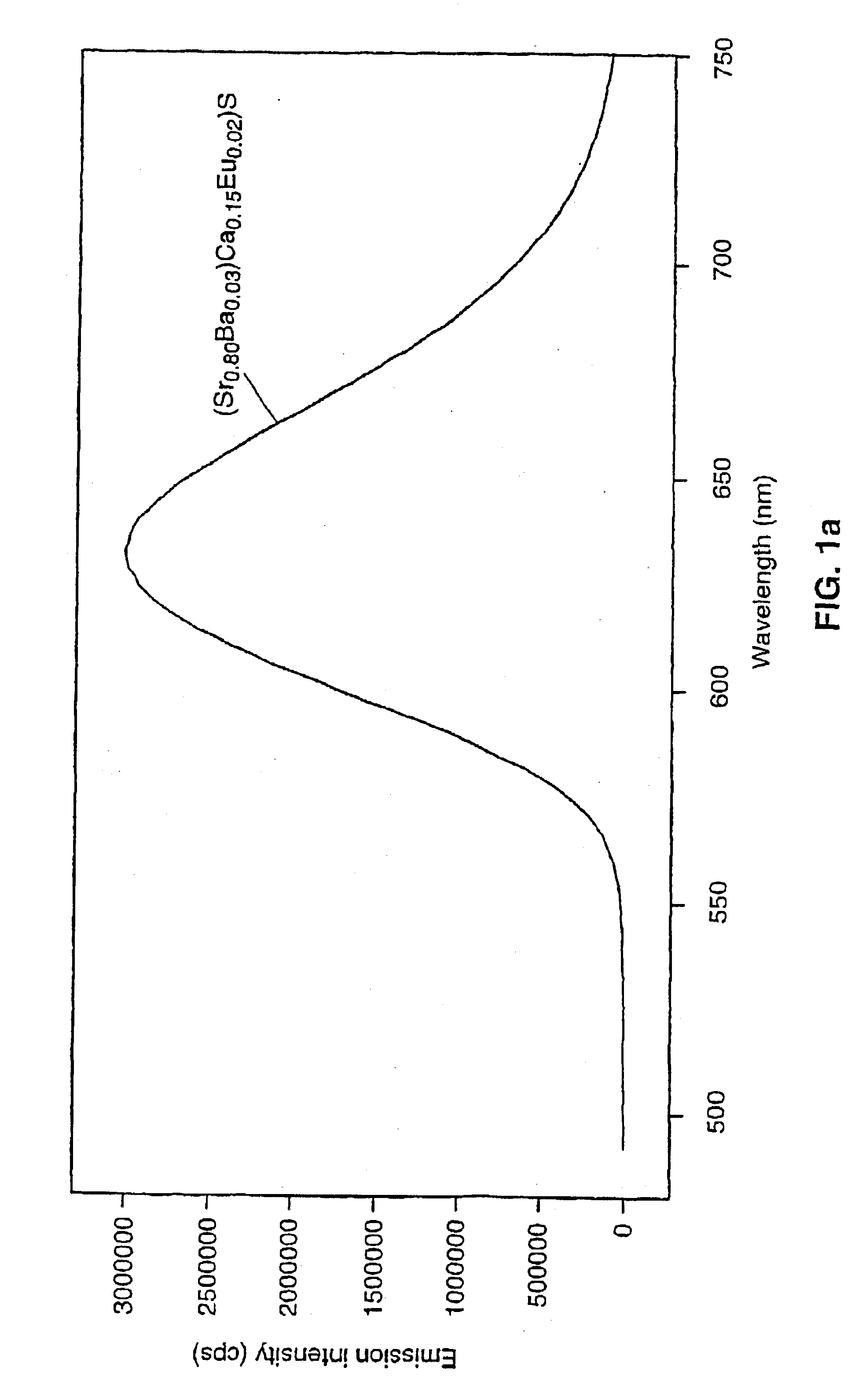
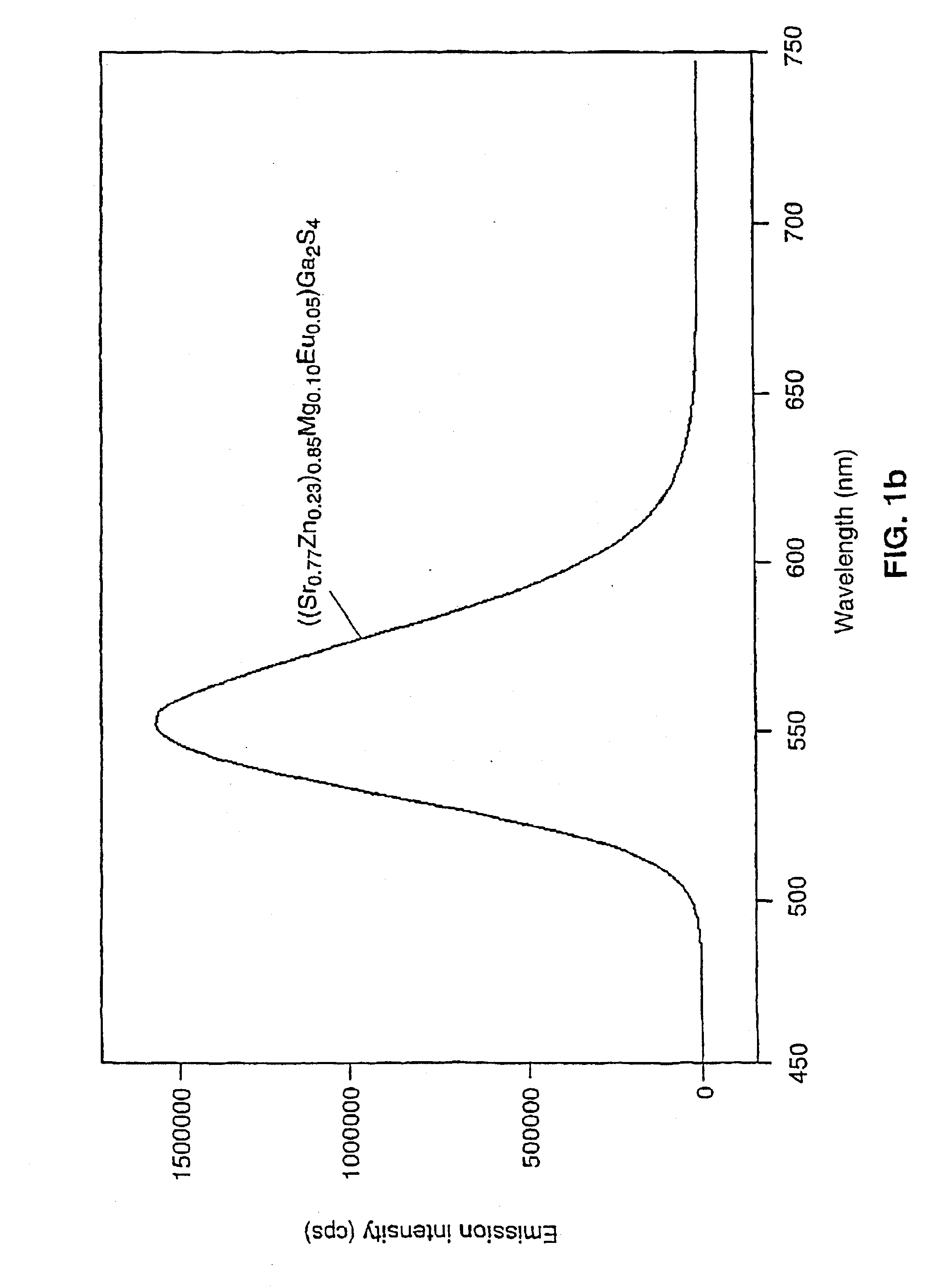
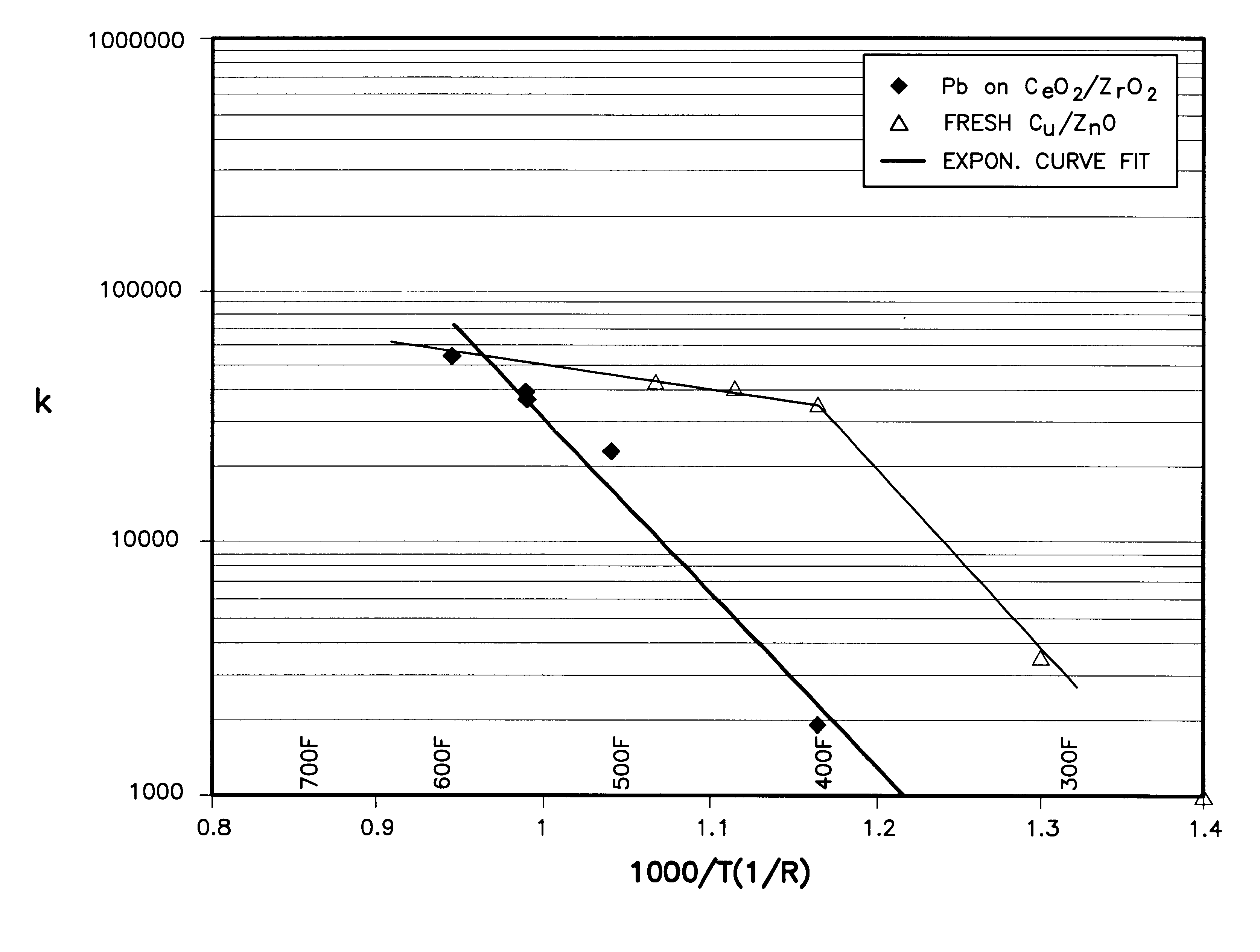
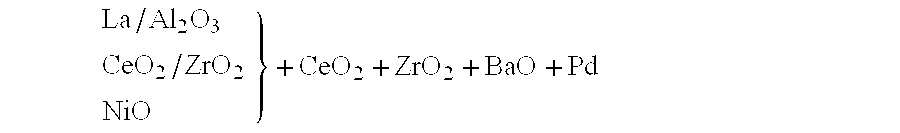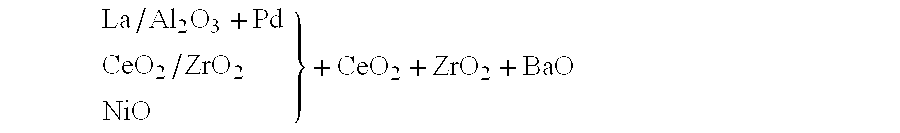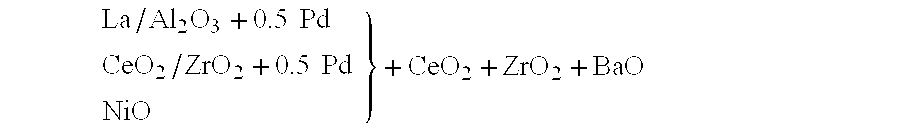Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4506 results about "Ce element" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cerium is a chemical element with symbol Ce and atomic number 58. Cerium is a soft, ductile and silvery-white metal that tarnishes when exposed to air, and it is soft enough to be cut with a knife.
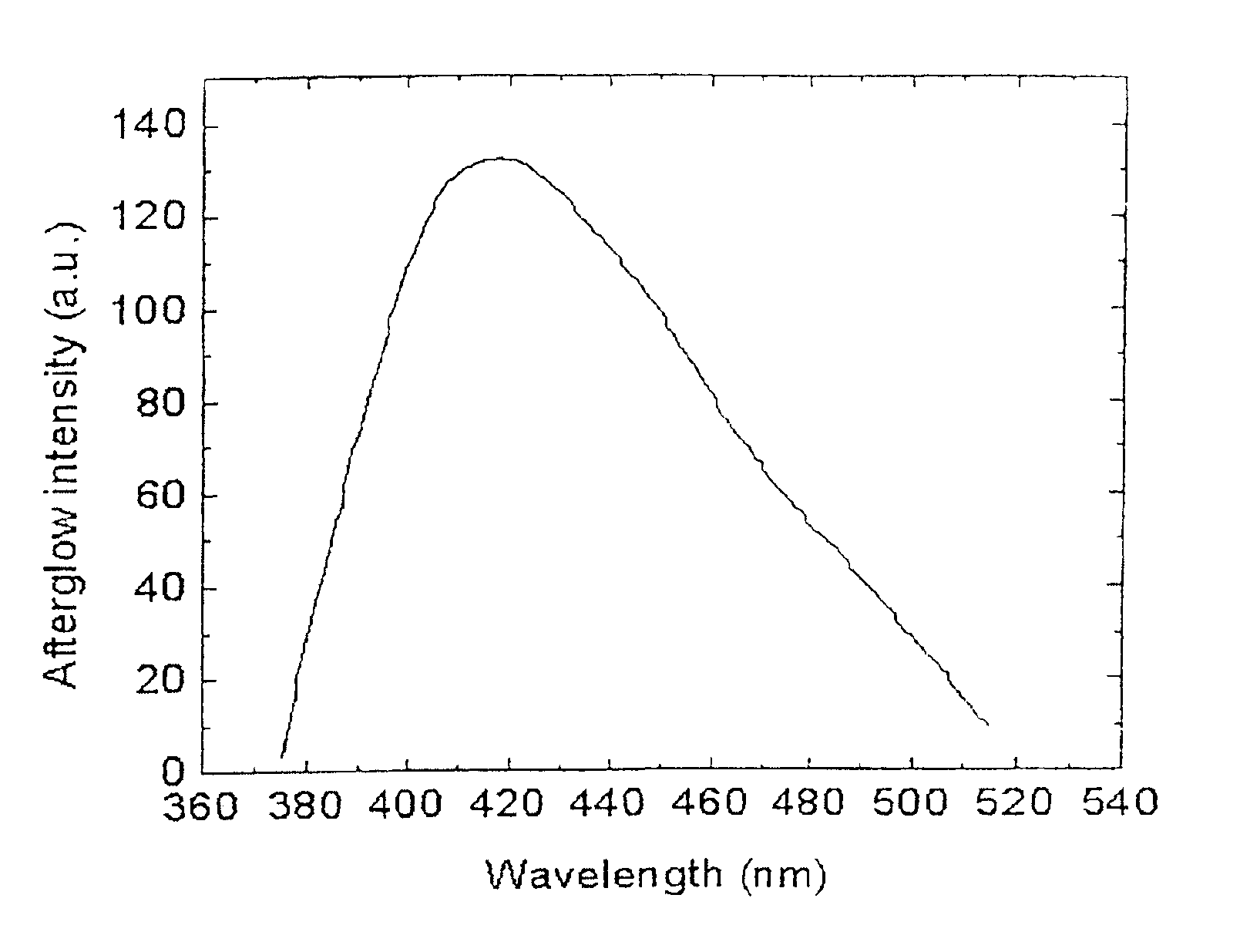
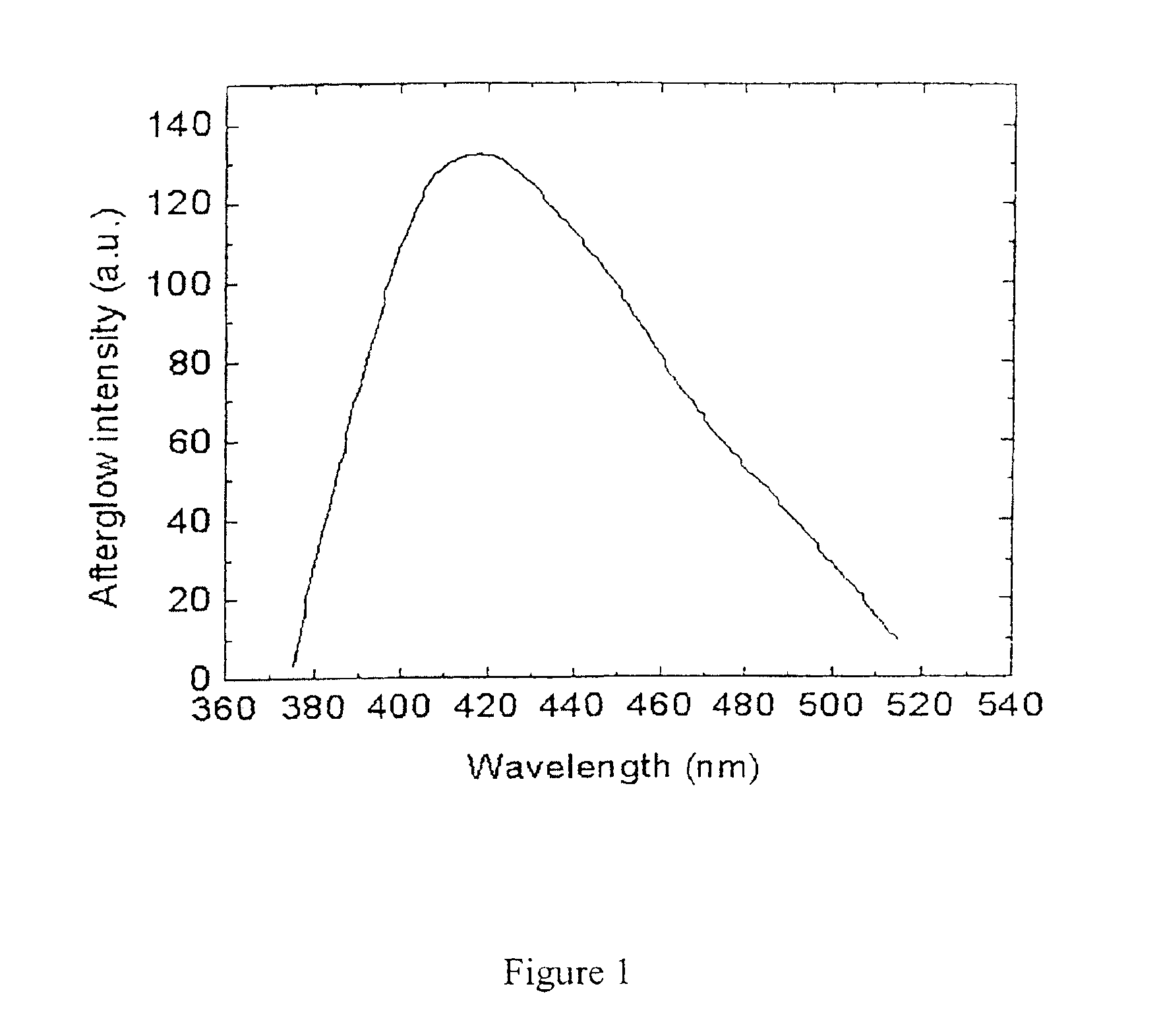
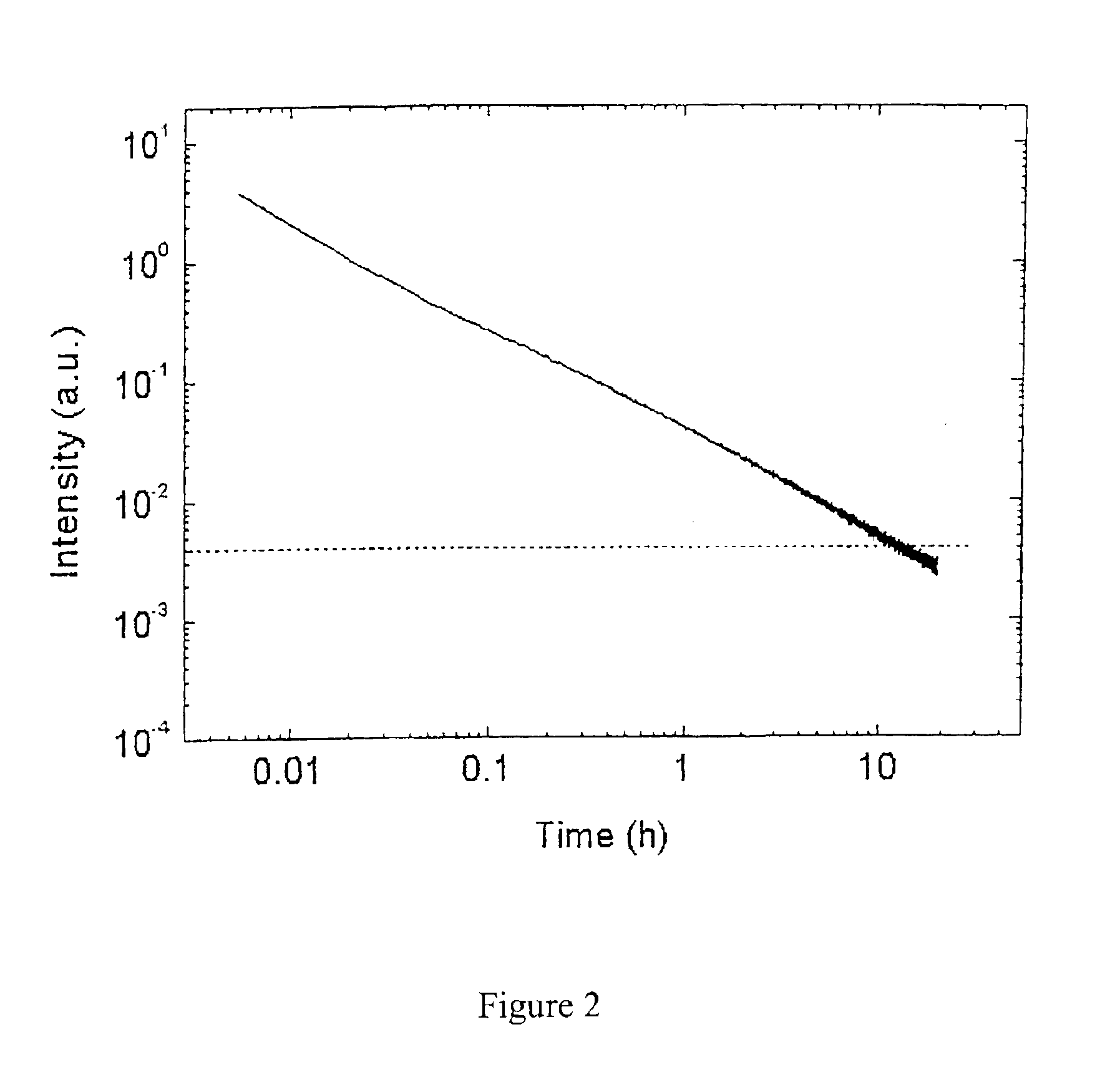
Long persistent phosphors and persistent energy transfer technique
The invention provides long-persistent phosphors, methods for their manufacture and phosphorescent articles. The invention also provides a method for generating a long-persistent phosphorescence at a selected color. The phosphors of the invention may be alkaline earth aluminates, alkaline earth silicates, and alkaline earth aluminosilicates. The phosphors include those activated by cerium. The phosphors also include those in which persistent energy transfer occurs from a donor ion to an acceptor ion, producing persistent emission largely characteristic of the acceptor ion.
Owner:UNIV OF GEORGIA RES FOUND INC +1
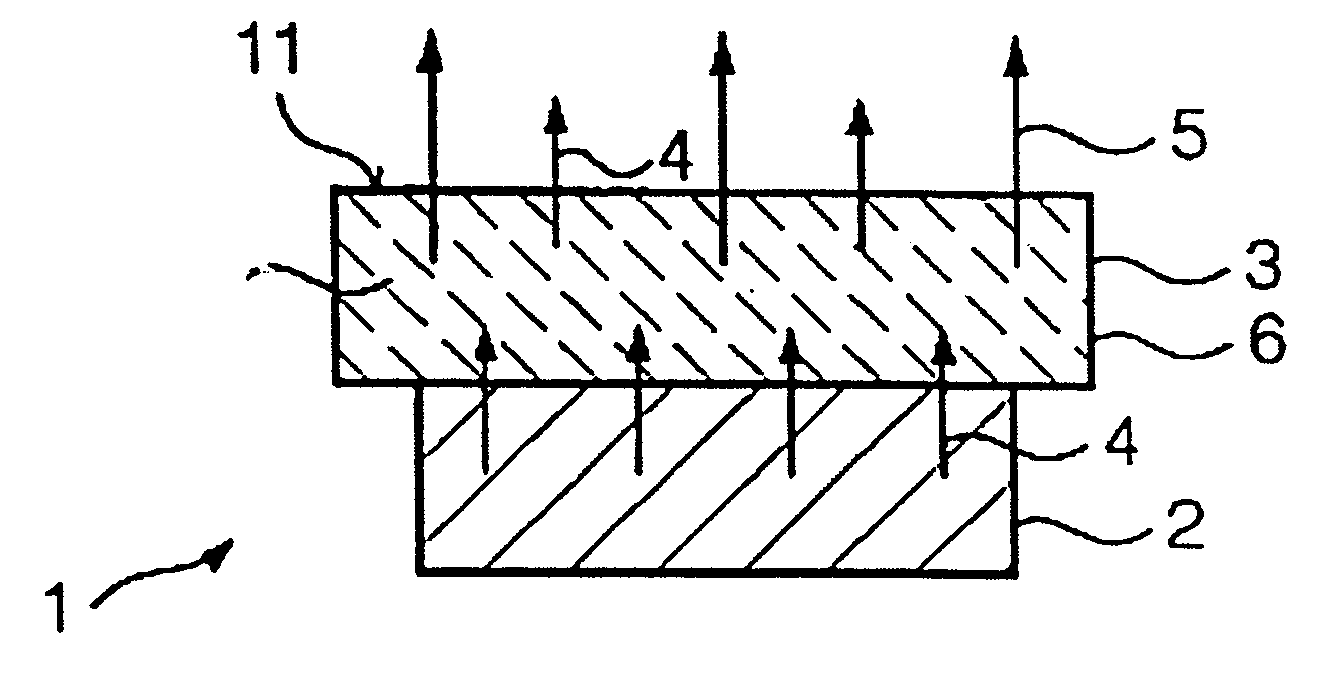
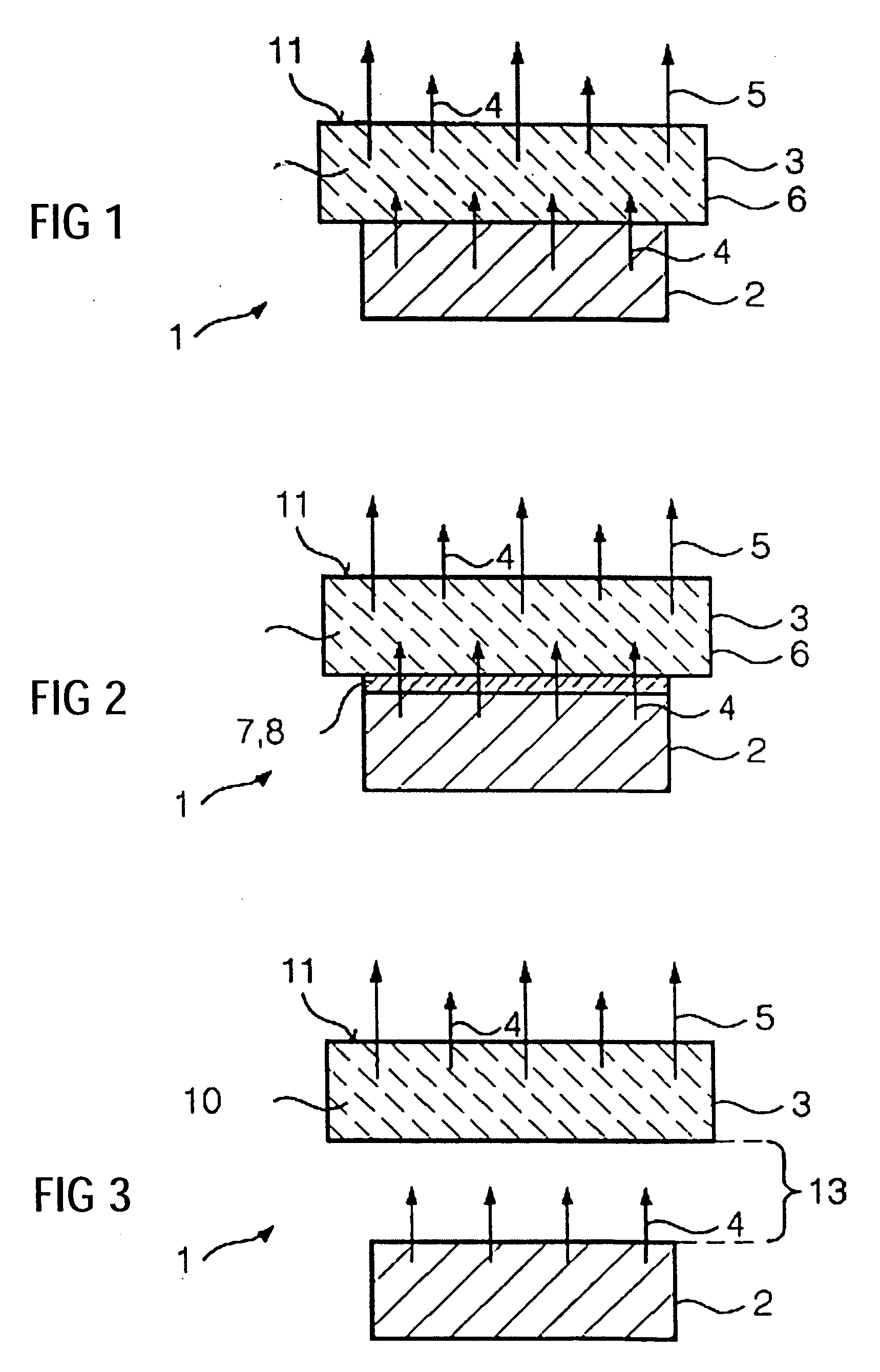
Light source having an LED and a luminescence conversion body and method for producing the luminescence conversion body
ActiveUS20040145308A1Efficient luminescence conversionDischarge tube luminescnet screensLamp detailsDopantSecondary radiation
The invention relates to a light source (1), having at least one LED (2) for emitting a primary radiation (4) and at least one luminescence conversion body (3) having at least one luminescent material for converting the primary radiation (4) into a secondary radiation (5). The luminescence conversion body is a polycrystalline ceramic body. The LED is based on GaInN and emits blue primary radiation. The ceramic body comprises for example a luminescent material based on a cerium-doped yttrium aluminum garnet. This luminescent material emits yellow secondary radiation. Blue primary radiation and yellow secondary radiation penetrate through the luminescence conversion body and are perceived as white light by the observer. In order to produce the luminescence conversion body, provision is made of a polycrystalline ceramic body which is united with a solution of a dopant. By means of a thermal treatment, the dopant (activator) diffuses into the ceramic body, the luminescent material being formed.
Owner:OSRAM OPTO SEMICONDUCTORS GMBH
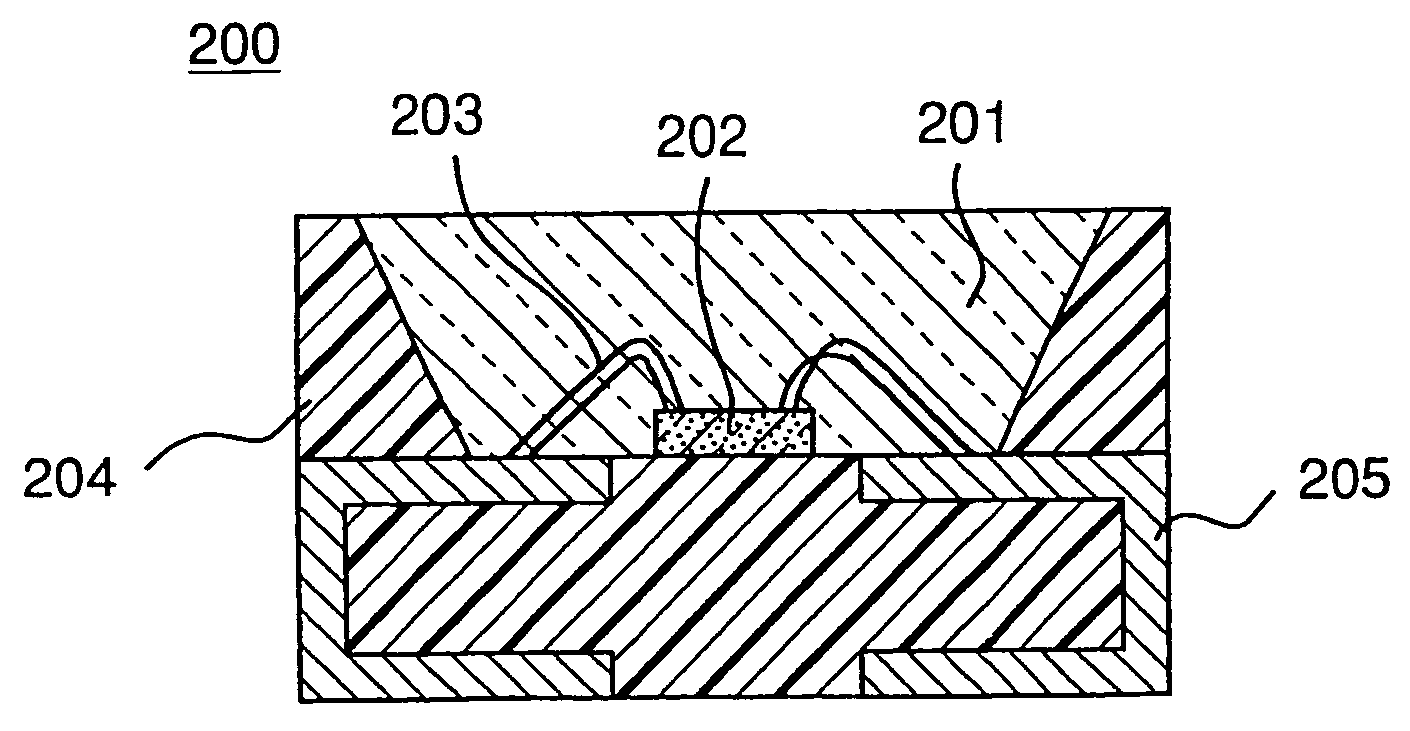
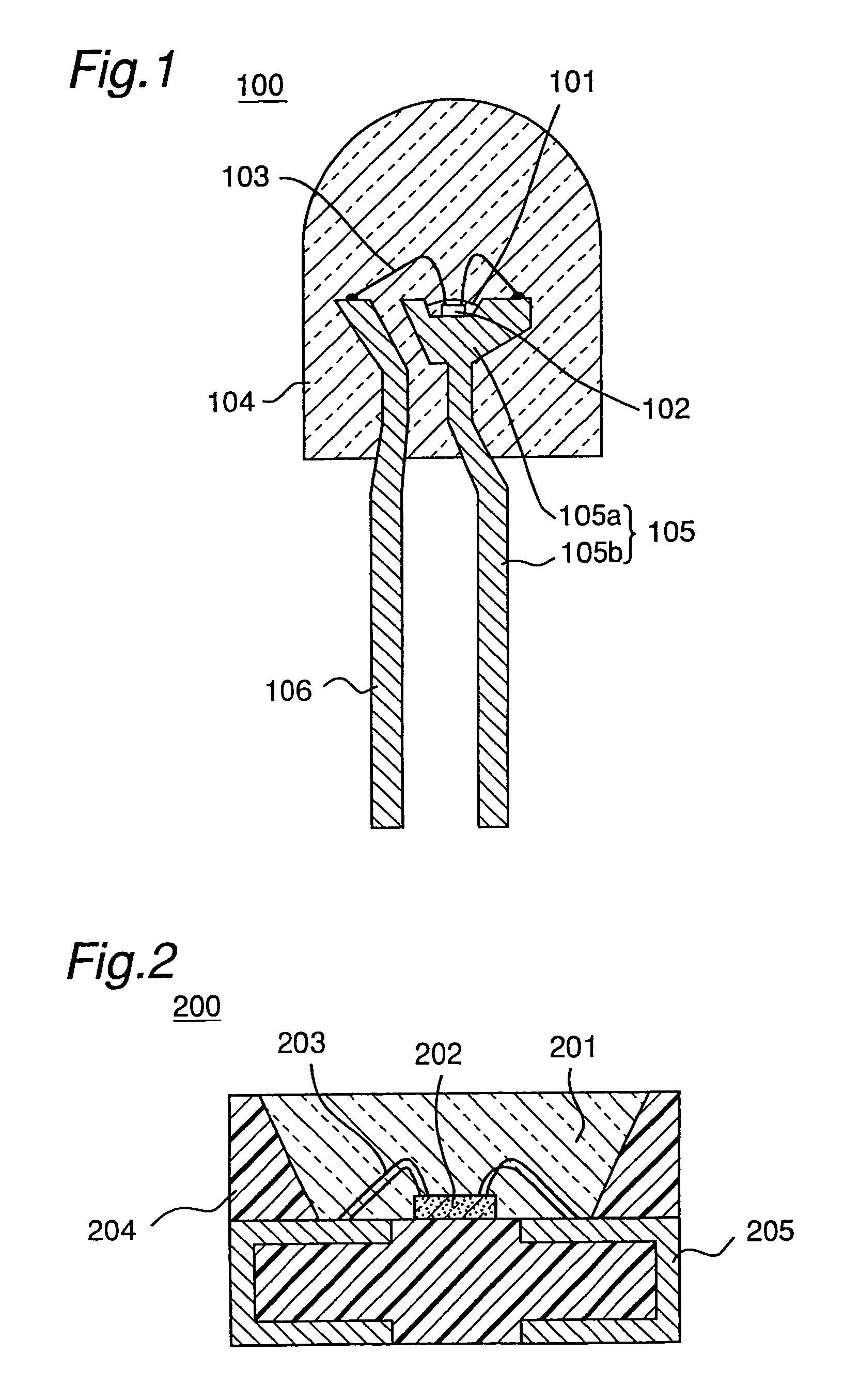
Light emitting device with blue light LED and phosphor components
InactiveUS7026756B2Low degree of deterioration in emission light intensityIncrease brightnessMechanical apparatusDischarge tube luminescnet screensIndiumPhosphor
A light emitting device includes a light emitting component having an active layer of a semiconductor and a phosphor capable of absorbing a part of light emitted from the light emitting component and emitting light of wavelength different from that of the absorbed light, wherein the light emitting component is a LED which has an active layer constituting a gallium nitride based semiconductor containing Indium and is capable of emitting a blue color light with a peak wavelength within the range from 420 to 490 nm. The phosphor is a garnet fluorescent material activated with cerium which is capable of absorbing a part of the blue color light and thereby emitting light having a broad emission spectrum with a peak wavelength existing around the range from 510 to 600 nm and a tail continuing into the region from 700 to 750 nm.
Owner:NICHIA CORP
Electroless plating processes
InactiveUS6861097B1Reducing problem encounteredSimple methodPaper/cardboard articlesDecorative surface effectsPolymeric surfaceOxidation state
The invention includes processes for combined polymer surface treatment and metal deposition. Processes of the invention include forming an aqueous solution containing a metal activator, such as an oxidized species of silver, cobalt, ruthenium, cerium, iron, manganese, nickel, rhodium, or vanadium. The activator can be suitably oxidized to a higher oxidation state electrochemically. Exposing a part to be plated (such as an organic resin, e.g. a printed circuit board substrate) to the solution enables reactive hydroxyl species (e.g. hydroxyl radicals) to be generated and to texture the polymer surface. Such texturing facilitates good plated metal adhesion. As part of this contacting process sufficient time is allowed for both surface texturing to take place and for the oxidized metal activator to adsorb onto said part. The part is then contacted with a reducing agent capable of reducing the metal activator to a lower ionic form, or a lower oxidation state. That reduction can result in the formation of metallic catalytic material over the surface of the part. The reduced metal activator can then function to catalyze the electroless deposition of metal such as copper from solution by contacting the part with the plating solution.
Owner:SHIPLEY CO LLC
Method for Preparing Fuel Element For Smoking Article
ActiveUS20100065075A1Good water solubilityTobacco treatmentTobacco smoke filtersCerium nitrateCopper nitrate
The invention provides a method for making a fuel element for a smoking article comprising forming a combustible carbonaceous material into a fuel element adapted for use in a smoking article; incorporating a metal-containing catalyst precursor into the fuel element or onto the surface thereof to form a treated fuel element, the incorporating step occurring before, during, or after said forming step; and optionally heating or irradiating the treated fuel element at a temperature and for a time sufficient to convert the catalyst precursor to a catalytic metal compound. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
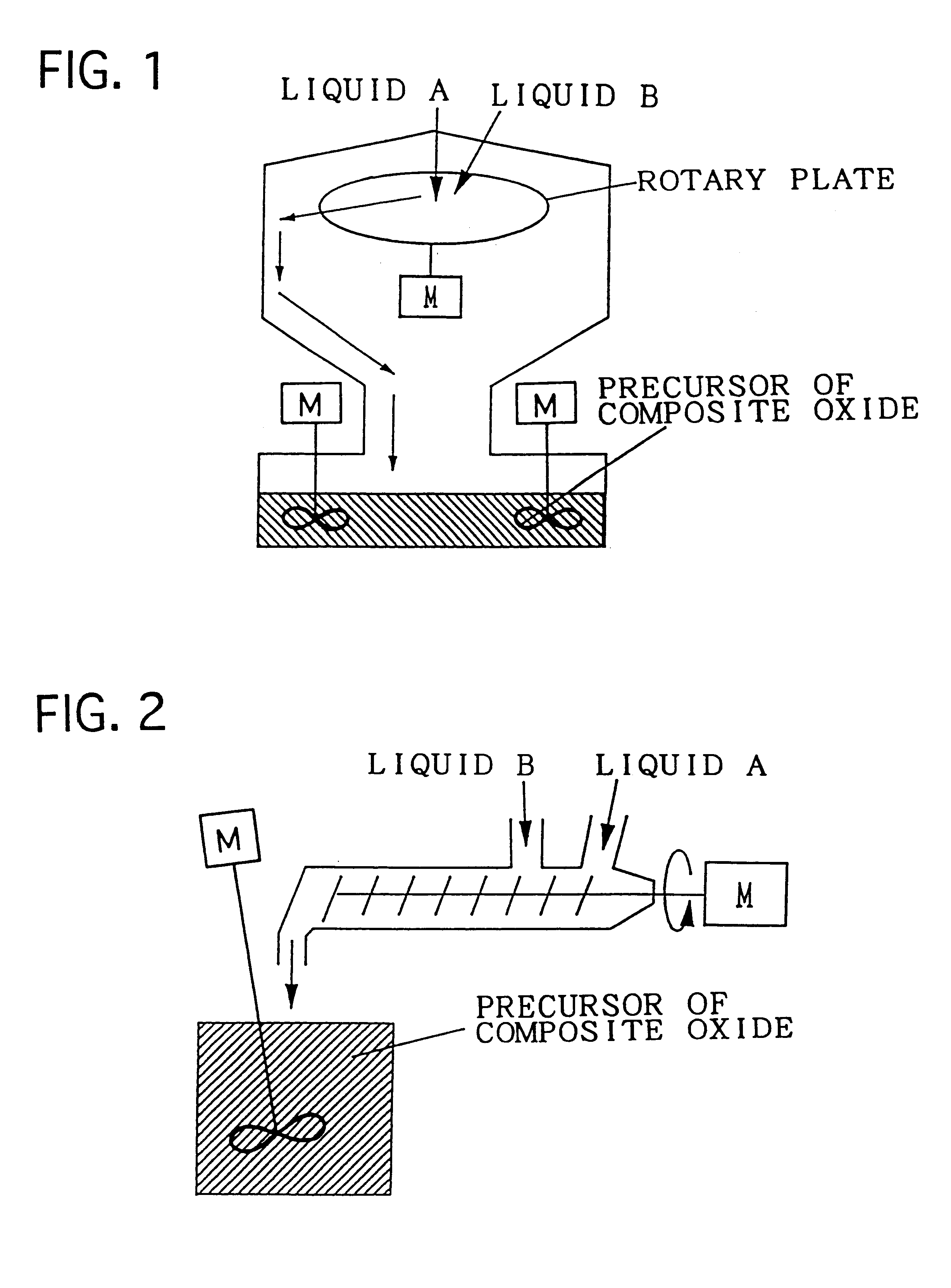
Composite oxide, composite oxide carrier and catalyst
InactiveUS6306794B1Improve heat resistanceImprove homogeneityInternal combustion piston enginesDispersed particle separationHeat resistanceCerium
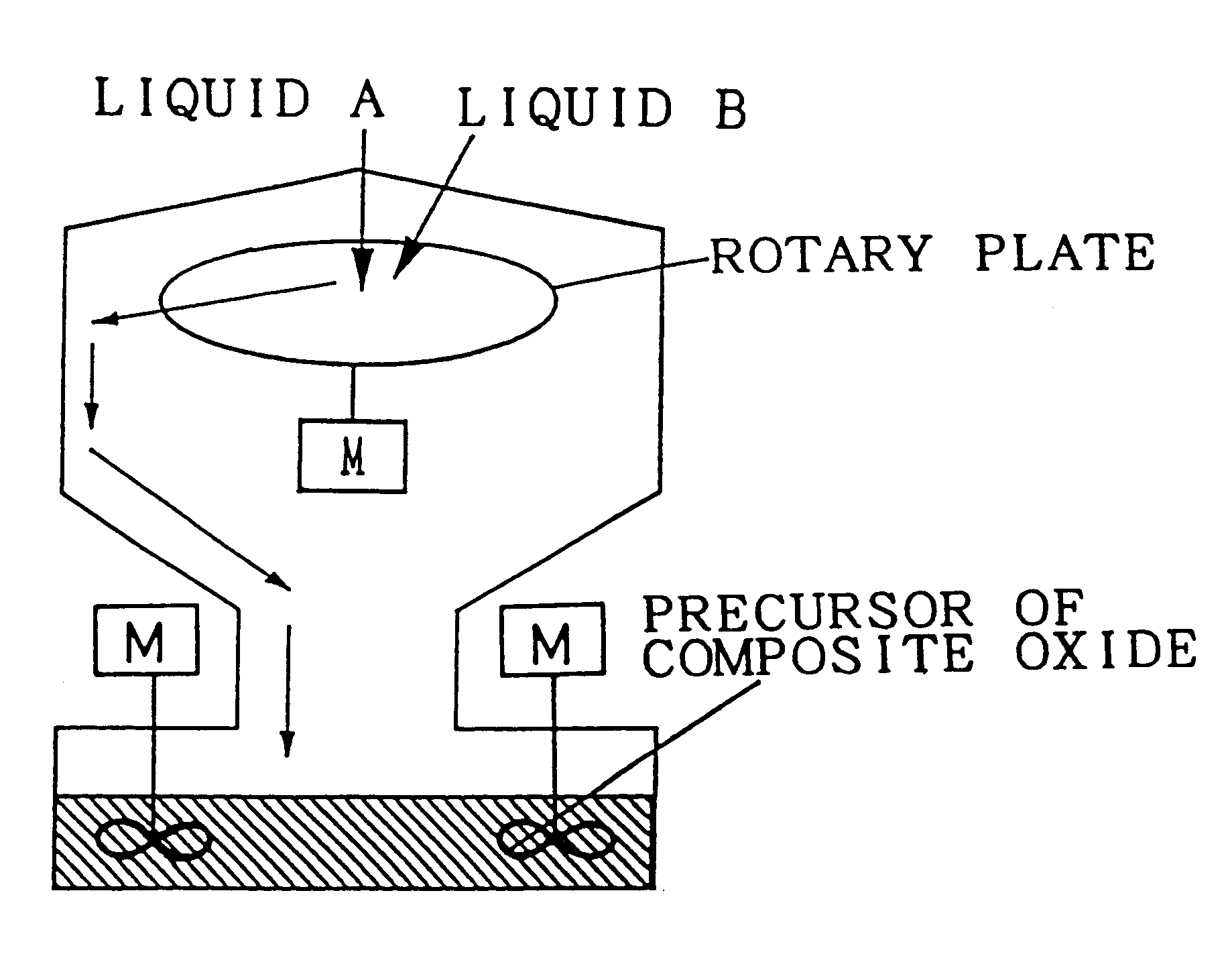
The composite oxide and the composite oxide carrier are manufactured by the precursor forming step and firing step. The composite oxide catalyst is obtained by preparing a composite of catalytic components simultaneously with the formation of the precursor of composite oxide in the step of forming the precursor of composite oxide. The composite oxide and the composite oxide carrier are composed of a composite oxide in which at least one of cerium and zirconium, and aluminium disperse with extremely high homogeneity. With this structure, the heat resistance of the carrier is improved and consequently, enlargement of particles of the composite oxide defining the carrier, and sintering of adjacent particles of the composite oxide can be restrained, whereby the catalyst using the composite oxide carrier in accordance with the present invention is excellent in heat resistance.
Owner:TOYOTA CENT RES & DEV LAB INC
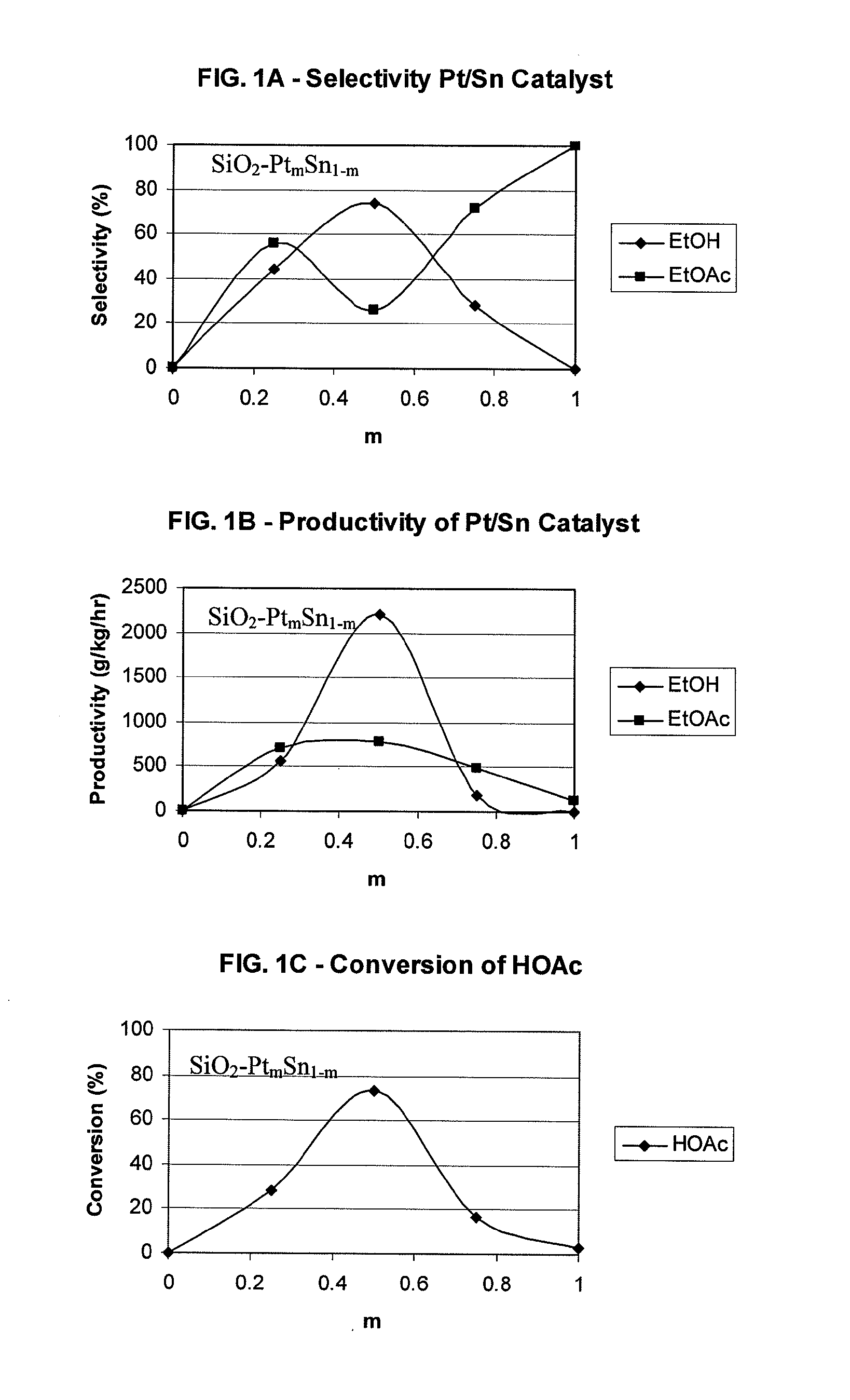
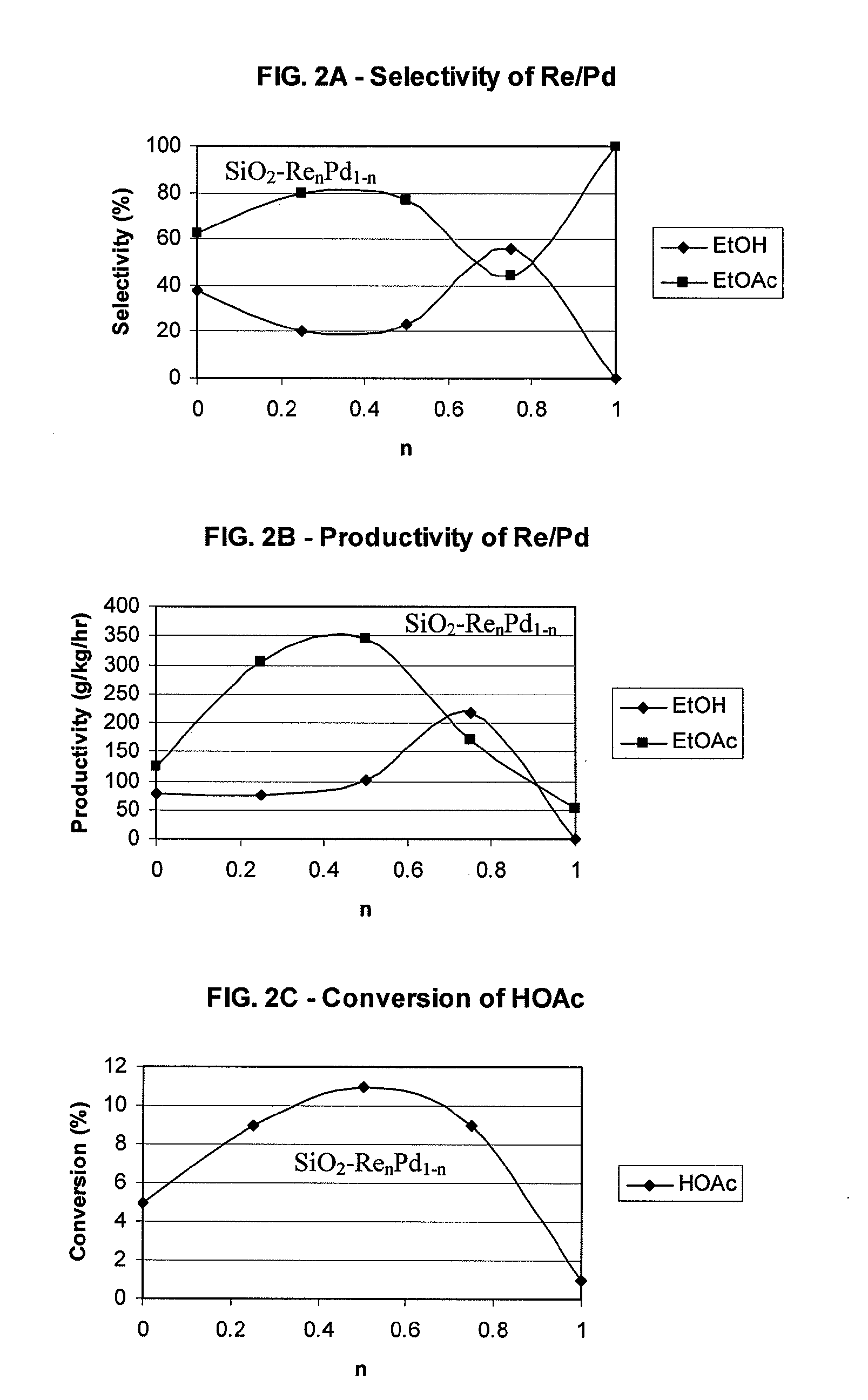
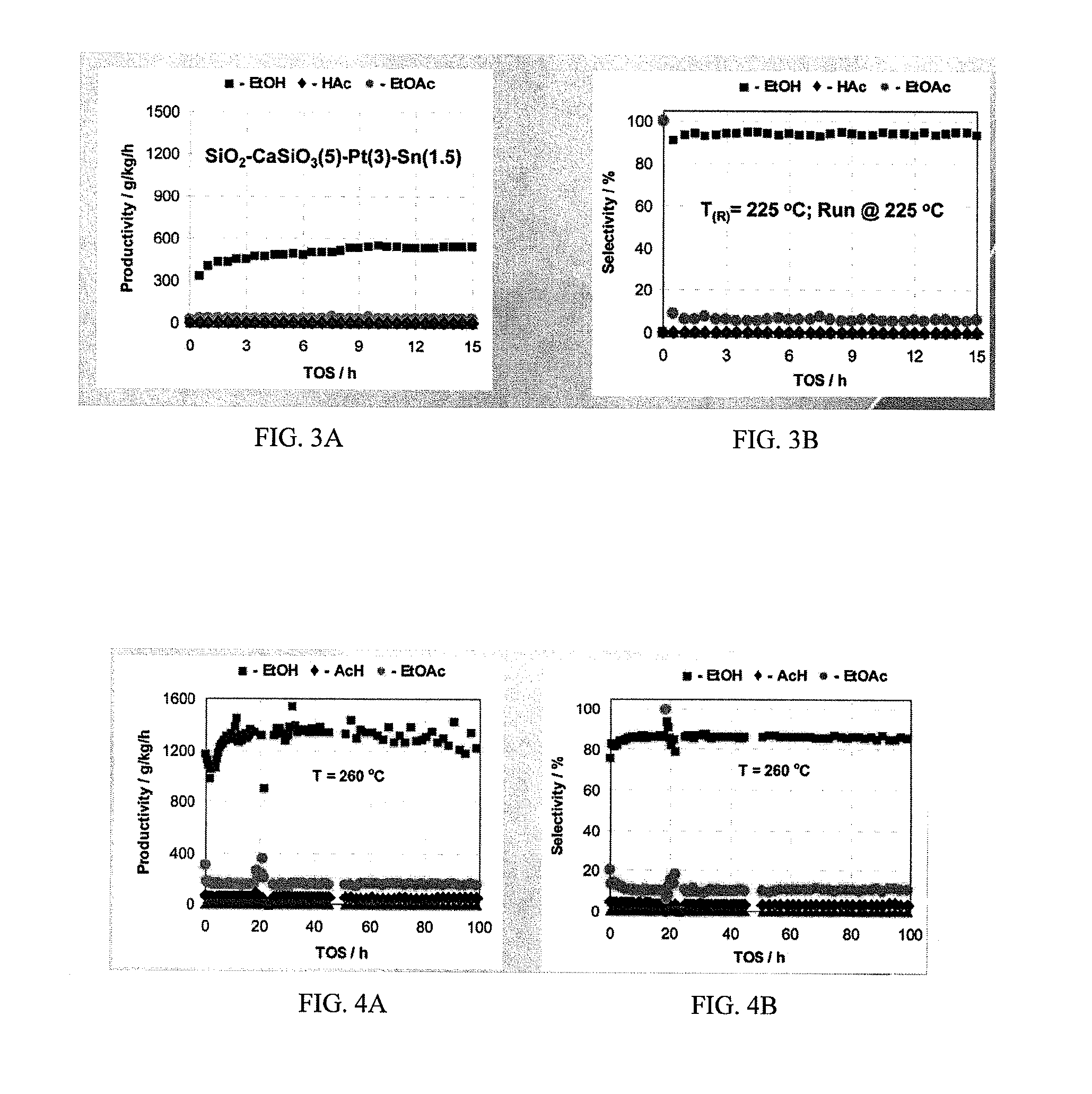
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
Exhaust gas treatment catalyst for internal combustion engines with two catalytically active layers on a carrier structure
InactiveUS6348430B1Improve heat resistanceHigh activityInorganic chemistryInternal combustion piston enginesParticulatesPartial oxidation
A catalyst for treating the exhaust gas from internal combustion engines is provided, wherein the catalyst contains two catalytically active layers supported on a support. The first catalytically active layer contains a platinum group metal in close contact with all of the constituents of the first catalytically active layer, wherein the constituents of the first catalytically active layer include particulate aluminum oxide; particulate oxygen storage material, such as cerium oxide, cerium / zirconium and zirconium / cerium mixed oxides, and alkaline earth metal oxides. The second catalytically active layer, which is in direct contact with the exhaust gas, contains particulate aluminum oxide and at least one particulate oxygen storage material, such as cerium oxide, cerium / zirconium and zirconium / cerium mixed oxides. Rhodium is supported on part of the aluminum oxides in the second catalytically active layer or on the particulate oxygen storage material in the second catalytically active layer. By providing the platinum group metal in close contact with all of the constituents of the first catalytically active layer, improved conversion efficiency of the impurities in the exhaust gas can be achieved.
Owner:UMICORE AG & CO KG +1
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
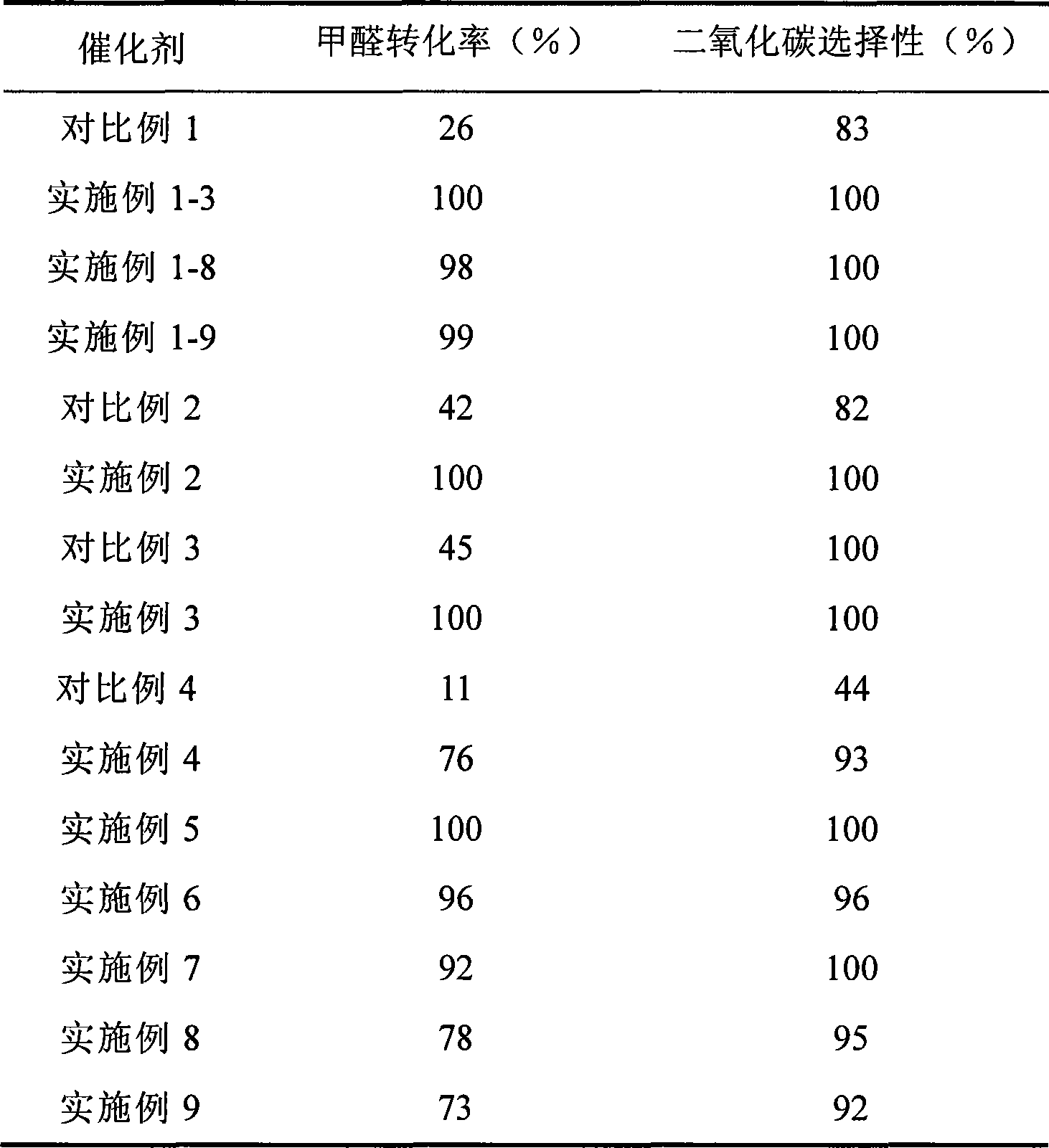
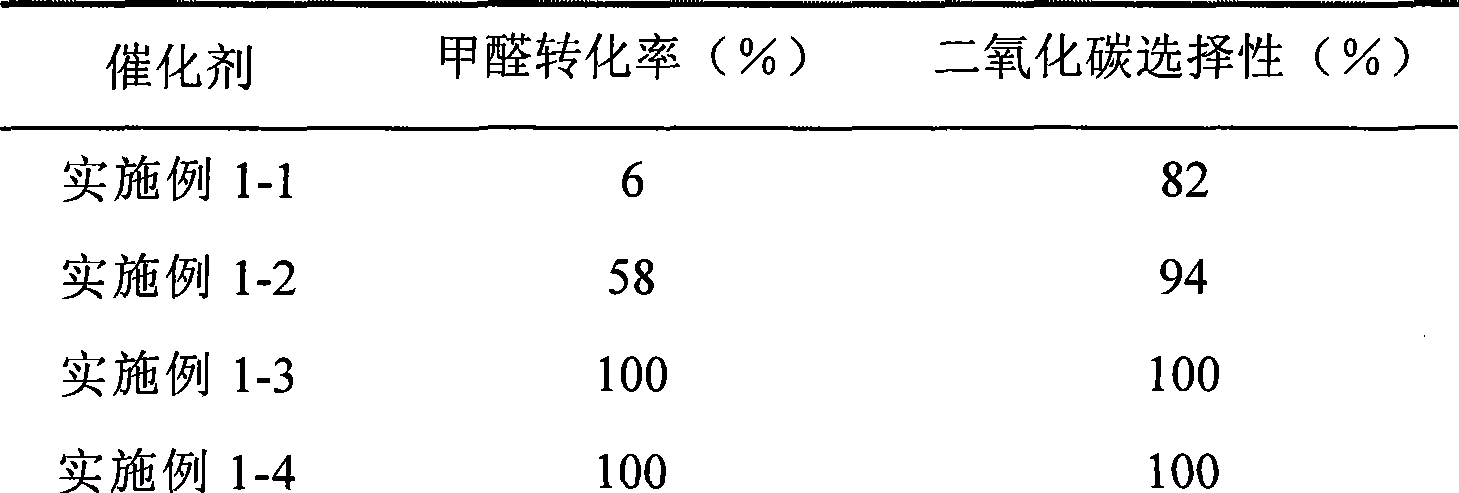
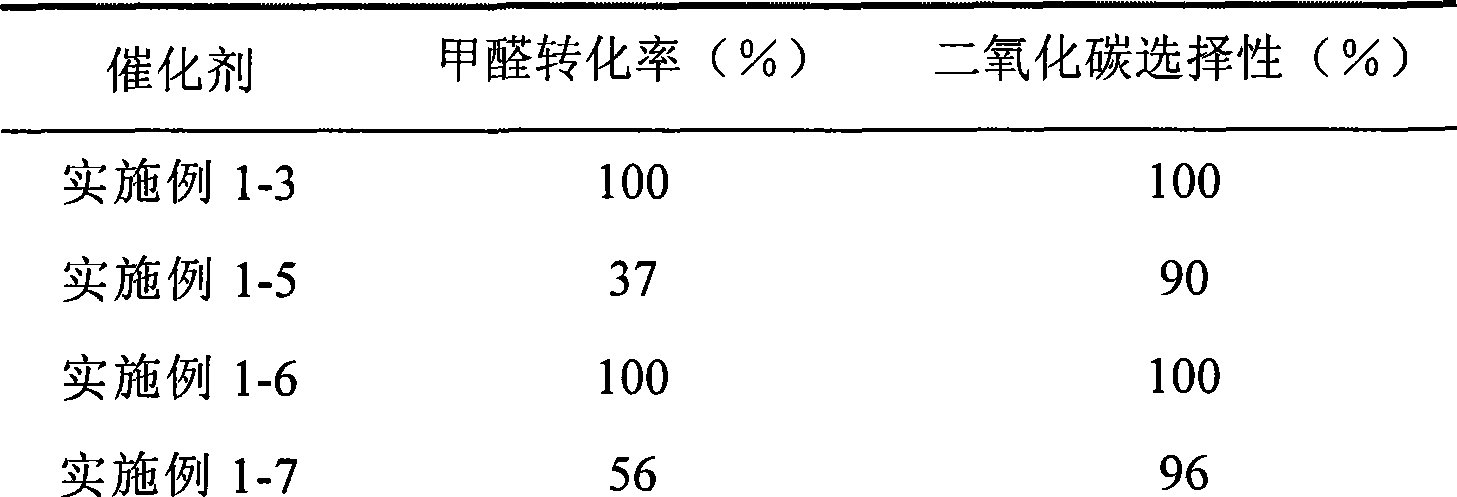
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Scintillating substance and scintillating wave-guide element
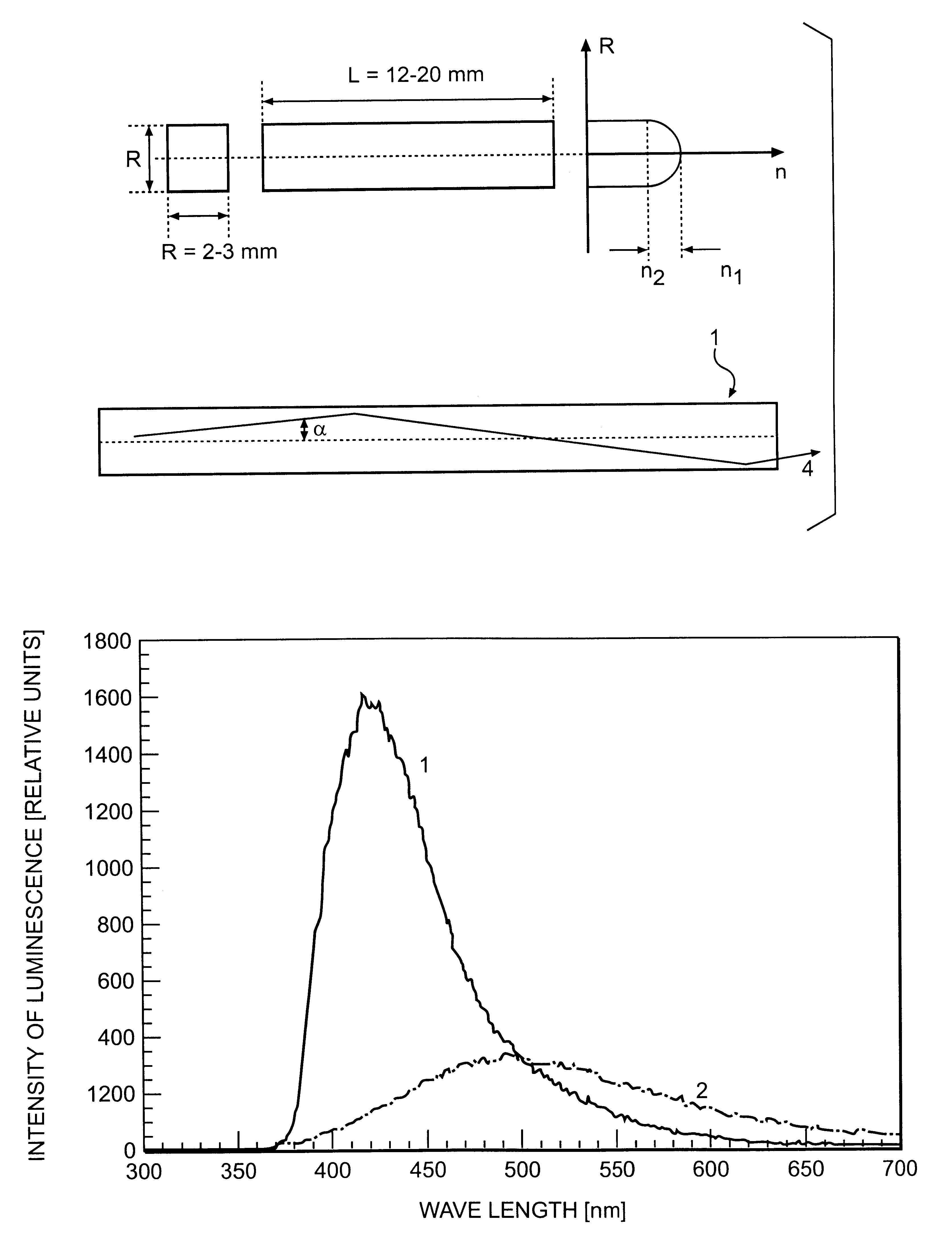
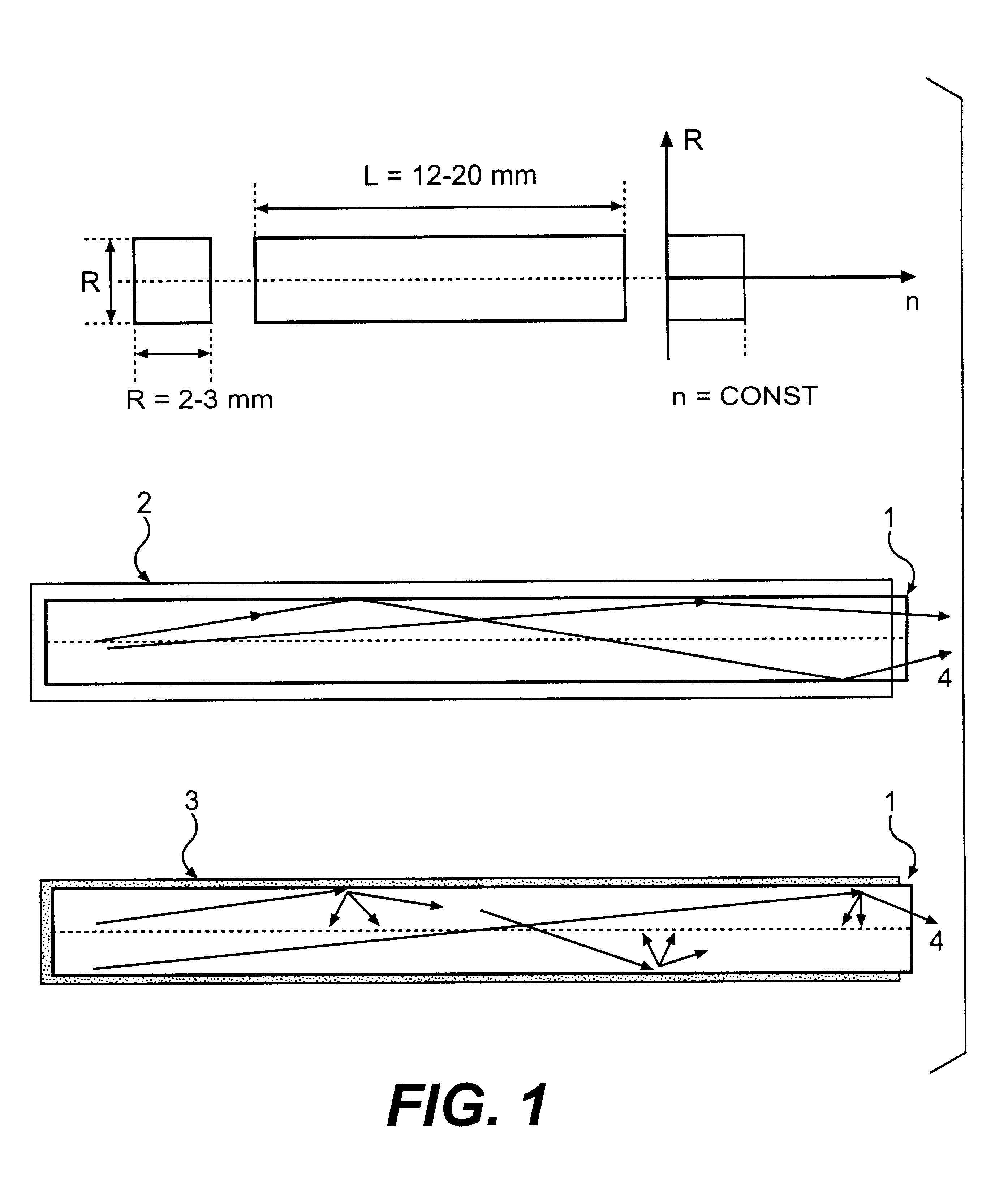
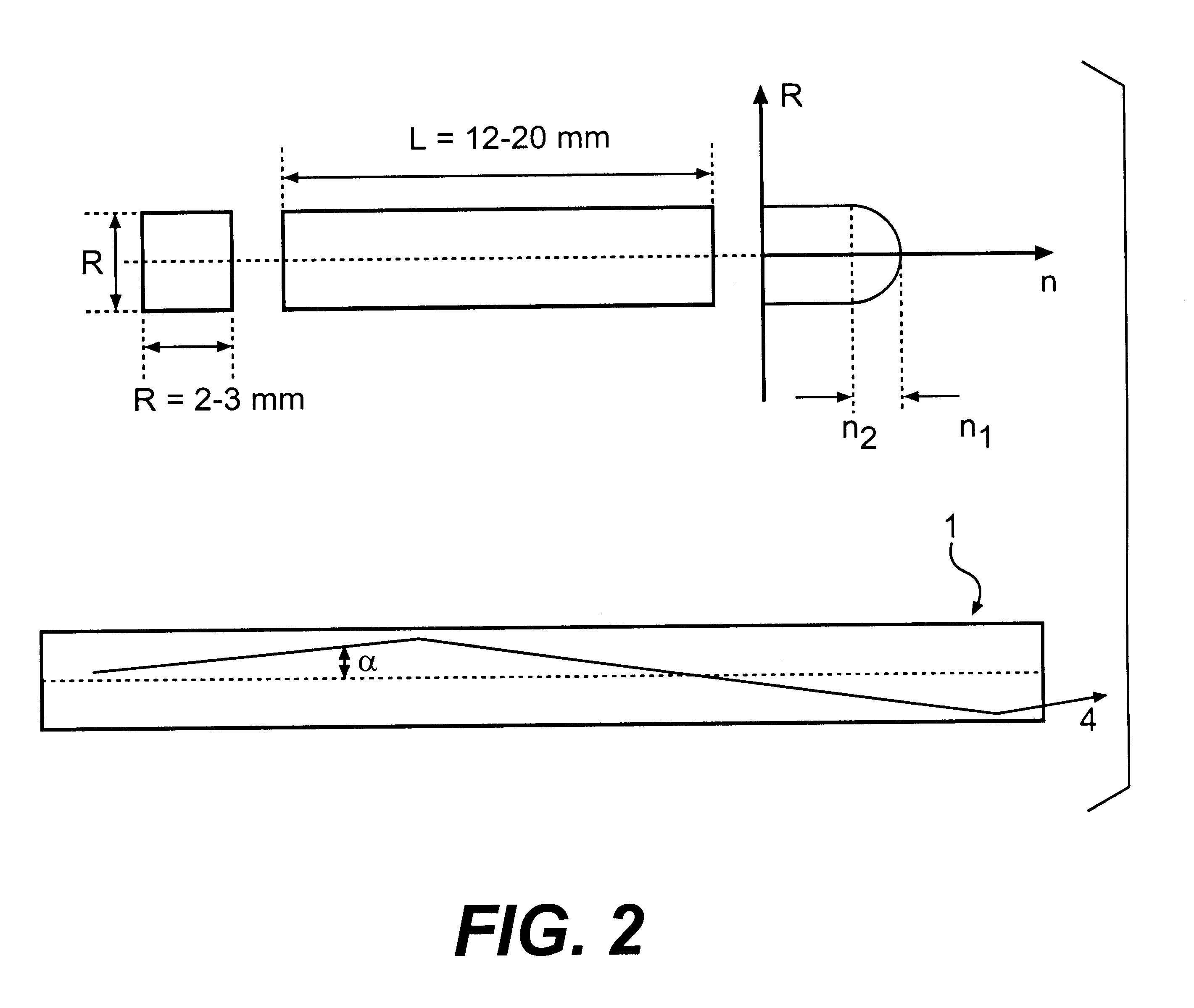
InactiveUS6278832B1Improve light outputShorten the timePolycrystalline material growthOptical fibre with graded refractive index core/claddingFiberAdditive ingredient
The invention is related to nuclear physics, medicine and oil industry, namely to the measurement of x-ray, gamma and alpha radiation; control for trans uranium nuclides in the habitat of a man; non destructive control for the structure of heavy bodies; three dimensional positron-electron computer tomography, etc.The essence of the invention is in additional ingredients in a chemical composition of a scintillating material based on crystals of oxyorthosilicates, including cerium Ce and crystallized in a structural type Lu2SiO5.The result of the invention is the increase of the light output of the luminescence, decrease of the time of luminescence of the ions Ce3+, increase of the reproducibility of grown crystals properties, decrease of the cost of the source melting stock for growing scintillator crystals, containing a large amount of Lu2O3, the raise of the effectiveness of the introduction of SCintillating crystal luminescent radiation into a glass waveguide fibre, prevention of cracking of crystals during the production of elements, creation of waveguide properties in scintillating elements, exclusion of expensive mechanical polishing of their lateral surface.
Owner:SOUTHBOURNE INVESTMENTS
Multifunctional composite absorbing material for purifying water and preparation method thereof
ActiveCN102350298AReduce wasteEfficient removalOther chemical processesWater/sewage treatment bu osmosis/dialysisAcrylic resinCerium
The invention provides a multifunctional composite absorbing material for purifying water and a preparation method thereof, relating to an absorbing material. The invention provides the multifunctional composite absorbing material for purifying water, which can be used for effectively removing a plurality of harmful substances in the water and has higher removal efficiency and lower production cost, and the preparation method thereof. The absorbing material is selected from at least one of absorbing materials A, B and C; the absorbing material A takes a mesoporous adsorption ceramic material as a carrier to load nano metals including nano silver, nano zinc, nano iron and nano cerium; the absorbing material B takes the mesoporous adsorption ceramic material as the carrier to load nano metal oxides including nano titanium dioxide, nano zinc oxide, nano ferric oxide and nano cerium dioxide; and the absorbing material C is prepared from the following raw materials according to the mass ratio: 100-200 parts of active carbon powder, 20-30 parts of polyethylene powder, 10-30 parts of calcium sulfite powder, 10-30 parts of natural zeolite powder, 20-40 parts of macroporous acrylic resin and 10-20 parts of attapulgite powder.
Owner:XIAMEN BAILIN WATER PURIFICATION TECH CO LTD
Method for preparing fuel element for smoking article
The invention provides a method for making a fuel element for a smoking article including the steps of mixing a metal-containing catalyst precursor with a filler material or graphite or a combination thereof to form a pre-treated fuel element component; optionally calcining the pre-treated fuel element component in order to convert the catalyst precursor to a catalytic metal compound; after the optional calcining step, combining the pre-treated fuel element component with a carbonaceous material and a binder to produce a fuel element composition; and forming the fuel element composition into a fuel element adapted for use in a smoking article. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Scintillator compositions, and related processes and articles of manufacture
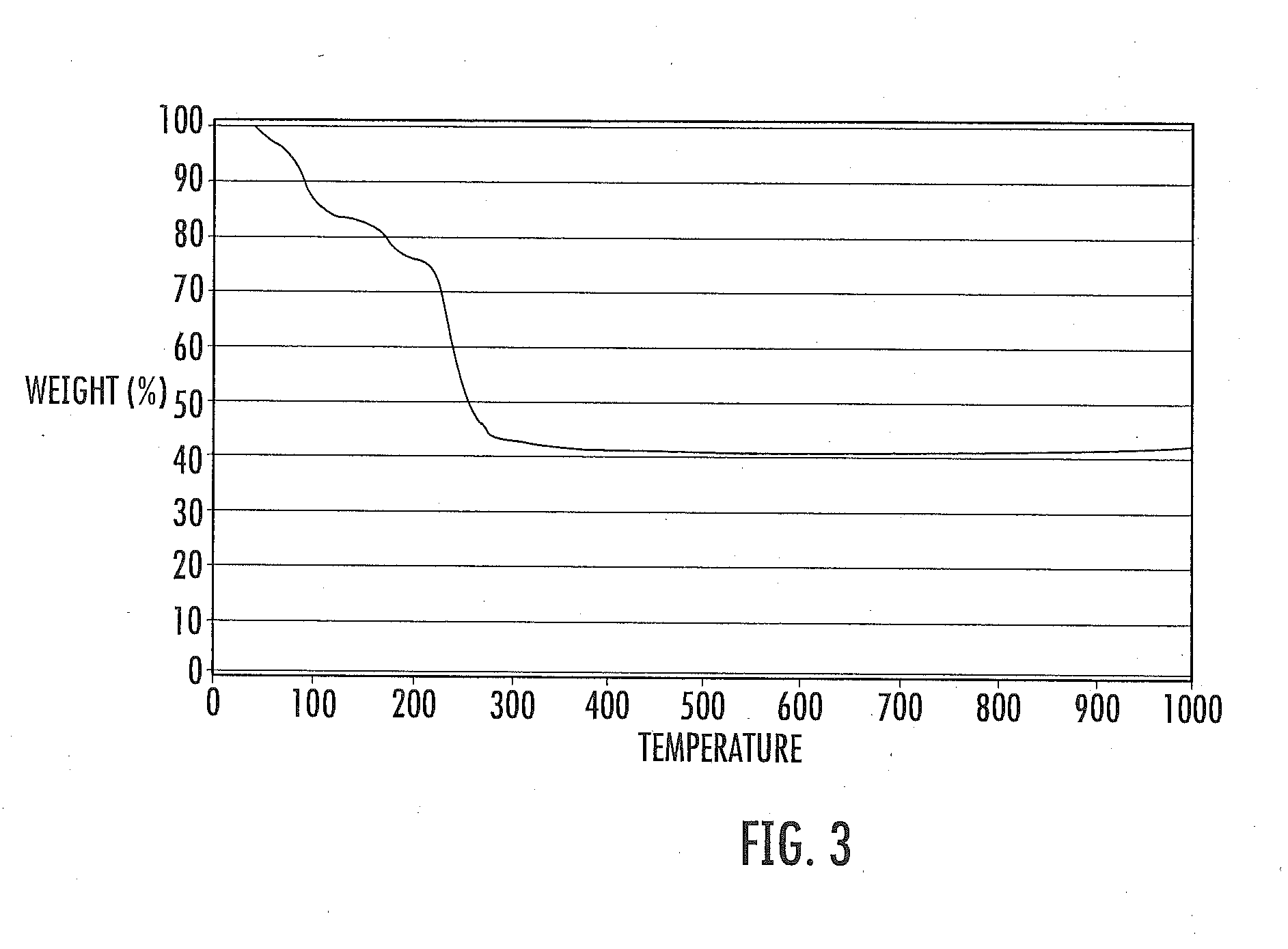
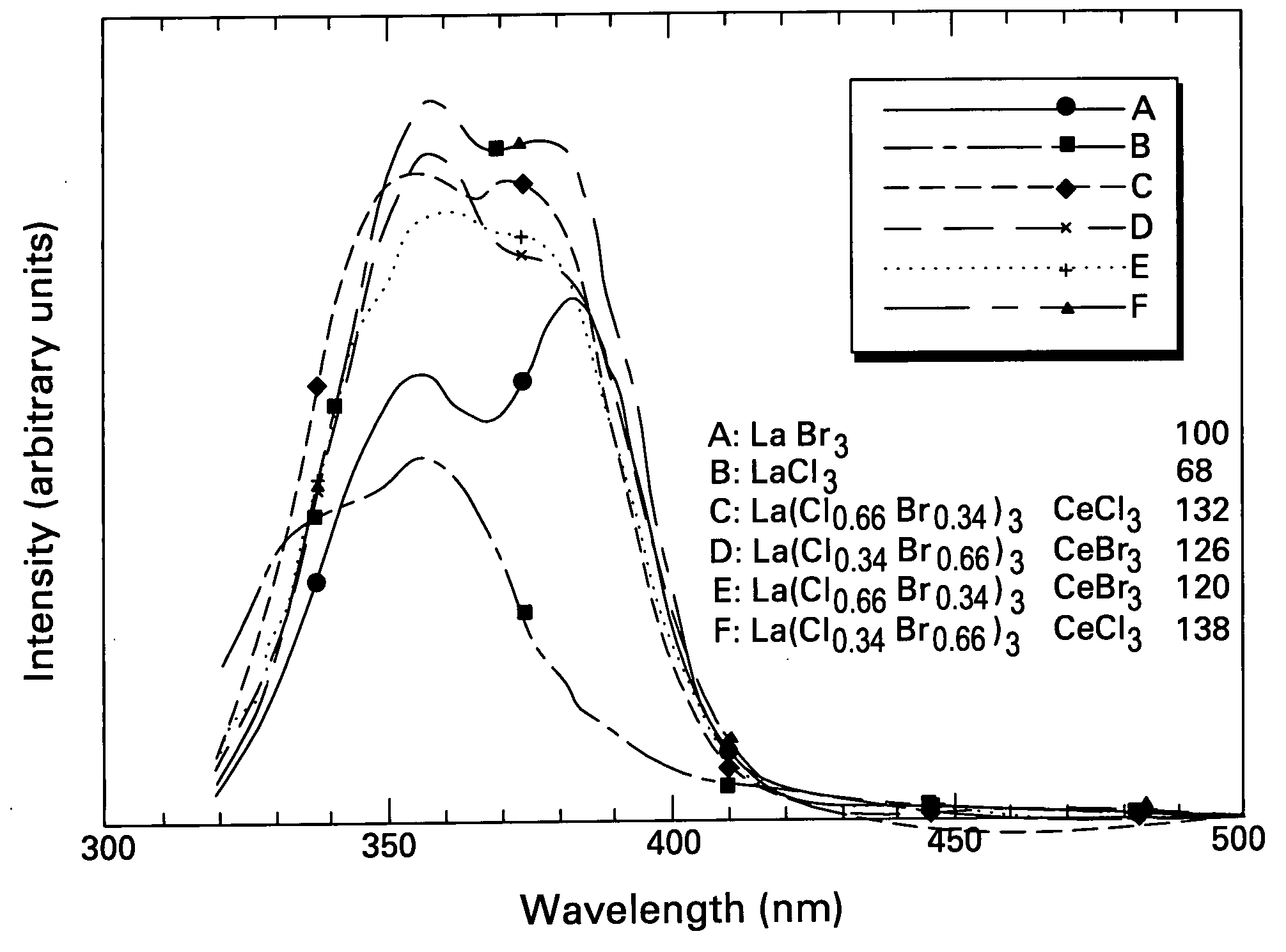
ActiveUS20050082484A1Improve performanceExcellent and reproducible scintillation responseMaterial analysis by optical meansLuminescent compositionsIodideHigh energy
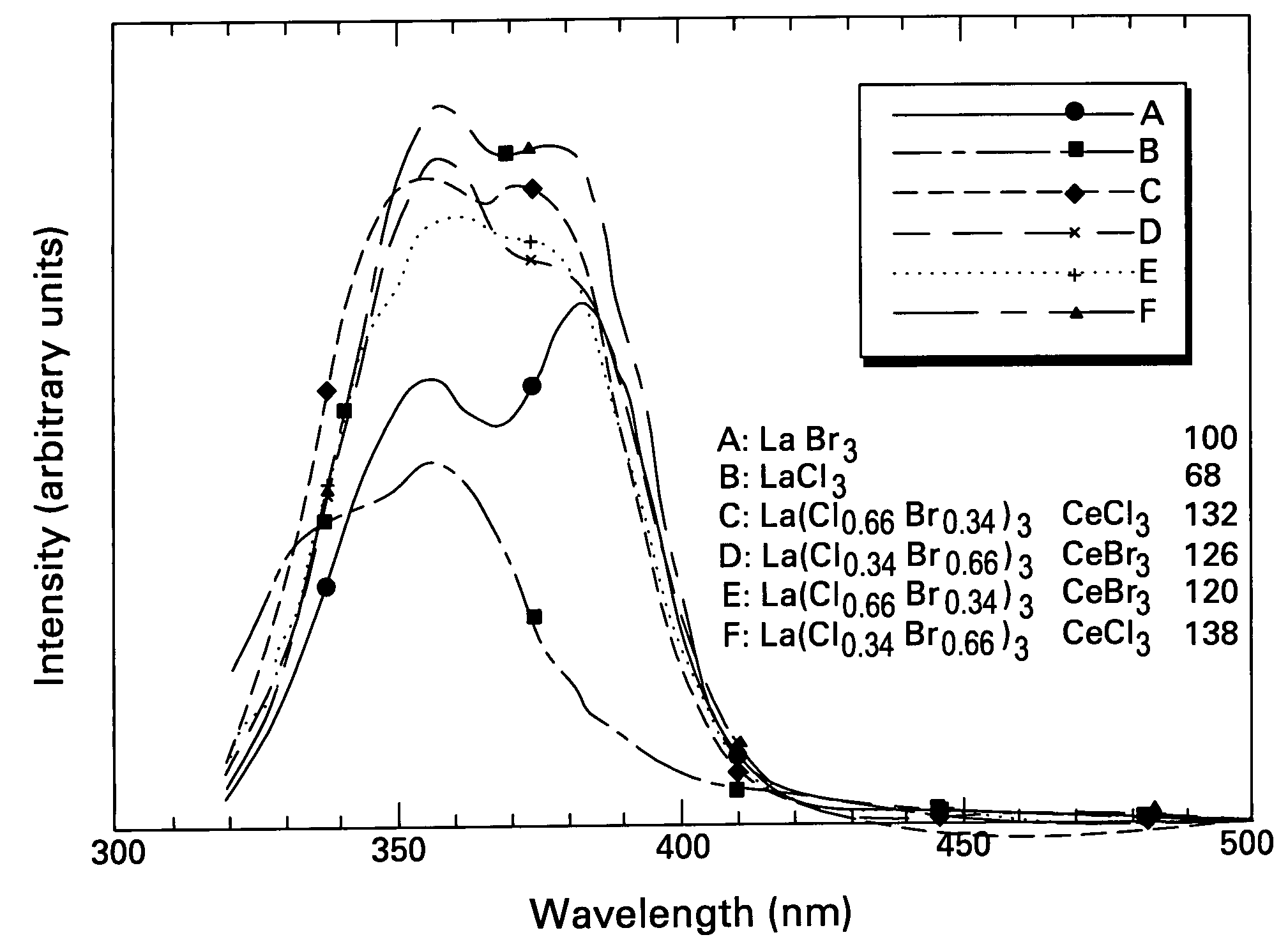
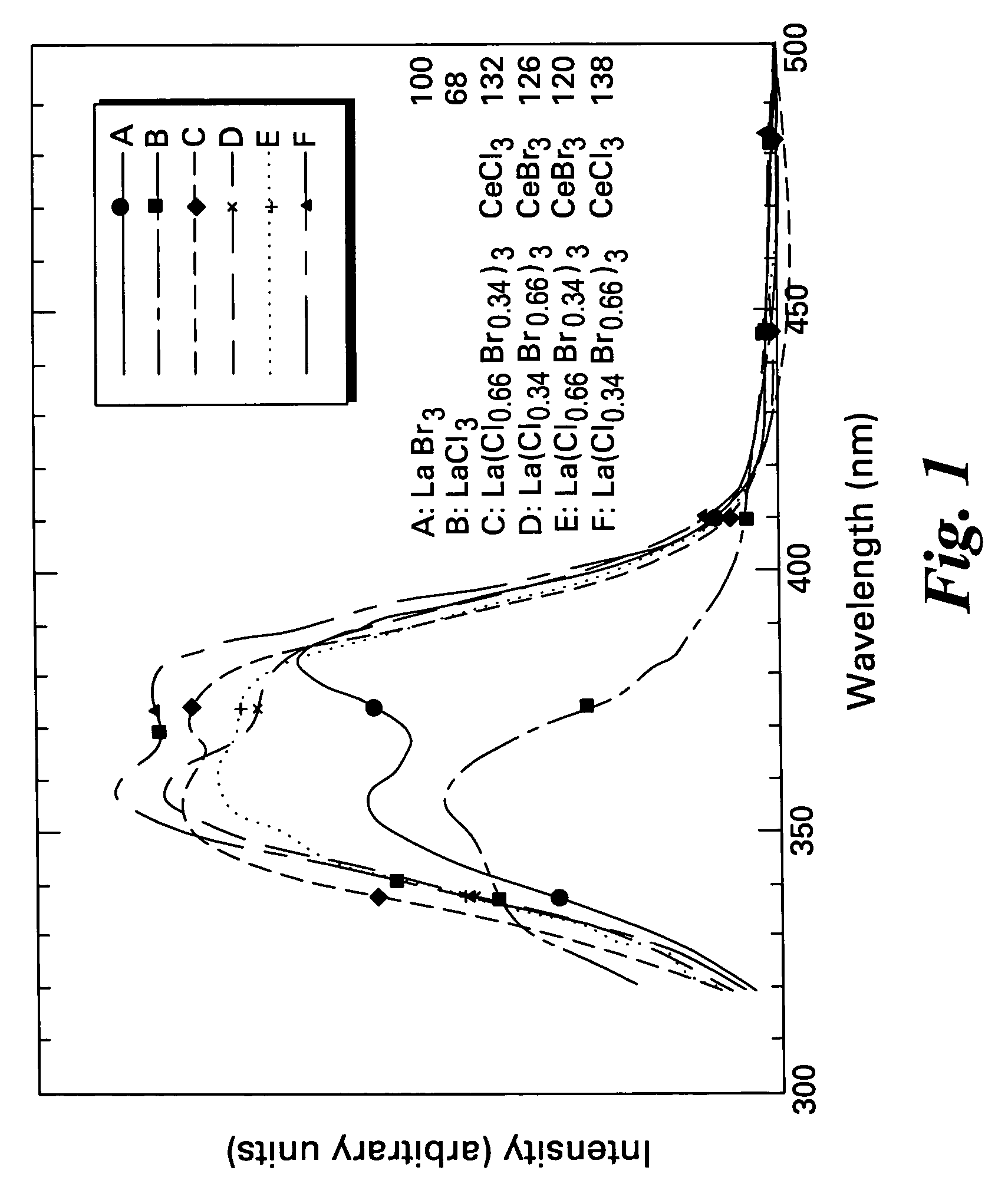
Scintillator materials based on certain types of halide-lanthanide matrix materials are described. In one embodiment, the matrix material contains a mixture of lanthanide halides, i.e., a solid solution of at least two of the halides, such as lanthanum chloride and lanthanum bromide. In another embodiment, the matrix material is based on lanthanum iodide alone, which must be substantially free of lanthanum oxyiodide. The scintillator materials, which can be in monocrystalline or polycrystalline form, also include an activator for the matrix material, e.g., cerium. Radiation detectors that use the scintillators are also described, as are related methods for detecting high-energy radiation.
Owner:BAKER HUGHES OILFIELD OPERATIONS LLC
Method for preparing fuel element for smoking article
The invention provides a method for making a fuel element for a smoking article comprising forming a combustible carbonaceous material into a fuel element adapted for use in a smoking article; incorporating a metal-containing catalyst precursor into the fuel element or onto the surface thereof to form a treated fuel element, the incorporating step occurring before, during, or after said forming step; and optionally heating or irradiating the treated fuel element at a temperature and for a time sufficient to convert the catalyst precursor to a catalytic metal compound. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Gas purifying process and gas purifying apparatus
A method is provided for removing water, carbon monoxide and carbon dioxide out of a gas, such as air, by passing the gas through a packed column so that the gas sequentially contacts a catalyst consisting of platinum or palladium and at least one member selected from the group consisting of iron, cobalt, nickel, manganese, copper, chromium, tin, lead and cerium wherein the catalyst is supported on alumina containing substantially no pores having pore diameters of 110 Angstroms or less under conditions which oxidize the carbon monoxide in the gas into carbon dioxide; an adsorbent selected from the group consisting of silica gel, activated alumina, zeolite and combinations thereof under conditions in which water is adsorbed and removed from the gas and an adsorbent selected from the group consisting of calcium ion exchanged A zeolite; calcium ion exchanged X zeolite; sodium ion exchanged X zeolite and mixtures thereof under conditions which carbon dioxide is adsorbed and removed from the gas. The gas may also be subjected to a catalyst / adsorbent in the packed column to effect oxidation and removal of hydrogen in the gas.
Owner:NIPPON SANSO CORP
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Light emitting device for generating specific colored light, including white light
InactiveUS6850002B2Improve efficiencyDischarge tube luminescnet screensElectric discharge tubesCeriumMetallic sulfide
A device for the generation of specific colored light including white light by luminescent down conversion and additive color mixing based on a light-emitting diode (LED) comprising a semiconductor light-emitting layer emitting near UV light about 370-420 nm or blue light about 420-480 nm and phosphors which absorb completely or partly the light emitted by the light-emitting component and emit light of wavelengths longer than that of the absorbed primary light, wherein the light emitting layer of the light emitting component is preferably a Ga(In)N-based semiconductor; and at least one of the phosphors contains a metal sulfide fluorescent material activated with europium containing at least one element selected from the group consisting of Ba, Sr, Ca, Mg and Zn; and / or at least another phosphor which contains a complex thiometalate fluorescent material activated with either europium, cerium or both europium and cerium containing 1) at least one element selected from the group consisting of Ba, Sr, Ca, Mg and Zn and 2) at least one element selected from the group consisting of Al, Ga, In, Y, La and Gd.
Owner:OSRAM OPTO SEMICONDUCTORS GMBH +1
Shift converter having an improved catalyst composition, and method for its use
InactiveUS6455182B1Reduce amountImproved catalyst compositionHydrogenCatalyst carriersFuel cellsCerium
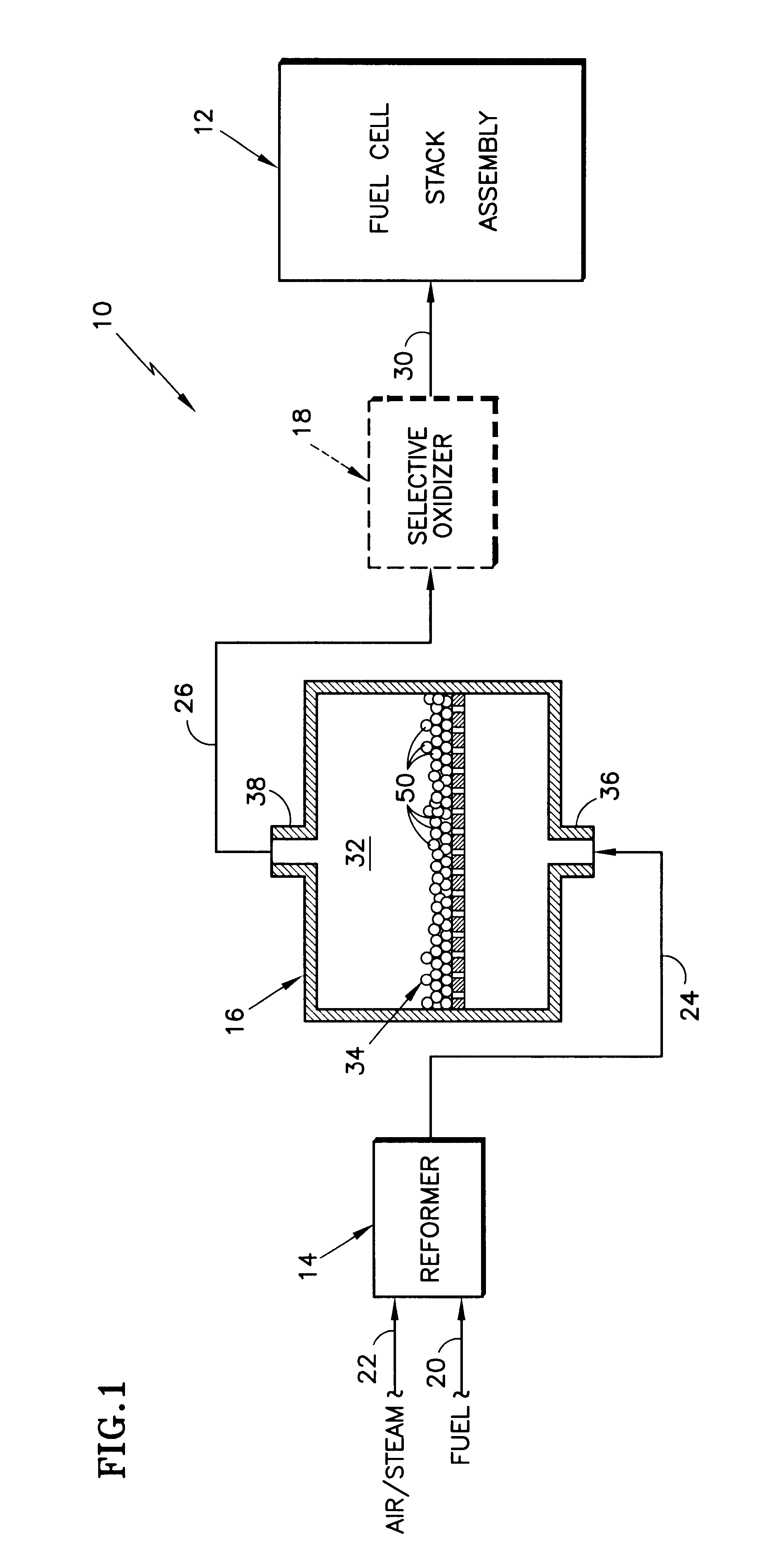
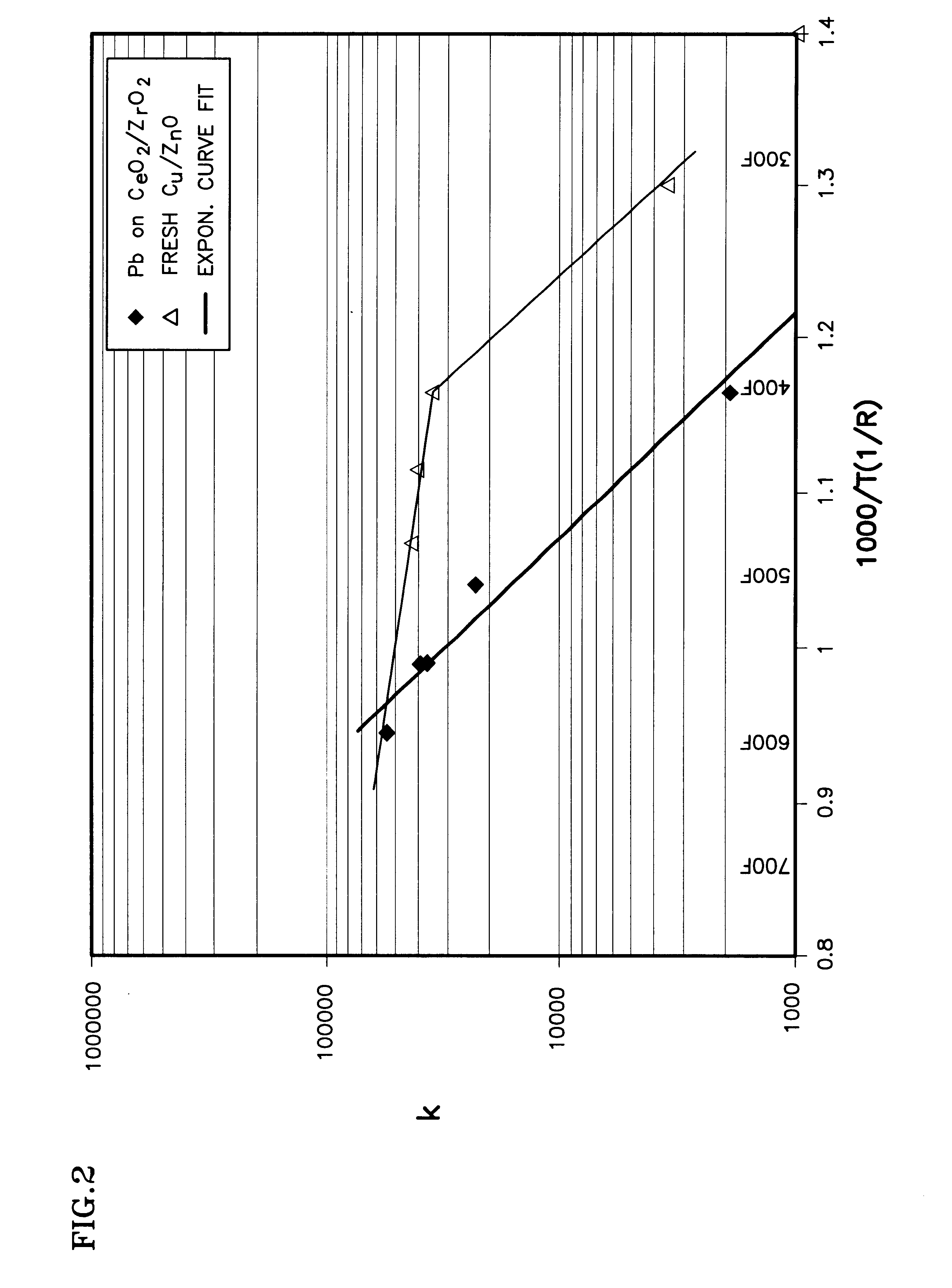
A shift converter (16) in a fuel processing subsystem (14, 16, 18) for a fuel cell (12) uses an improved catalyst composition (50) to reduce the amount of carbon monoxide in a process gas for the fuel cell (12). The catalyst composition (50) is a noble metal catalyst having a promoted support of mixed metal oxide, including at least both ceria and zirconia. Cerium is present in the range of 30 to 50 mole %, and zirconium is present in the range of 70 to 50 mole %. Additional metal oxides may also be present. Use of the catalyst composition (50) obviates the requirement for prior reducing of catalysts, and minimizes the need to protect the catalyst from oxygen during operation and / or shutdown.
Owner:HYAXIOM INC
Layered thermal barrier coatings containing lanthanide series oxides for improved resistance to CMAS degradation
InactiveUS20070160859A1Avoid damageElimination of expensiveBlade accessoriesEfficient propulsion technologiesReaction layerCerium
A coating applied as a two layer system. The outer layer is an oxide of a group IV metal selected from the group consisting of zirconium oxide, hafnium oxide and combinations thereof, which are doped with an effective amount of a lanthanum series oxide. These metal oxides doped with a lanthanum series addition comprises a high weight percentage of the outer coating. As used herein, lanthanum series means an element selected from the group consisting of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu) and combinations thereof, and lanthanum series oxides are oxides of these elements. When the zirconium oxide is doped with an effective amount of a lanthanum series oxide, a dense reaction layer is formed at the interface of the outer layer of TBC and the CMAS. This dense reaction layer prevents CMAS infiltration below it. The second layer, or inner layer underlying the outer layer, comprises a layer of partially stabilized zirconium oxide.
Owner:GENERAL ELECTRIC CO
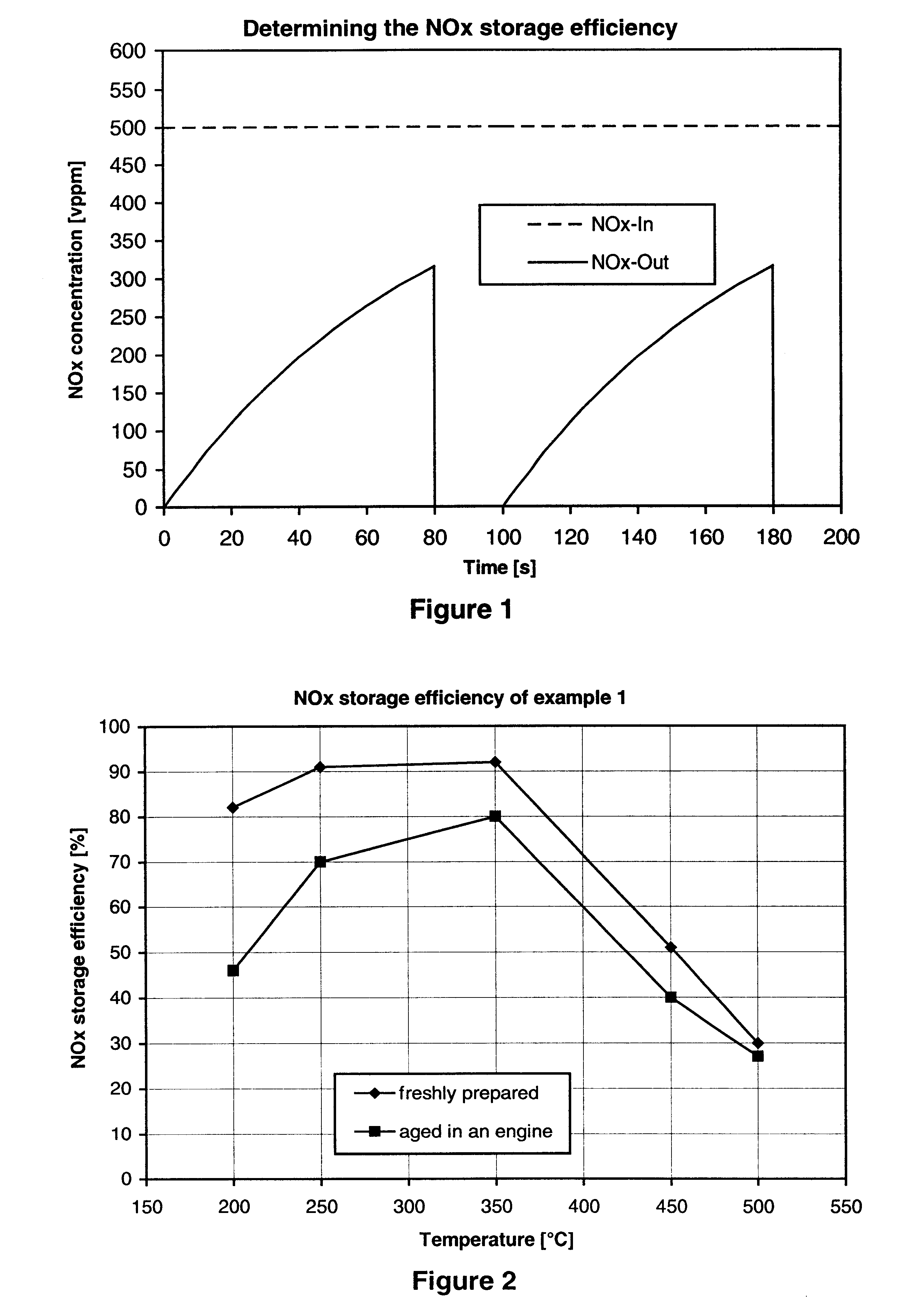
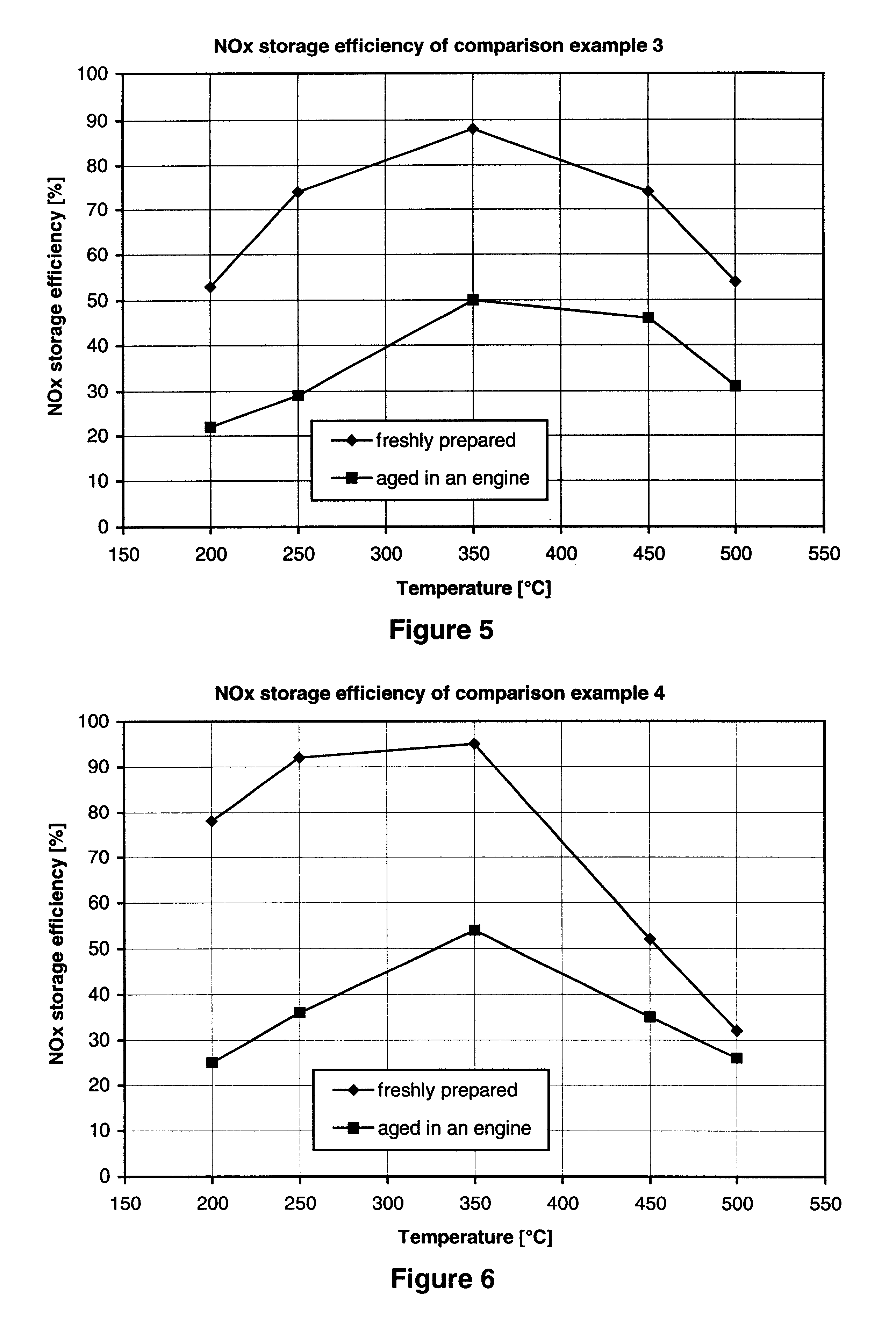
Nitrogen oxide storage material and nitrogen oxide storing catalyst prepared therefrom
InactiveUS6350421B1Determine efficiencyNitrogen compoundsExhaust apparatusAlkaline earth metalCuprate
A nitrogen oxide storage material is disclosed which contains at least one storage component for nitrogen oxides in the form of an oxide, mixed oxide, carbonate or hydroxide of the alkaline earth metals magnesium, calcium, strontium and barium and the alkali metals potassium and caesium on a high surface area support material. The support material can be doped cerium oxide, cerium / zirconium mixed oxide, calcium titanate, strontium titanate, barium titanate, barium stannate, barium zirconate, magnesium oxide, lanthanum oxide, praseodymium oxide, samarium oxide, neodymium oxide, yttrium oxide, zirconium silicate, yttrium barium cuprate, lead titanate, tin titanate, bismuth titanate, lanthanum cobaltate, lanthanum manganate and barium cuprate or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
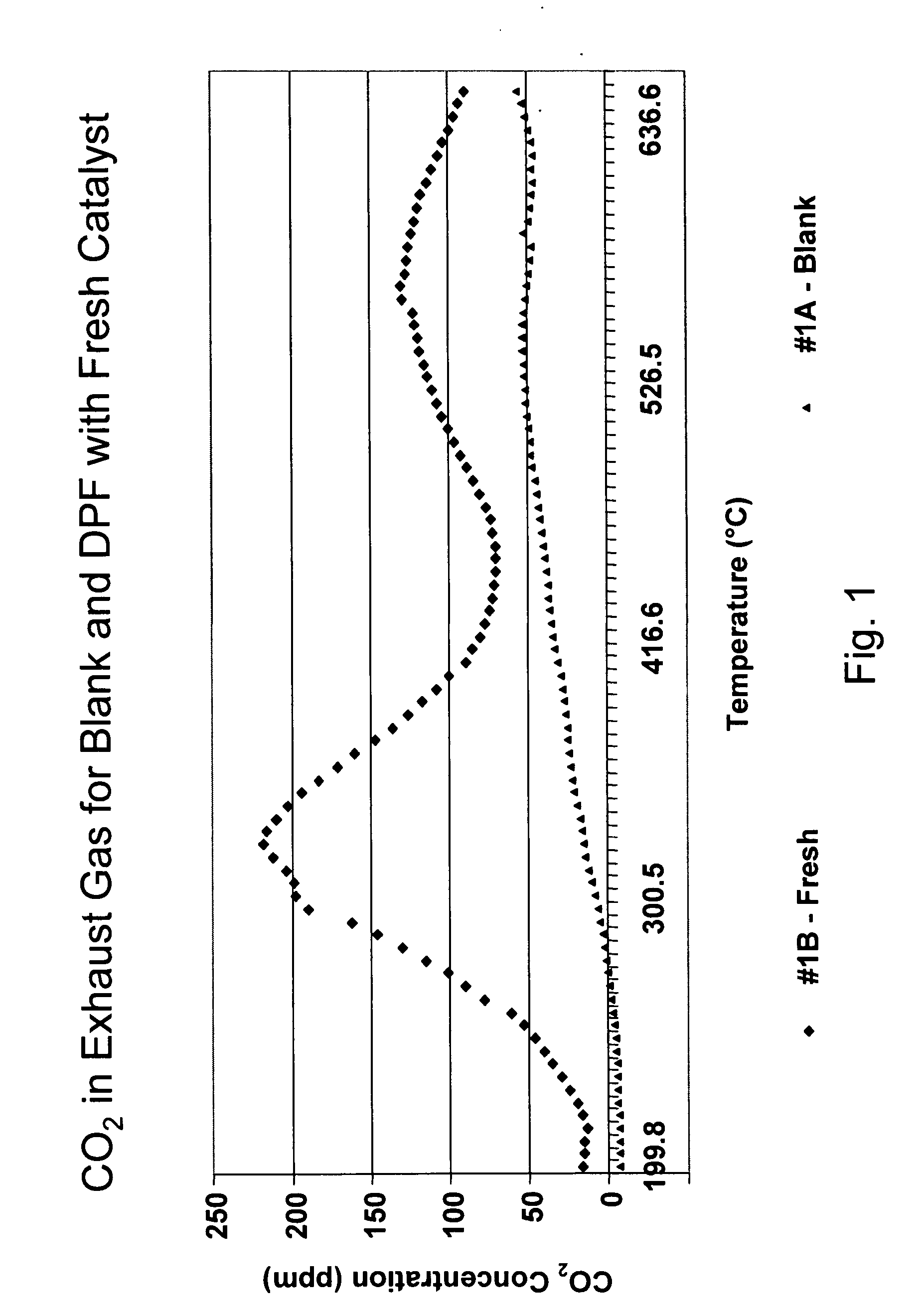
Platinum group metal-free catalysts for reducing the ignition temperature of particulates on a diesel particulate filter
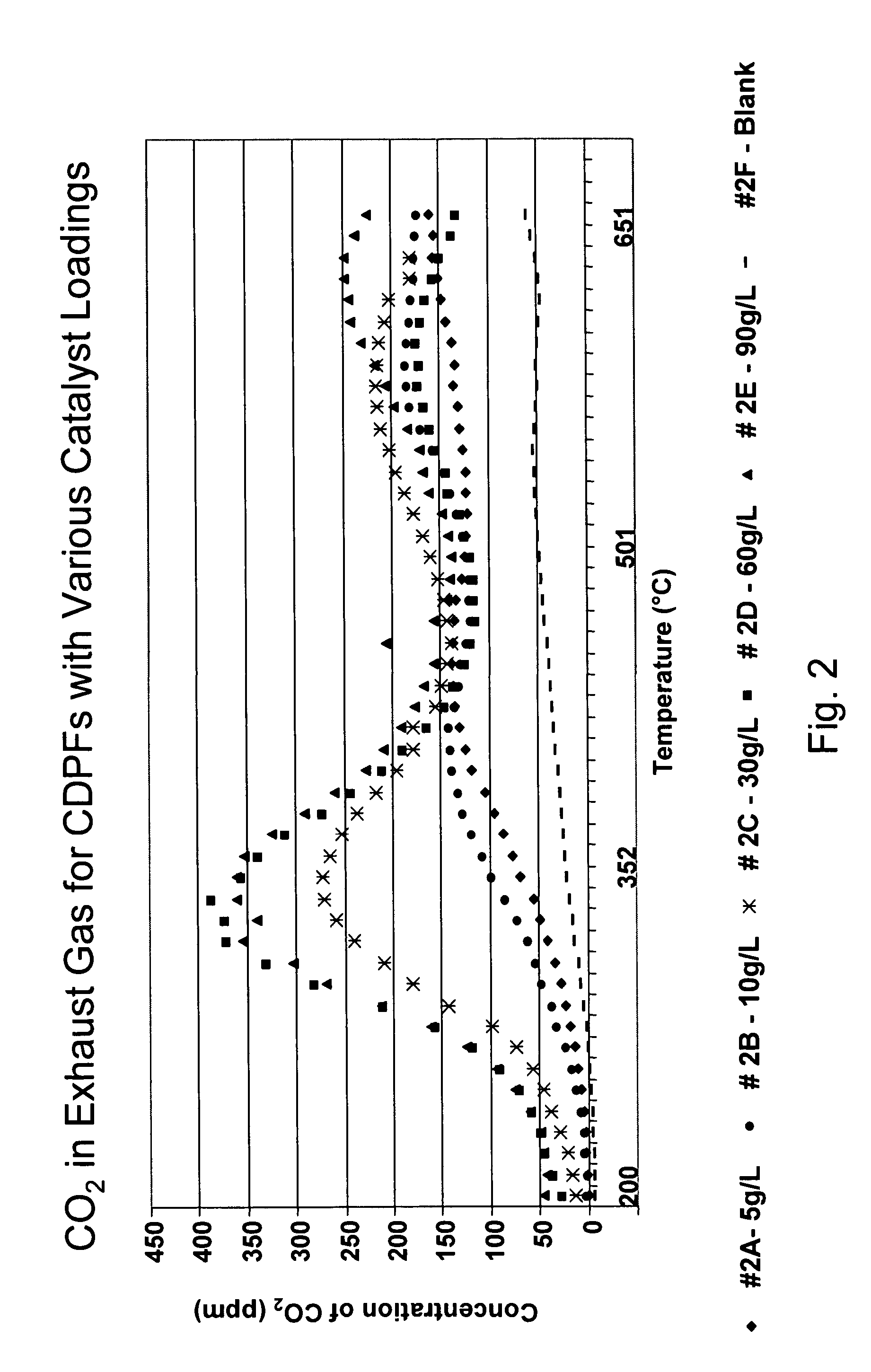
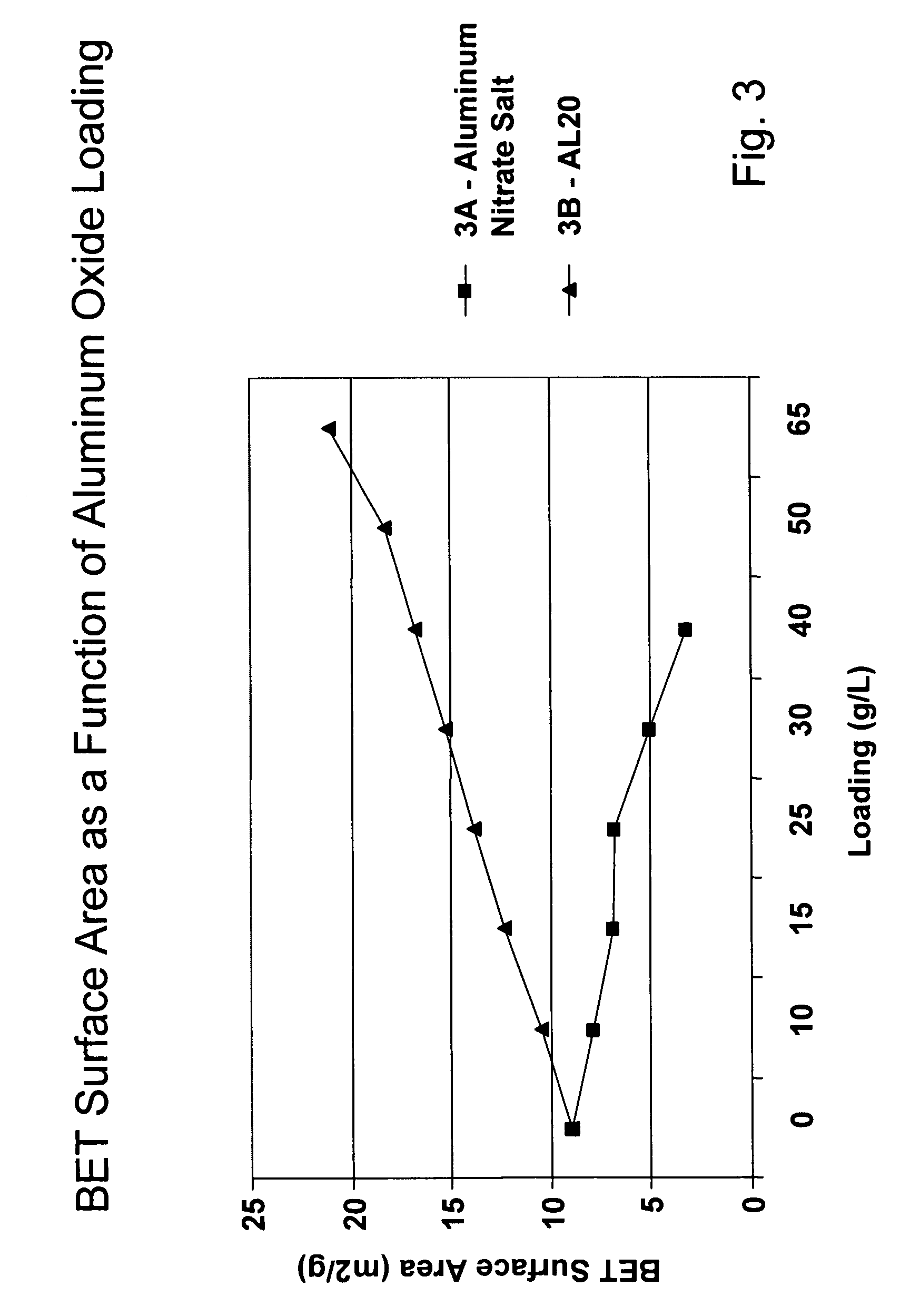
A catalyzed diesel particulate filter (CDPF) and a method for filtering particulates from diesel engine exhaust are provided, where the catalyzed diesel particulate filter includes a substrate and a catalyst composition, where the catalyst composition contains at least one first component, at least one second component, and at least one third component, where the first component is at least one first component selected from the group consisting of cerium and a lanthanide and mixtures thereof, the at least one second component is selected from the group consisting of cobalt, copper, manganese and mixtures thereof; and the third component comprises strontium, where the first component, the second component, and the third component are in an oxide form after calcination. The catalyst on the catalyzed diesel particulate filter lowers the temperature at which particulates are removed from the CDPF by oxidizing the particulates on the filter. The catalyzed diesel particulate filter may also include a washcoat. Washcoats prepared from colloidal aluminum oxide may have higher surface areas and pore volumes loadings than washcoats containing aluminum oxide prepared from aluminum nitrate.
Owner:CATALYTIC SOLUTIONS INC
Catalyst and method for reduction of nitrogen oxides
InactiveUS20060029535A1Efficient workSelective catalytic reductionNitrous oxide captureNitrogen compoundsCerium nitrateIron salts
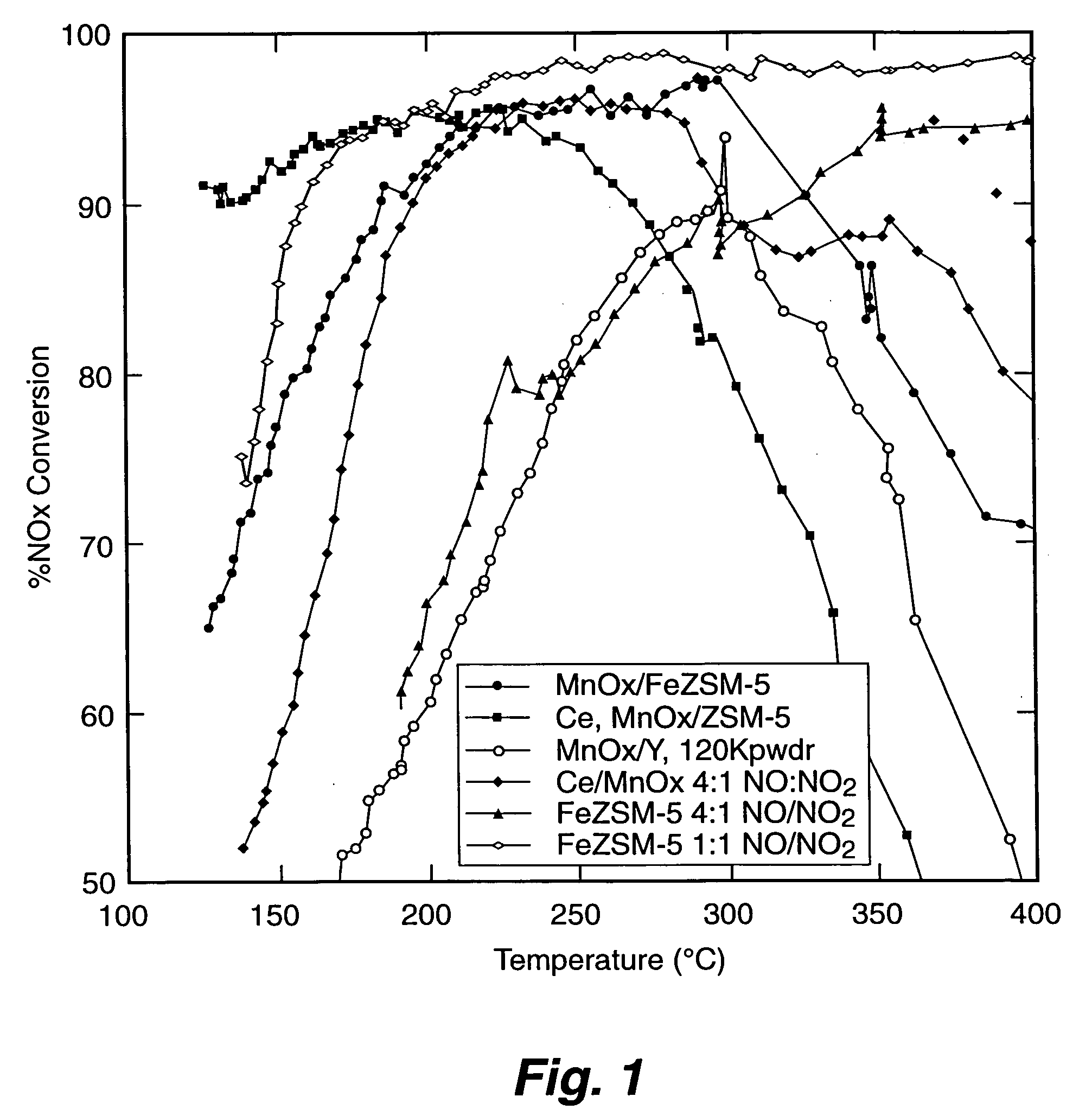
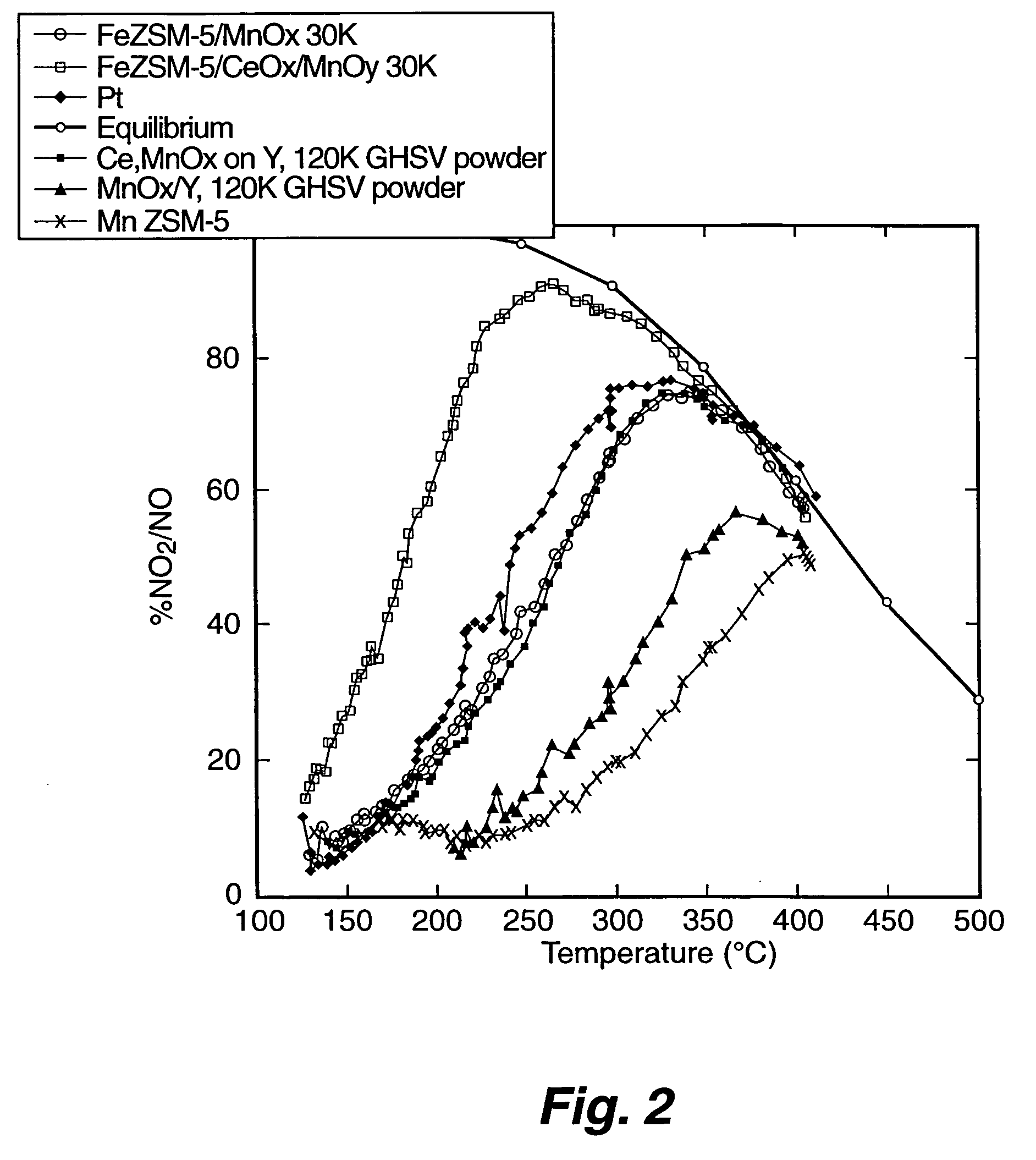
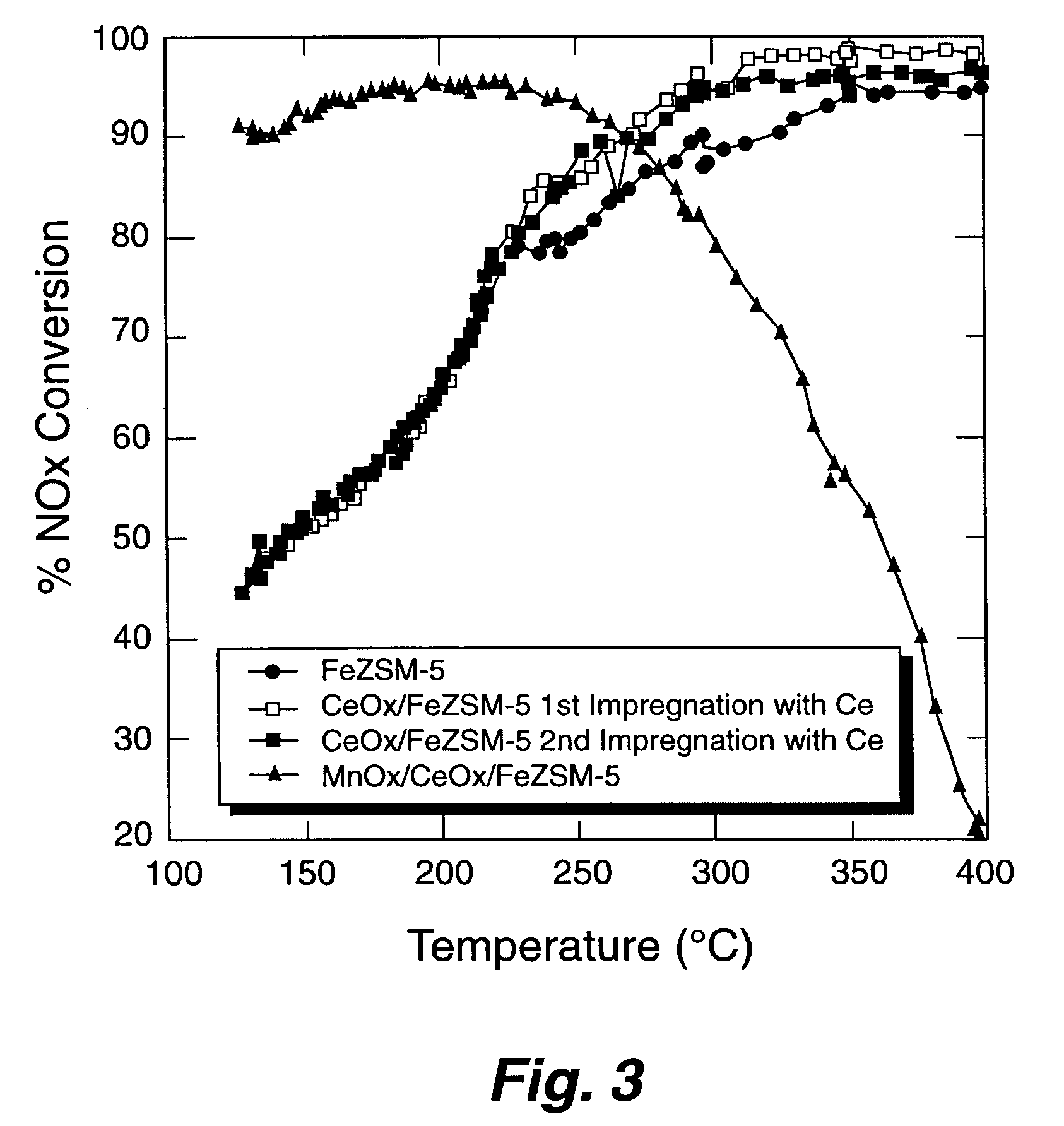
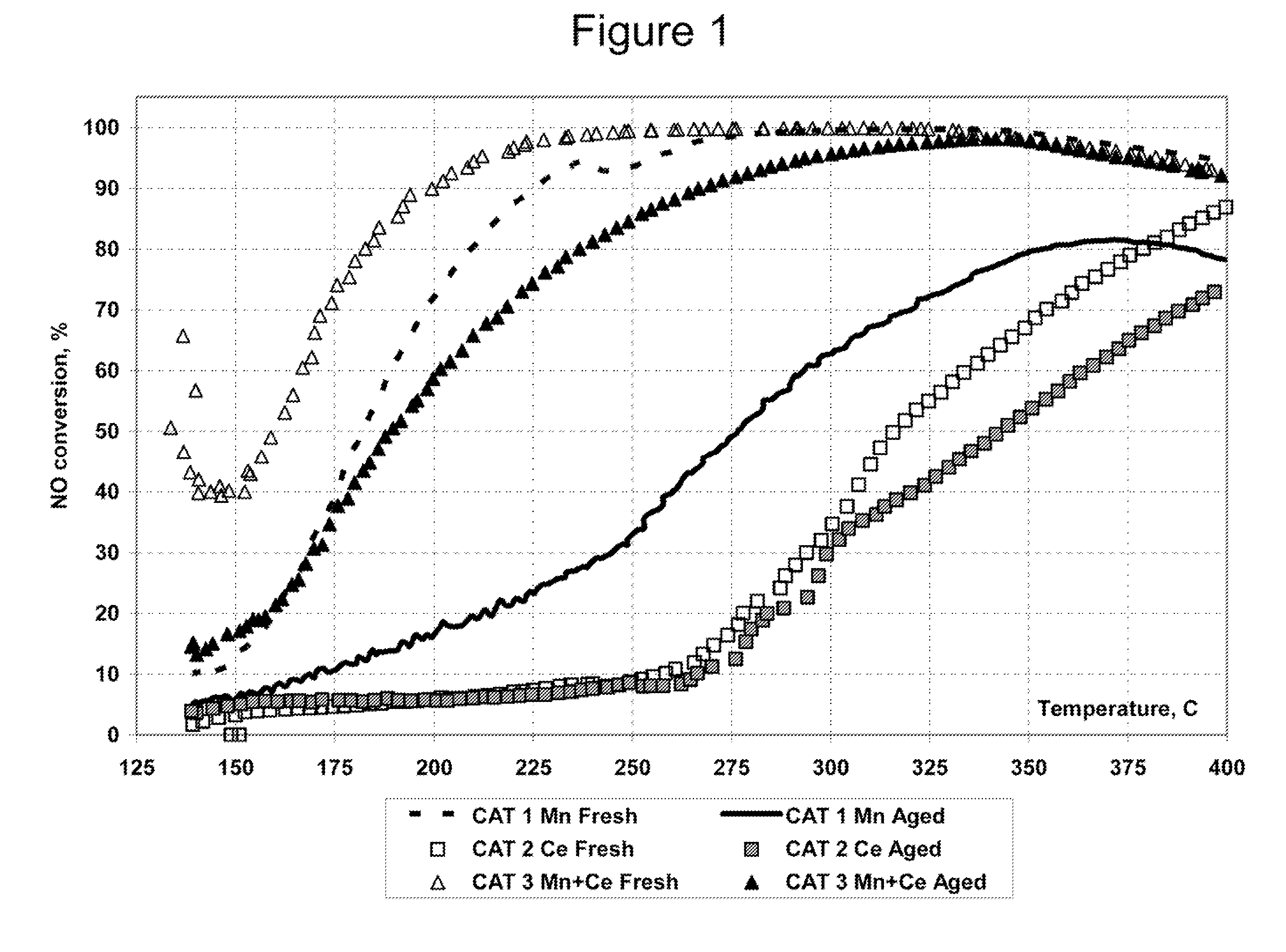
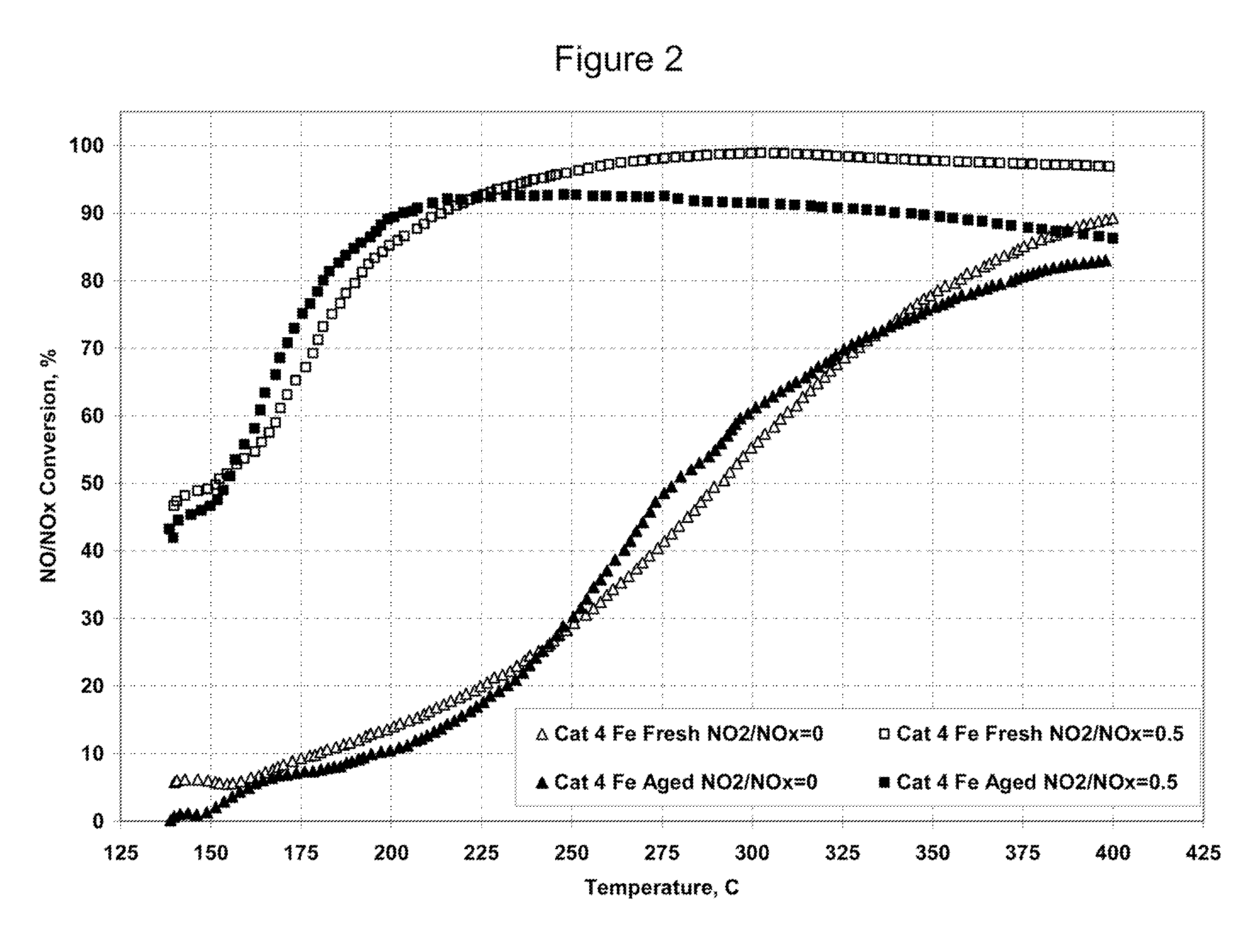
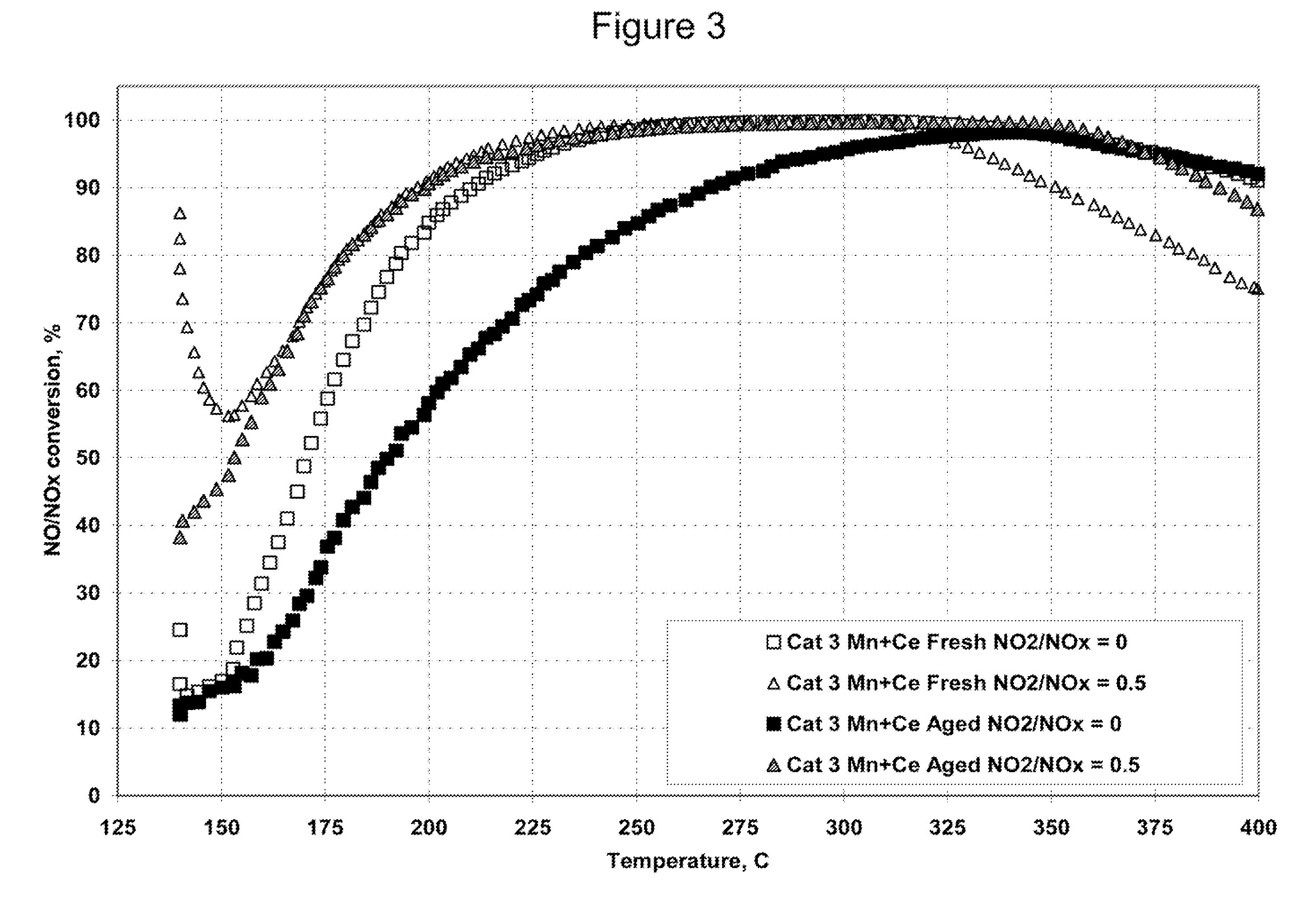
A Selective Catalytic Reduction (SCR) catalyst was prepared by slurry coating ZSM-5 zeolite onto a cordierite monolith, then subliming an iron salt onto the zeolite, calcining the monolith, and then dipping the monolith either into an aqueous solution of manganese nitrate and cerium nitrate and then calcining, or by similar treatment with separate solutions of manganese nitrate and cerium nitrate. The supported catalyst containing iron, manganese, and cerium showed 80 percent conversion at 113 degrees Celsius of a feed gas containing nitrogen oxides having 4 parts NO to one part NO2, about one equivalent ammonia, and excess oxygen; conversion improved to 94 percent at 147 degrees Celsius. N2O was not detected (detection limit: 0.6 percent N2O).
Owner:LOS ALAMOS NATIONAL SECURITY
Ammonia scr catalyst and method of using the catalyst
ActiveUS20090304566A1Enhanced Surface AcidityCombination devicesOrganic chemistryParticulatesNiobium
A DPF with an SCR catalyst and a method for selectively reducing nitrogen oxides with ammonia, filtering particulates, and reducing the ignition temperature of soot on a DPF are provided. The catalyst includes a first component of copper, chromium, cobalt, nickel, manganese, iron, niobium, or mixtures thereof, a second component of cerium, a lanthanide, a mixture of lanthanides, or mixtures thereof, and a component characterized by increased surface acidity. The catalyst may also include strontium as an additional second component. The catalyst selectively reduces nitrogen oxides to nitrogen with ammonia and oxidizes soot at low temperatures. The catalyst has high hydrothermal stability.
Owner:CATALYTIC SOLUTIONS INC
Dehydrogenation catalyst for feed gas containing carbon monoxide, preparation method and application method thereof
ActiveCN101543776AWide range of CO content requirementsGood choiceCarbon monoxideMetal/metal-oxides/metal-hydroxide catalystsPlatinum saltsCerium
The invention discloses a dehydrogenation catalyst for feed gas containing carbon monoxide, a preparation method and an application method thereof. The catalyst takes alumina as a carrier, palladium and / or platinum as an active component and 2 to 4 MOxes as an additive, M is sodium, potassium, magnesium, titanium, zirconium, vanadium, manganese, iron, nickel, cobalt, copper, molybdenum, tungsten or cerium, and components of the catalyst (calculated by carrier mass) are: 0.01 to 2 percent of the palladium and / or 0.01 to 1 percent of the platinum, and 1 to 2 percent of MOxes. The preparation method for the catalyst comprises the following steps that: firstly, an additive metal salt solution is used to impregnate the carrier; a palladium and / or platinum salt solution is used to impregnate the carrier after the carrier is dried and roasted; and the impregnated carrier is roasted at a temperature of between 450 and 850 DEG C to obtain the catalyst. Before the use, H2-N2 mixed gas containing more than 10 percent of hydrogen or pure hydrogen is activated by the catalyst at a temperature of between 450 and 650 DEG C. The catalyst can perform deep removal on less than 5 percent of hydrogen in the feed gas with the CO content of between 10 and 99 percent, the using temperature is between 100 and 300 DEG C, the space velocity is between 500 and 9,000h<-1>, the dehydrogenation rate is more than 99 percent, the content of outlet hydrogen is less than 100 ppm, and the loss of the carbon monoxide is less than 0.5 percent.
Owner:HAISO TECH
Catalytic converter for cleaning exhaust gas
InactiveUS6261989B1Reduce and preventEasy to cleanNitrogen compoundsInternal combustion piston enginesCeriumCe element
A catalytic converter for cleaning exhaust gas includes a heat-resistant support, and a catalytic coating formed on the heat-resistant support. The catalytic coating contains Pd-carrying particles of a cerium complex oxide, Pt & Rh-carrying particles of zirconium complex oxide, and particles of a heat-resistant inorganic oxide.
Owner:DAIHATSU MOTOR CO LTD +1
Liquid composition, process for its production and process for producing membrane-electrode assembly for polymer electrolyte fuel cells
ActiveUS20060019140A1Improve the immunityIncreased durabilityIon-exchanger regenerationFinal product manufacturePolymer scienceHigh energy
An electrolyte membrane is prepared from a liquid composition comprising at least one member selected from the group consisting of trivalent cerium, tetravalent cerium, bivalent manganese and trivalent manganese; and a polymer with a cation-exchange group. The liquid composition is preferably one containing water, a carbonate of cerium or manganese, and a polymer with a cation-exchange group, and a cast film thereof is used as an electrolyte membrane to prepare a membrane-electrode assembly. The present invention successfully provides a membrane-electrode assembly for polymer electrolyte fuel cells being capable of generating the electric power in high energy efficiency, having high power generation performance regardless of the dew point of the feed gas, and being capable of stably generating the electric power over a long period of time.
Owner:ASAHI GLASS CO LTD
Illumination system comprising a radiation source and a fluorescent material
ActiveUS20060255710A1High temperature resistancePointing stableDischarge tube luminescnet screensElectroluminescent light sourcesCeriumEuropium
The invention concerns an illumination system for generation of colored, especially amber or red light, comprising a radiation source and a fluorescent material comprising at least one phosphor capable of absorbing a part of light emitted by the radiation source and emitting light of wavelength different from that of the absorbed light; wherein said at least one phosphor is a amber to red emitting a rare earth metal-activated oxonitridoalumosilicate of general formula (Ca1−x−y−zSrxBayMgz)1−n(Al1−a+bBa)Si1−bN3−bOb:REn, wherein 0≦x≦1, 0≦y≦1, 0≦z≦1, 0≦a≦1, 0<b≦1 and 0.002≦n≦0.2 and RE is selected from europium(II) and cerium(III).
Owner:PHILIPS LUMILEDS LIGHTING CO LLC +1
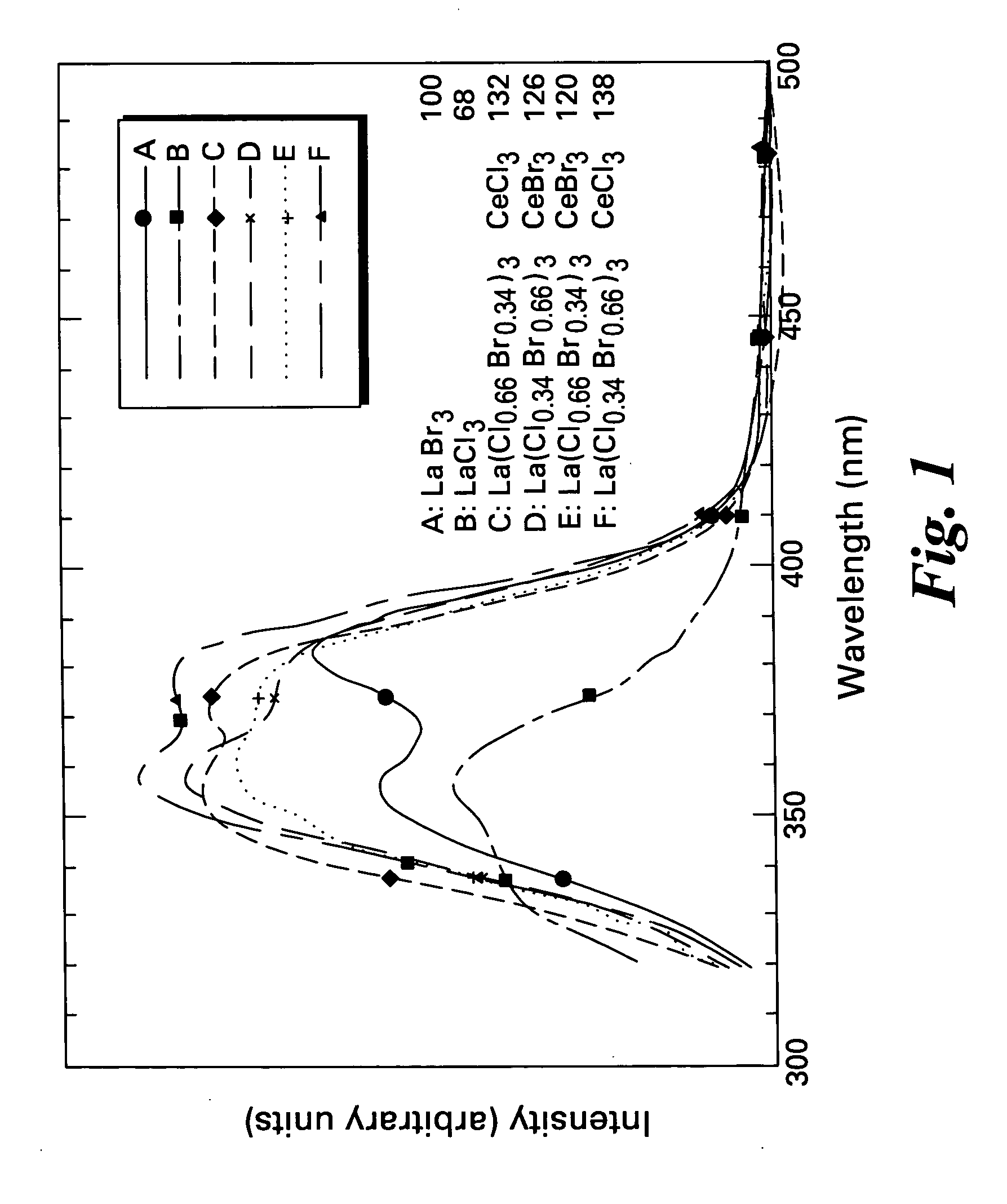
Scintillator compositions, and related processes and articles of manufacture
ActiveUS7084403B2Excellent and reproducible scintillation responseImprove performanceMaterial analysis by optical meansLuminescent compositionsHigh energyIodide
Scintillator materials based on certain types of halide-lanthanide matrix materials are described. In one embodiment, the matrix material contains a mixture of lanthanide halides, i.e., a solid solution of at least two of the halides, such as lanthanum chloride and lanthanum bromide. In another embodiment, the matrix material is based on lanthanum iodide alone, which must be substantially free of lanthanum oxyiodide. The scintillator materials, which can be in monocrystalline or polycrystalline form, also include an activator for the matrix material, e.g., cerium. Radiation detectors that use the scintillators are also described, as are related methods for detecting high-energy radiation.
Owner:BAKER HUGHES OILFIELD OPERATIONS LLC
Thermal protective coating for ceramic surfaces
ActiveUS6921431B2Extended shelf lifeConvenience to workFireproof paintsOther chemical processesColloidal silicaSodium Bentonite
A coating admixture, method of coating and substrates coated thereby, wherein the coating contains colloidal silica, colloidal alumina, or combinations thereof; a filler such as silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides, copper chromium oxides, cerium oxides, terbium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide is also added.
Owner:WESSEX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com