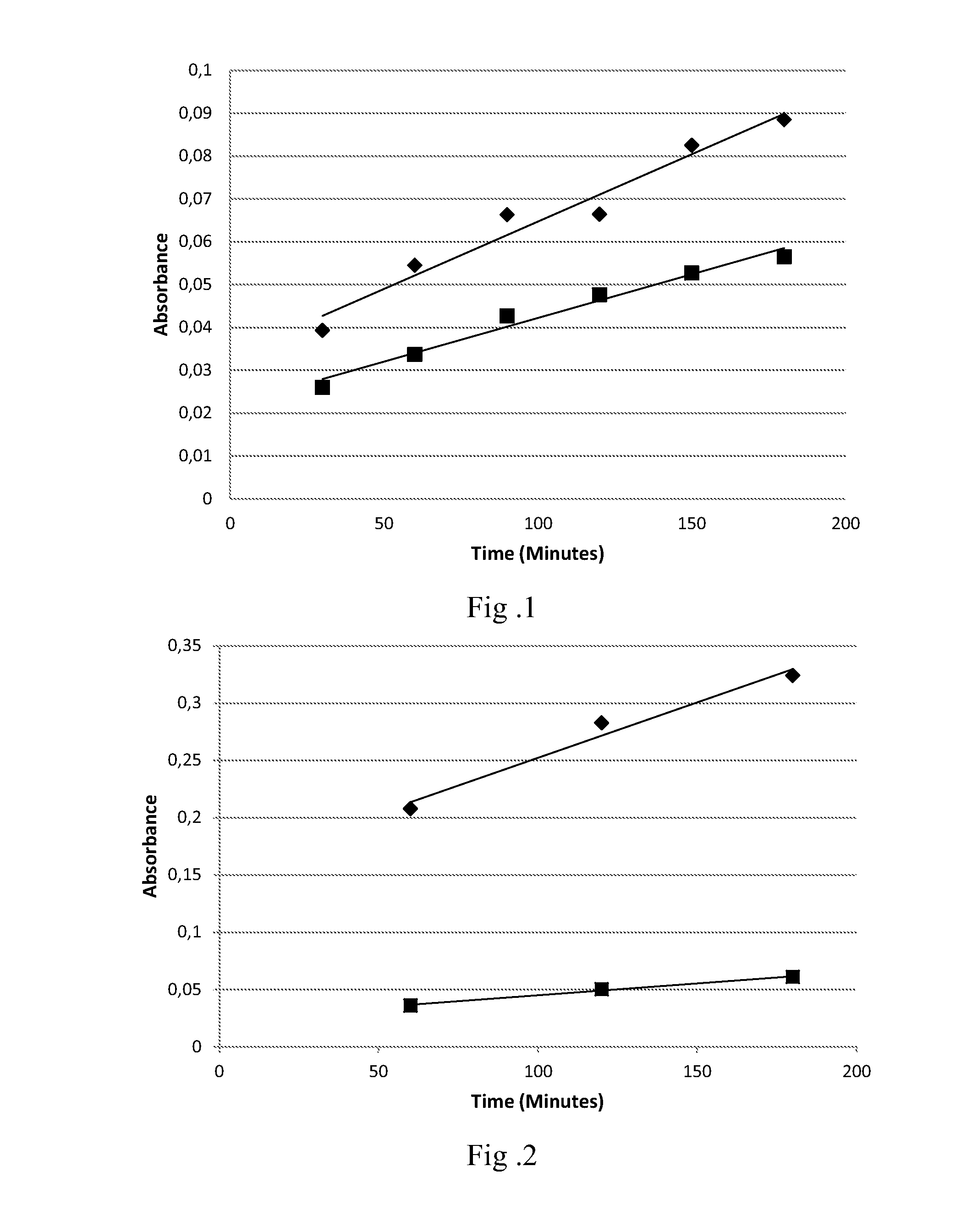
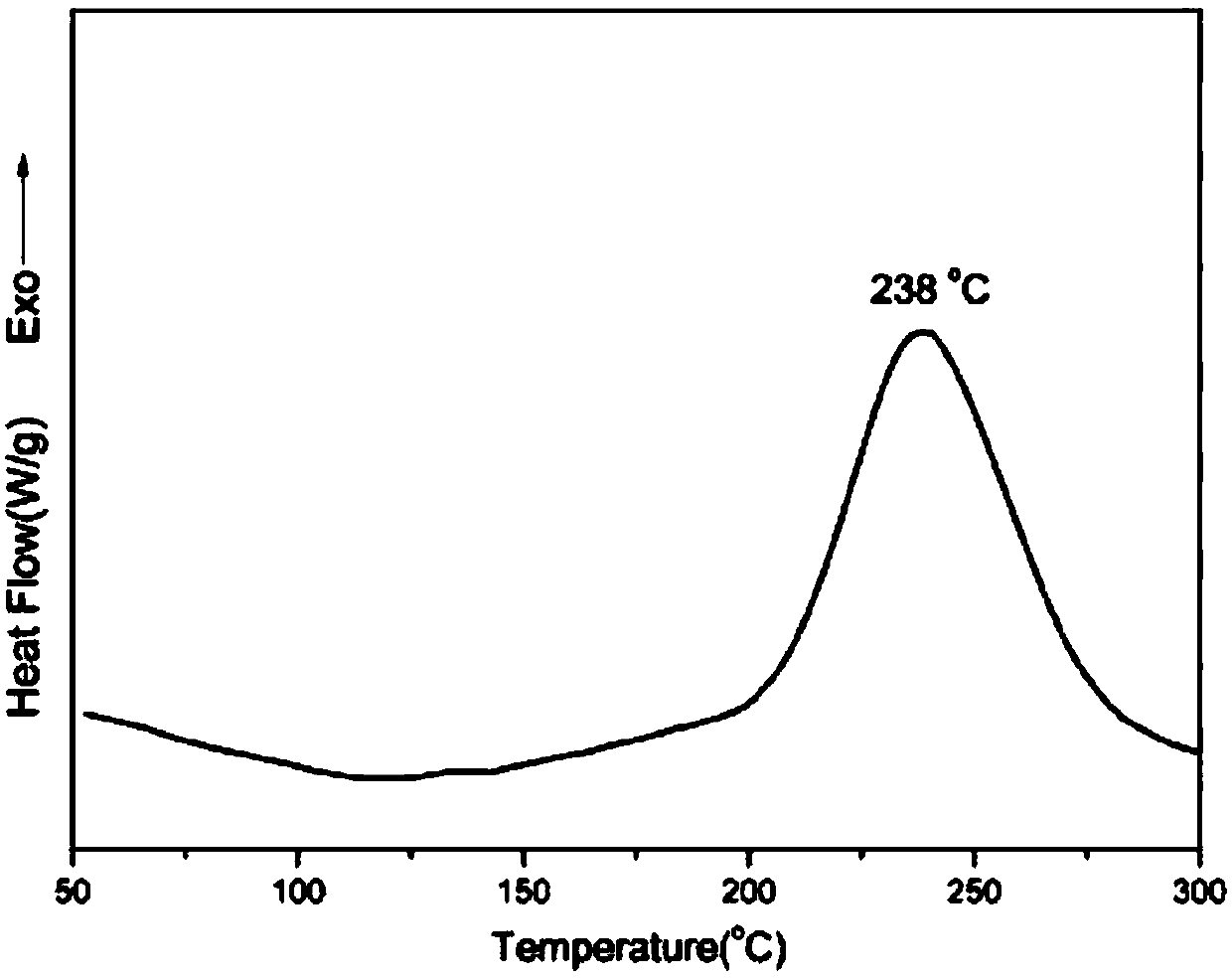
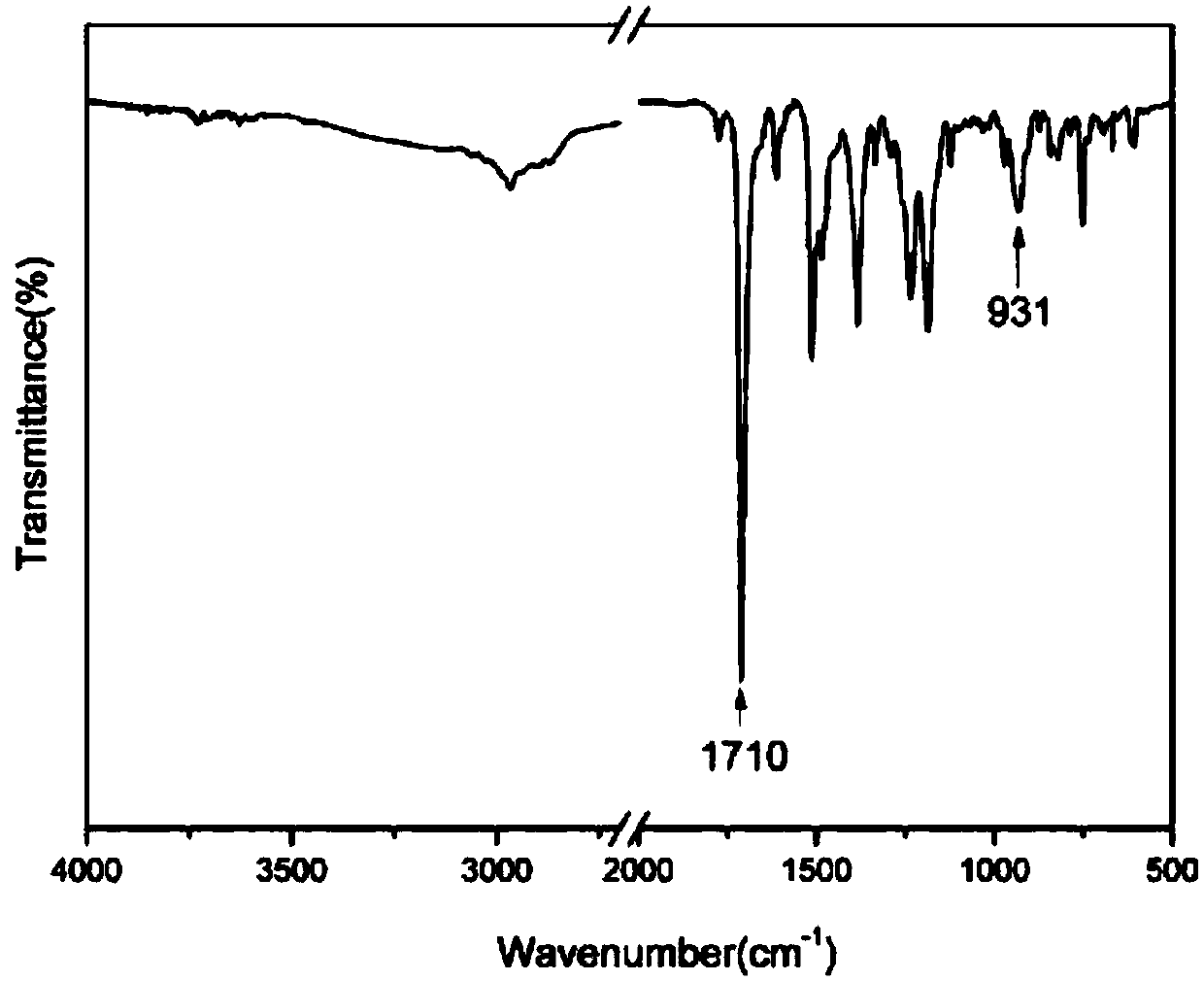
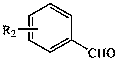
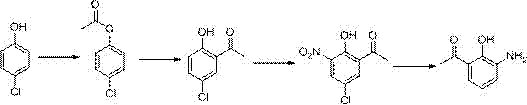
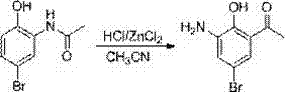
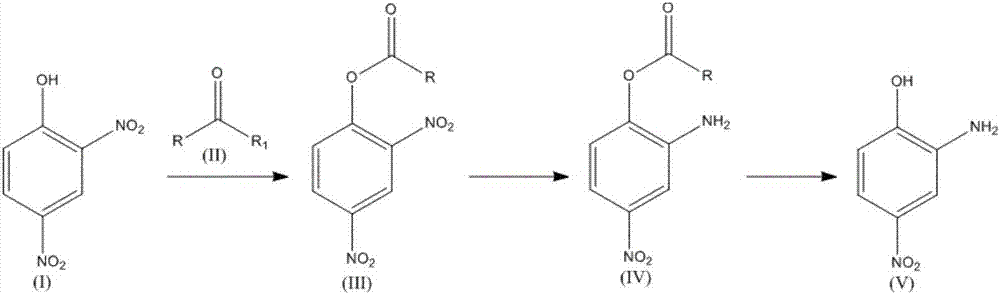
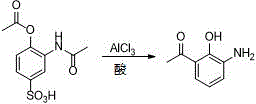
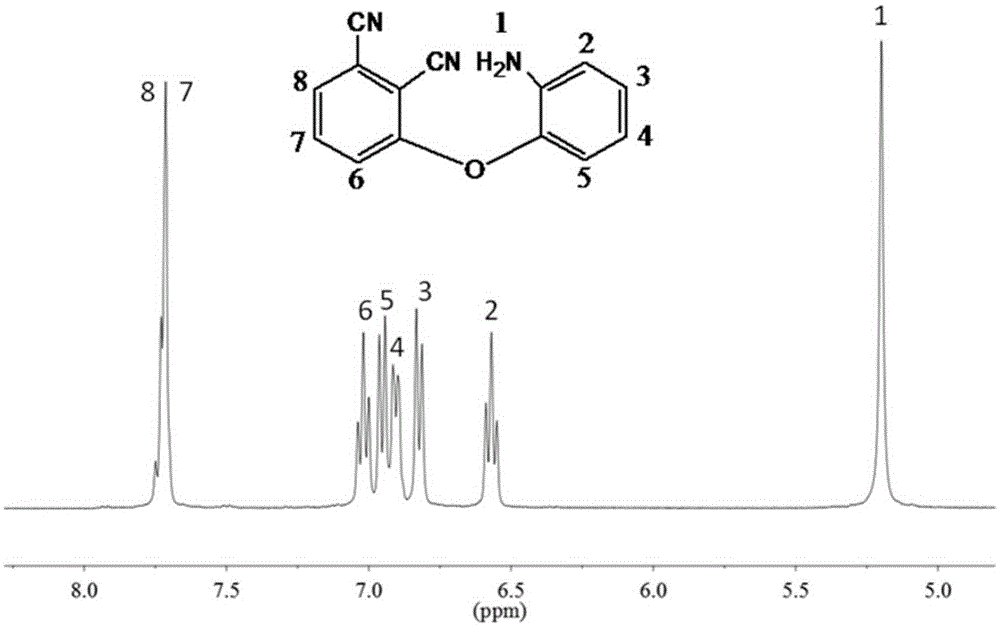
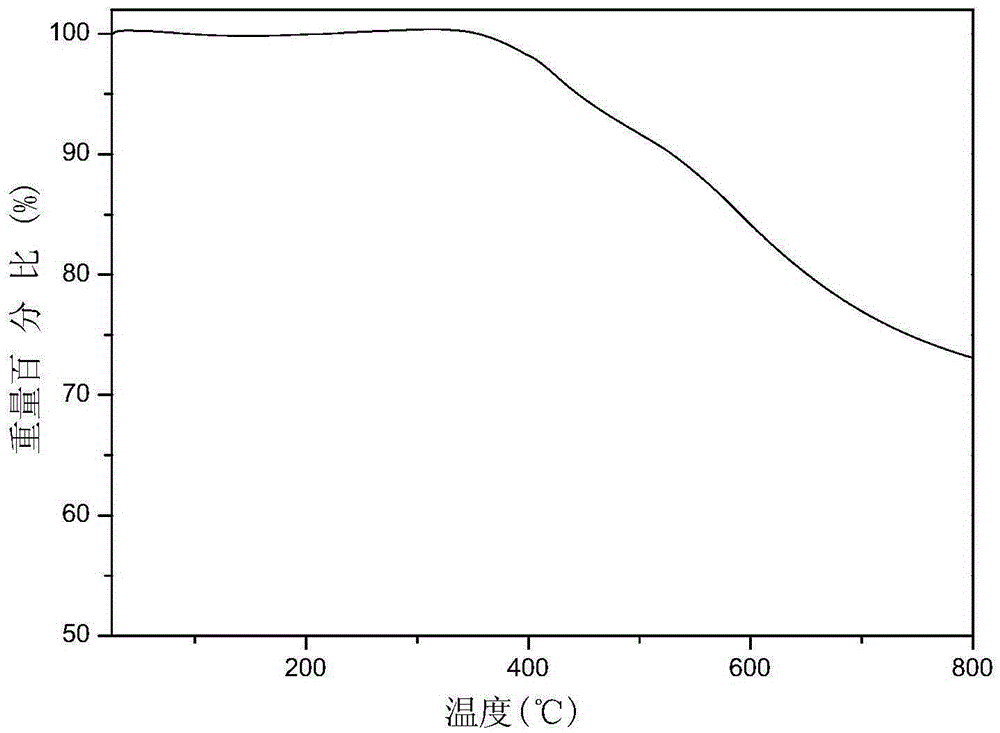
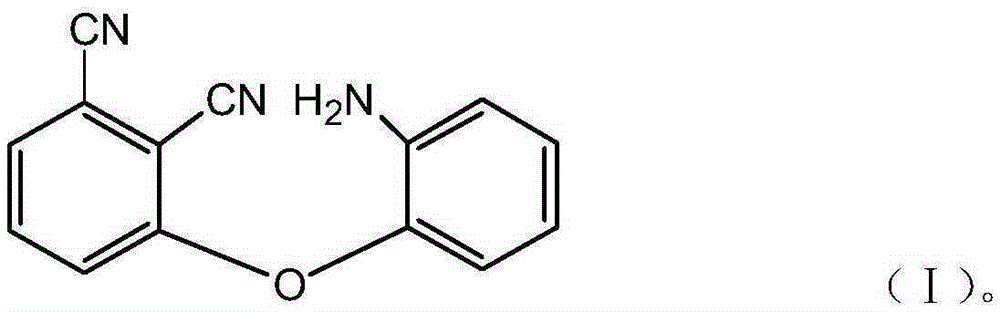
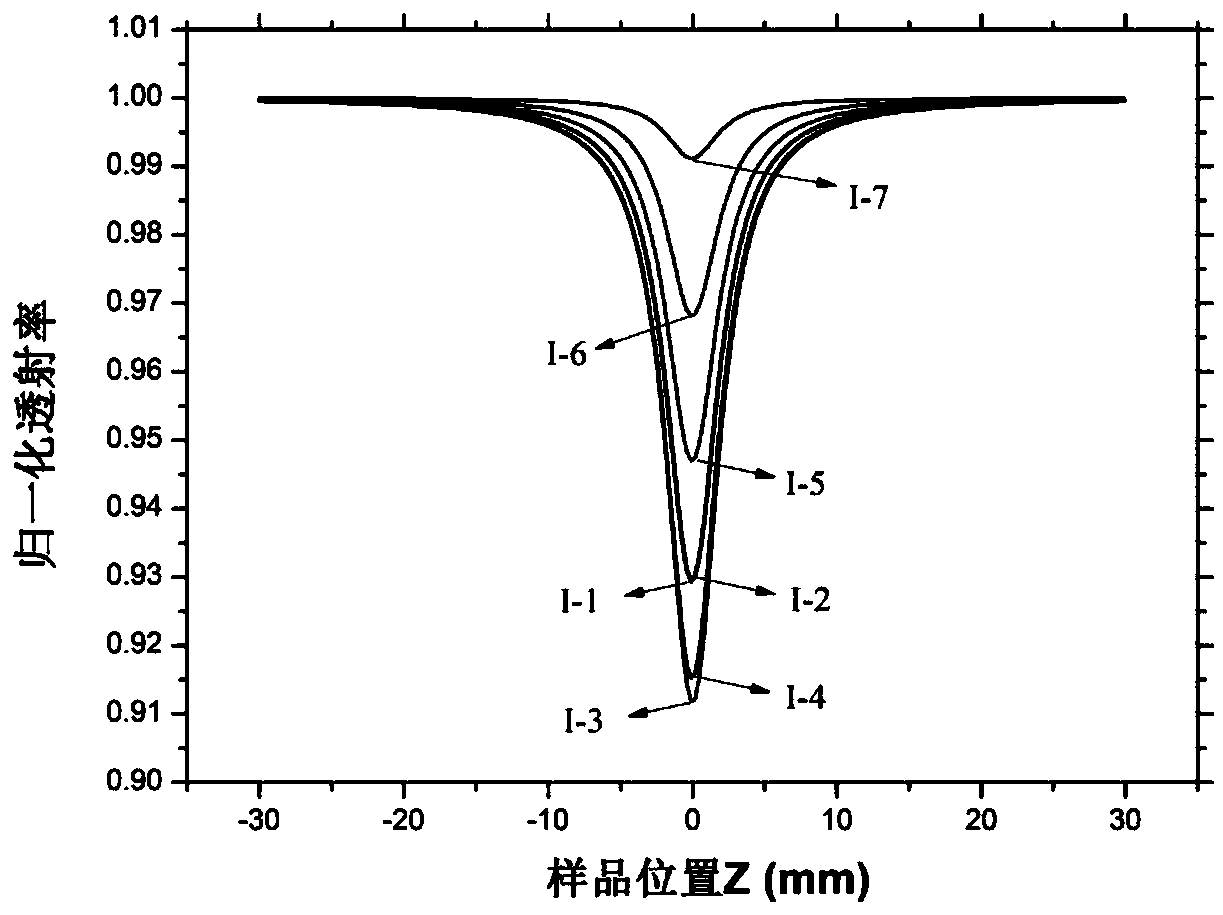
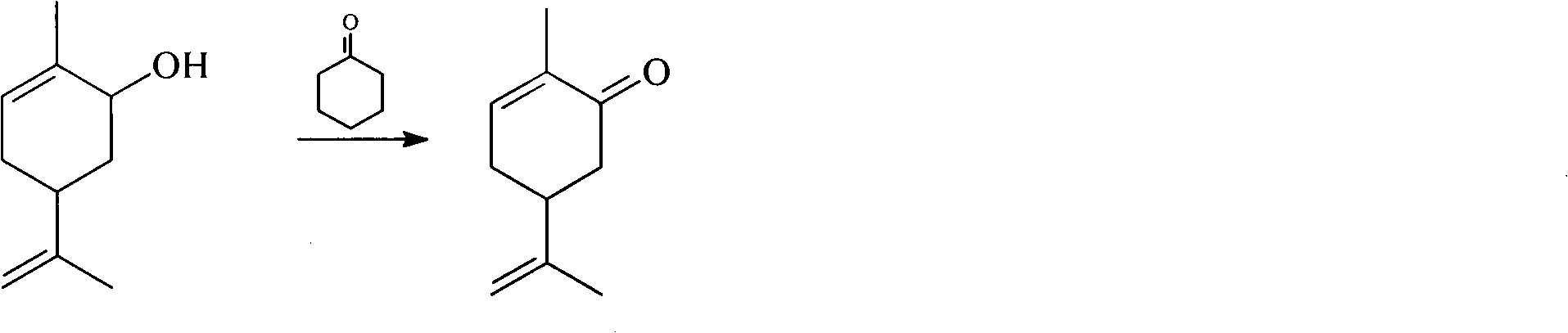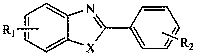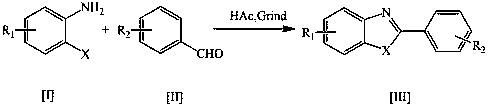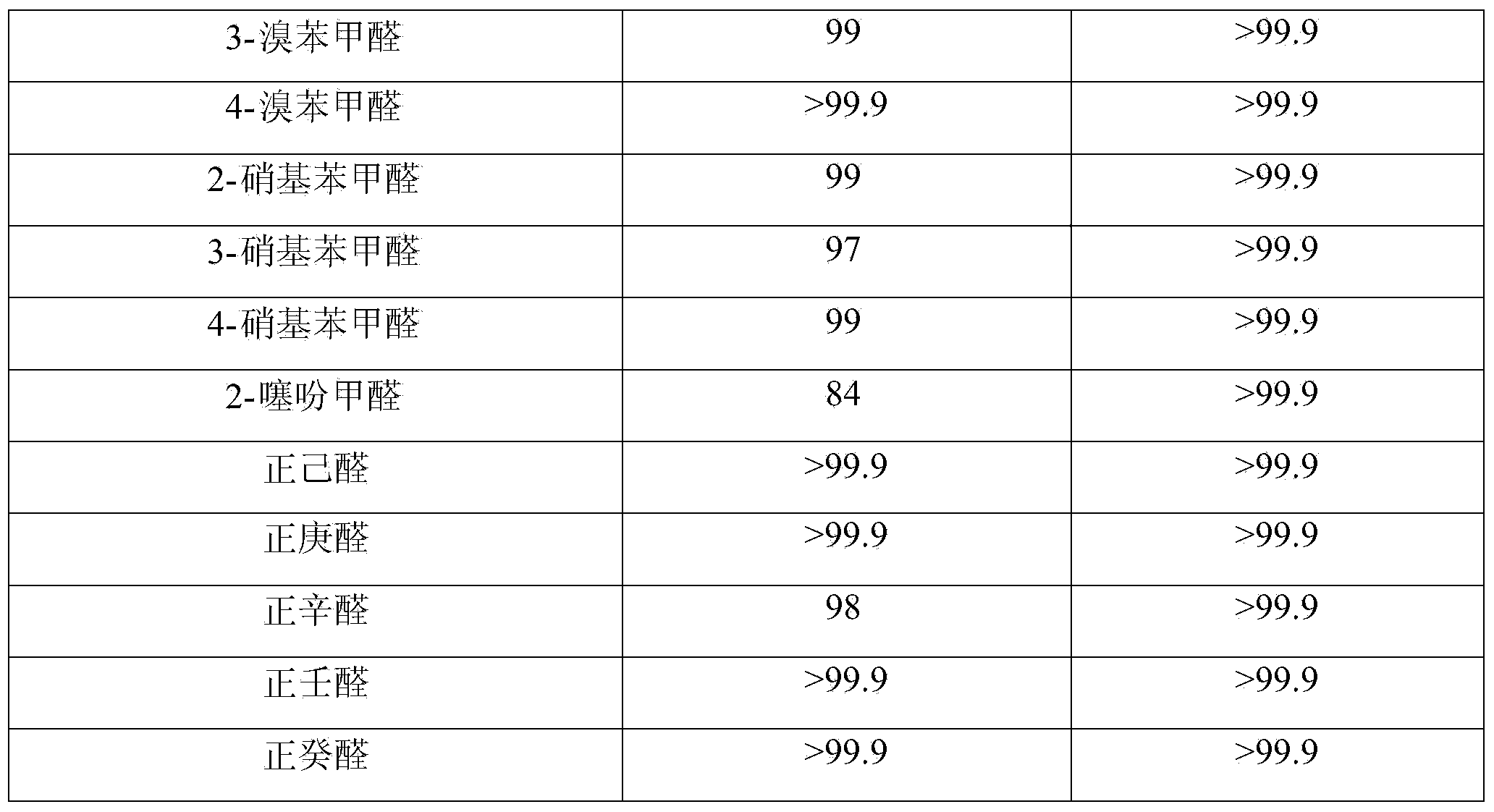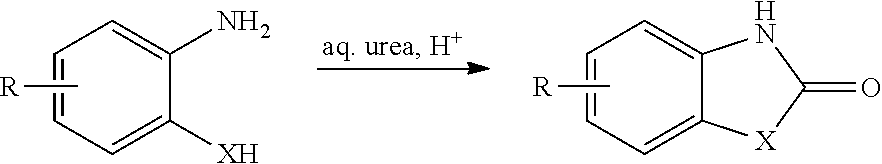Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "2-Aminophenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
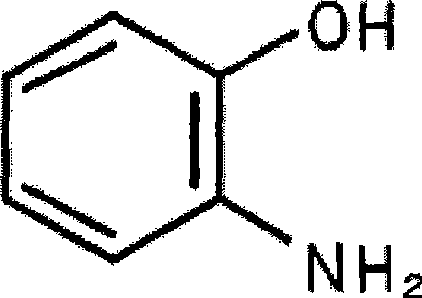
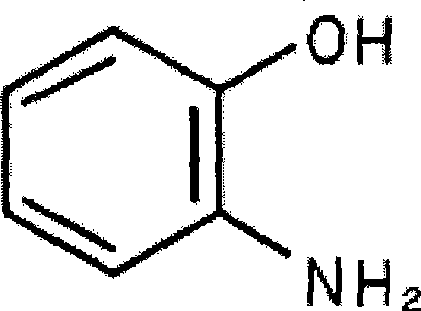
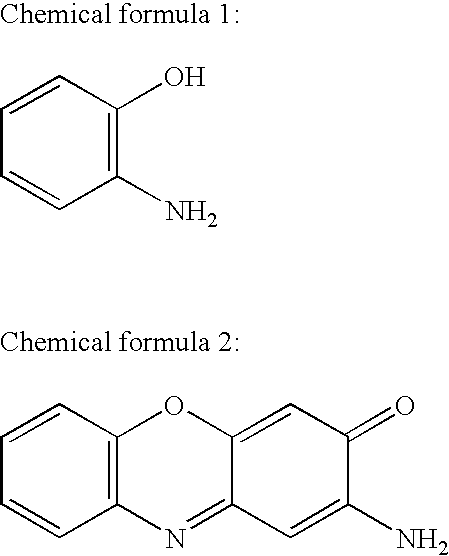
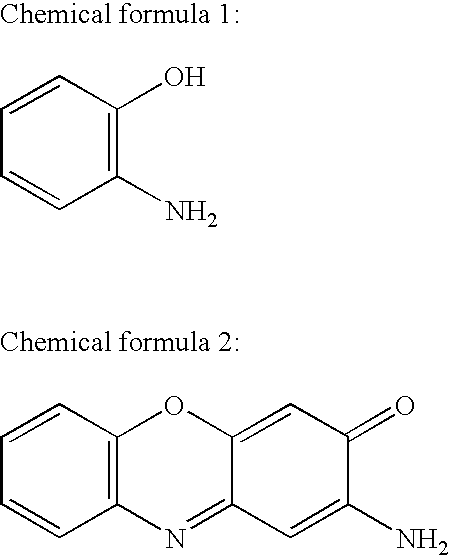
2-Aminophenol is an organic compound with the formula C₆H₇NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds. Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water.
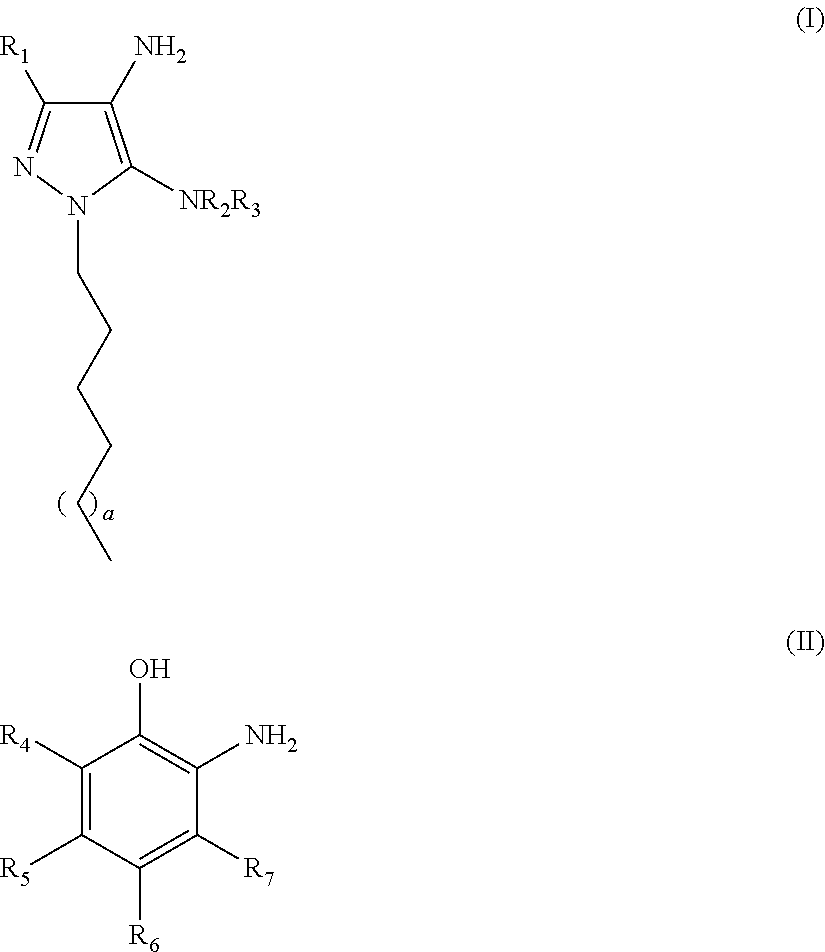
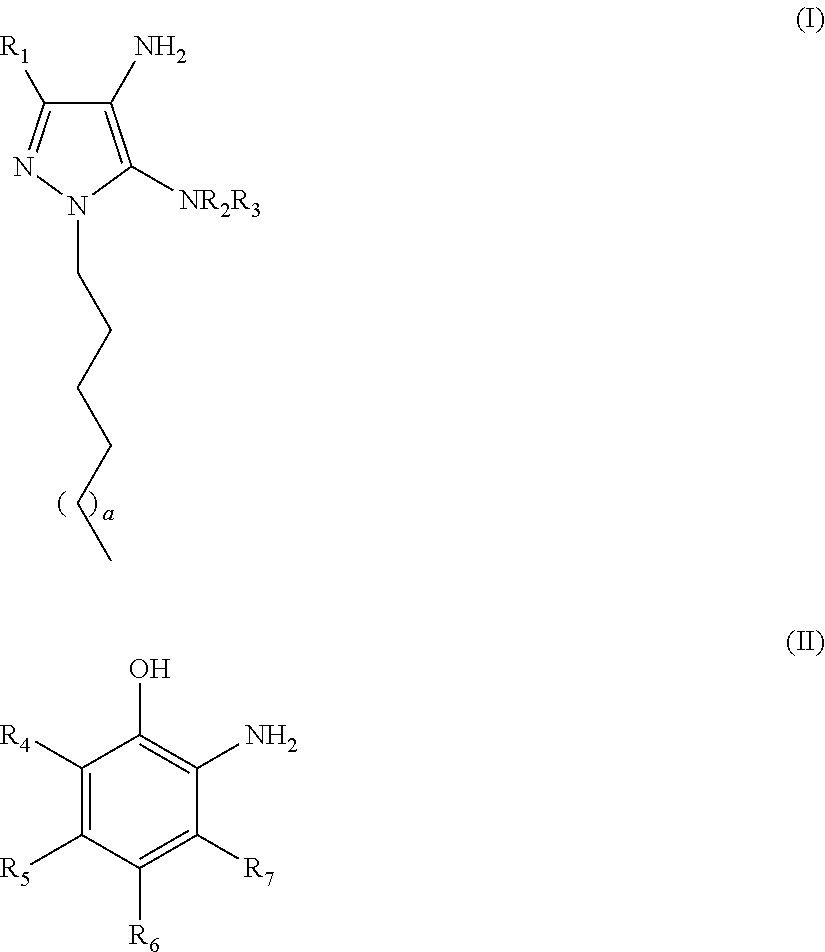
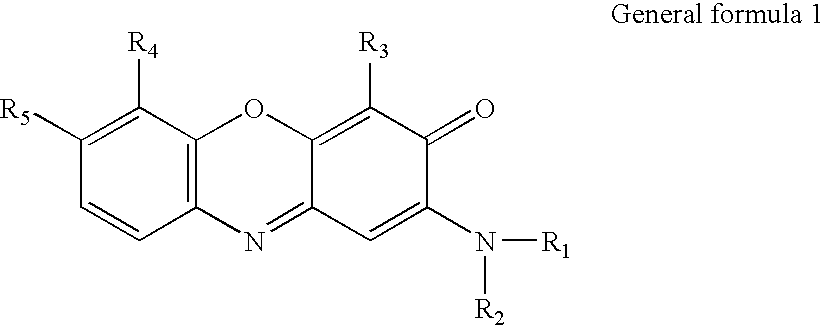
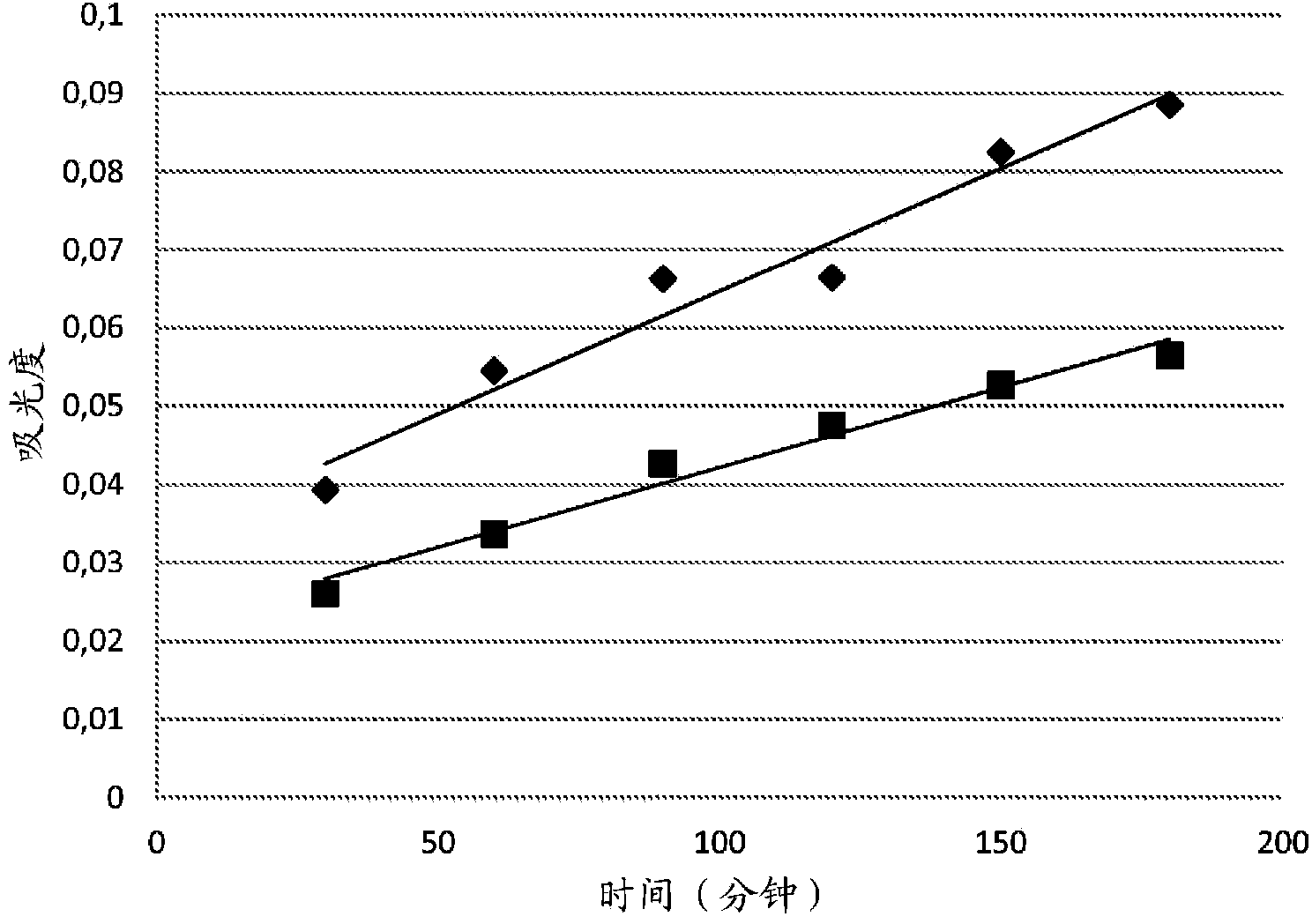
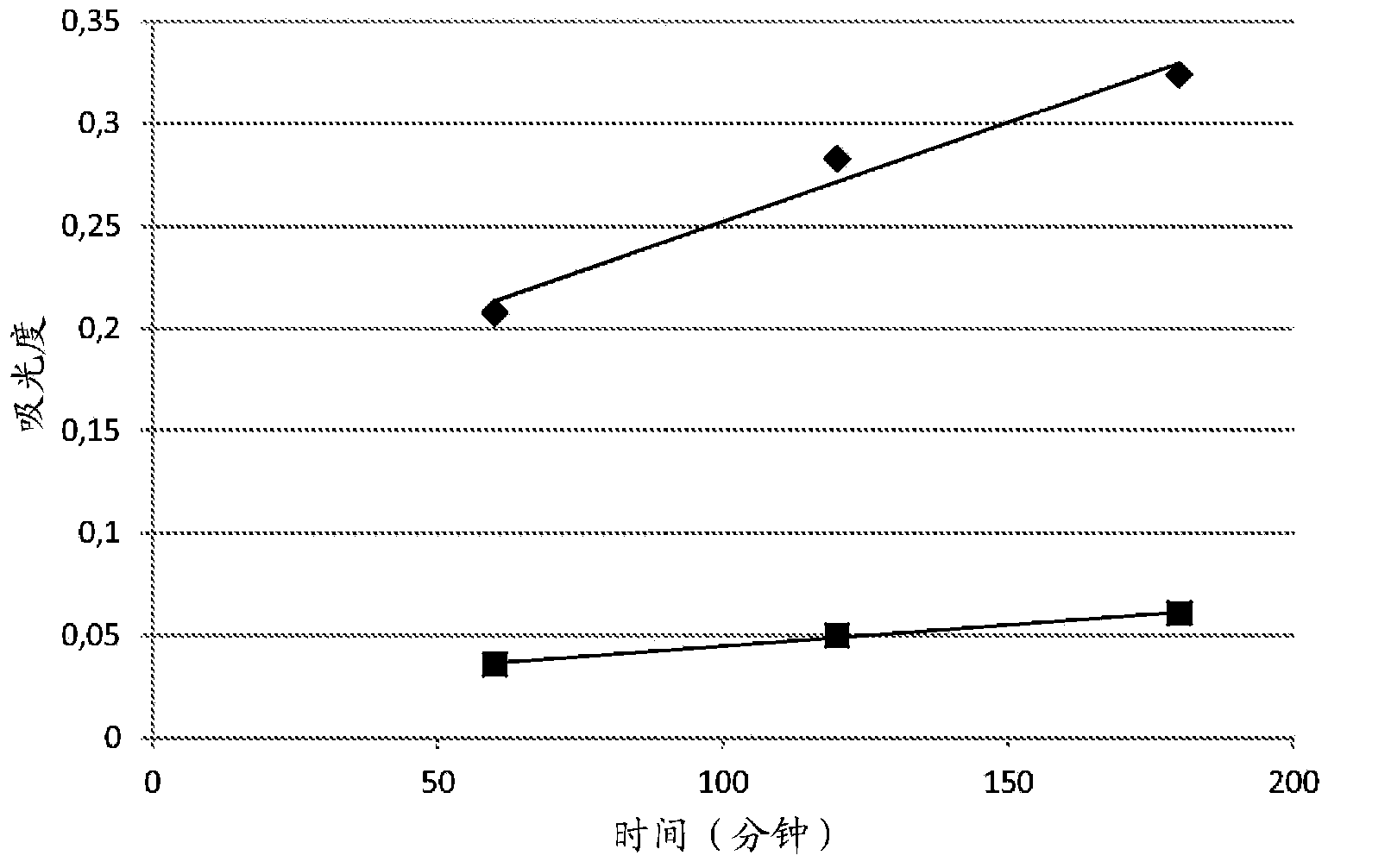
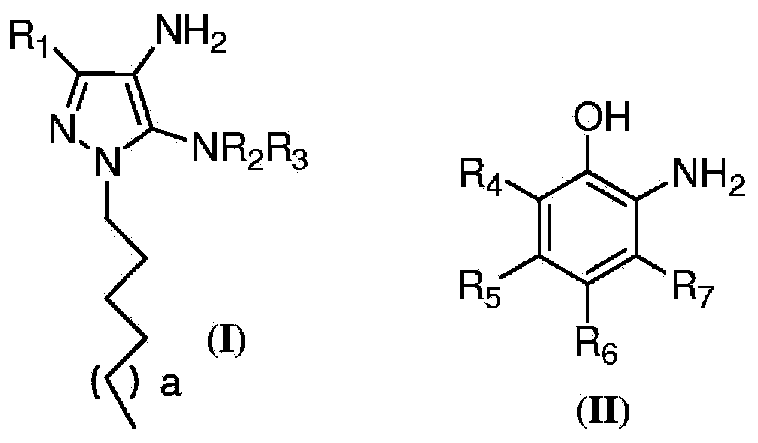
Oxidative Dyeing Compositions Comprising an 1-Hexyl/Heptyl-4,5-diaminopyrazole and a 2-Aminophenol and Derivatives Thereof
An oxidative dyeing composition comprising a 1-hexyl-4,5-diaminopyrazole compound or a 1-heptyl-4,5-diaminopyrazole compound of formula (I) in combination with a 2-aminophenol compound of formula (II):wherein R1, R2, R3, R4, R5, R6 and R7 are as defined herein and a=1 or 2.The composition provides good hair color intensity together with good wash and bleeding fastness.
Owner:WELLA OPERATIONS US LLC
Para-chloro nitrobenzene degrading testosterone coma monad and its use
The present invention relates to one Comamonas testosteroni CNB1CGMCC No. 1028 strain, whose growing cell, cell suspension and immobile cell can degrade para-chloronitrobenzene. The strain can grow with para-chloronitrobenzene as unique carbon source, nitrogen source and energy source, so as to degrade and utilize para-chloronitrobenzene completely. During its growth, the strain first reduces nitro group into hydroxylamine, and produces 2-aminophenol 1, 6-bioxygenase to open the ring of 2-amino-5-chlorophenol into hydrocarbon through meta ring opening. The strain is suitable for the biological treatment of industrial effluent from para-chloronitrobenzene production and the biological repair of para-chloronitrobenzene polluted soil.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
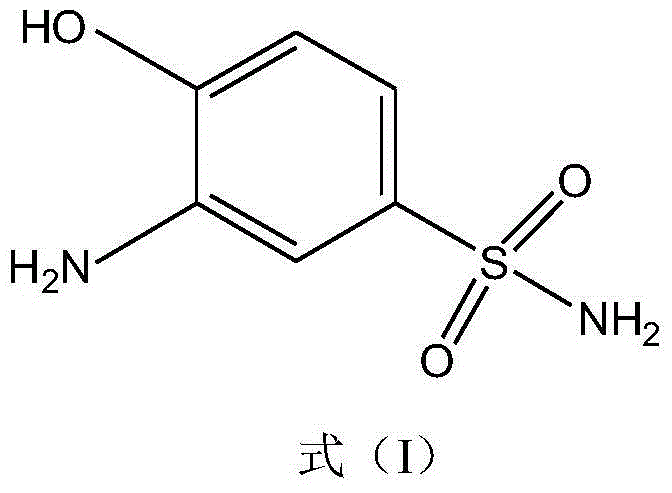
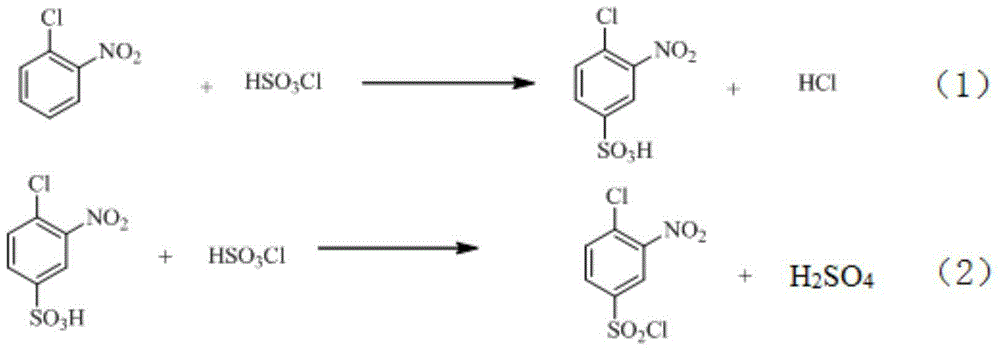
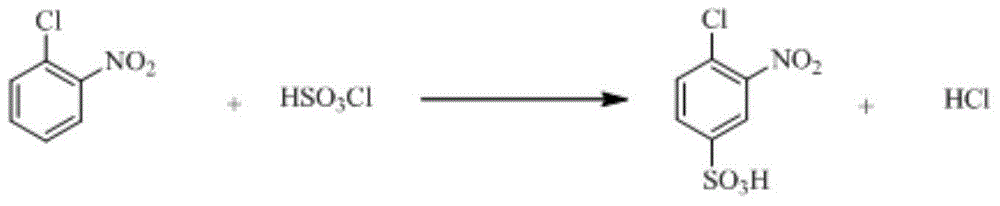
Synthetic method of 2-aminophenol-4-sulfonamide
InactiveCN104592064AReduce dosageReduce wasteOrganic compound preparationSulfonic acid amide preparationO-nitrochlorobenzeneChlorosulfuric acid
The invention relates to a synthetic method of 2-aminophenol-4-sulfonamide. The synthetic method comprises the steps of chlorosulfonation, ammoniation, hydrolysis, acidification and reduction, wherein the chlorosulfonation comprises the following two steps: (a) adding chlorosulfonic acid into p-nitrochlorobenzene which serves as a raw material, and carrying out sulfonation reaction to obtain 4-chloro-3-nitrobenzene sulfonic acid; and (b) adding sulfoxide chloride into 4-chloro-3-nitrobenzene sulfonic acid obtained in the step (a), and carrying out chlorination to obtain 4-chloro-3-nitrobenzenesulfonyl chloride. According to the synthetic method, a chlorosulfonation process is improved, and the chlorosulfonation is carried out in two steps, namely p-nitrochlorobenzene is taken as the raw material, is firstly sulfonated by chlorosulfonic acid and is then chloridized by sulfoxide chloride, so that the consumption of chlorosulfonic acid is reduced, the unnecessary wasting is reduced, the production cost is saved, and the yield of products is increased to reach up to 96.88%.
Owner:QINGDAO DOUBLE PEACH SPECIALTY CHEM GRP
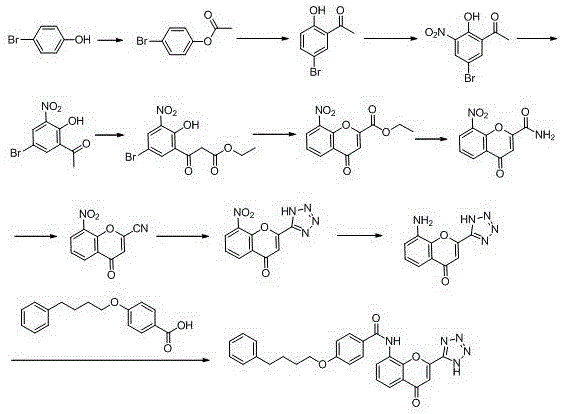
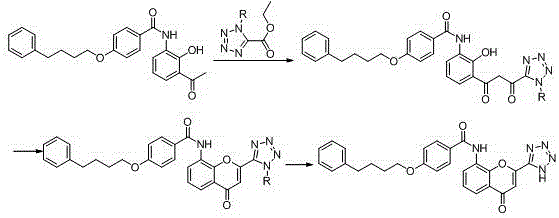
New preparation method of Pranlukast
The invention provides a new preparation method of drug Pranlukast for treating asthma. The new preparation method includes the specific steps that with 2-aminophenol-4-sulfonic acid as a starting material, a key intermediate 3-amino-2-hydroxyacetophenone is prepared by means of acylation, Fries rearrangement and deprotection, then reacts with 4-(phenylbutoxy)benzoic acid, and then is subjected to condensation with ethyl 1H-tetrazole-5-acetate, and finally preparation is achieved through ring closing under the acidic condition. Compared with the prior art, the raw material used for the new preparation method is low in price and easy to obtain, industrialization of a process can be achieved easily, and the obtained final product is high in purity; and no dangerous process exists, equipment is simple, and the route is novel.
Owner:上海微巨实业有限公司
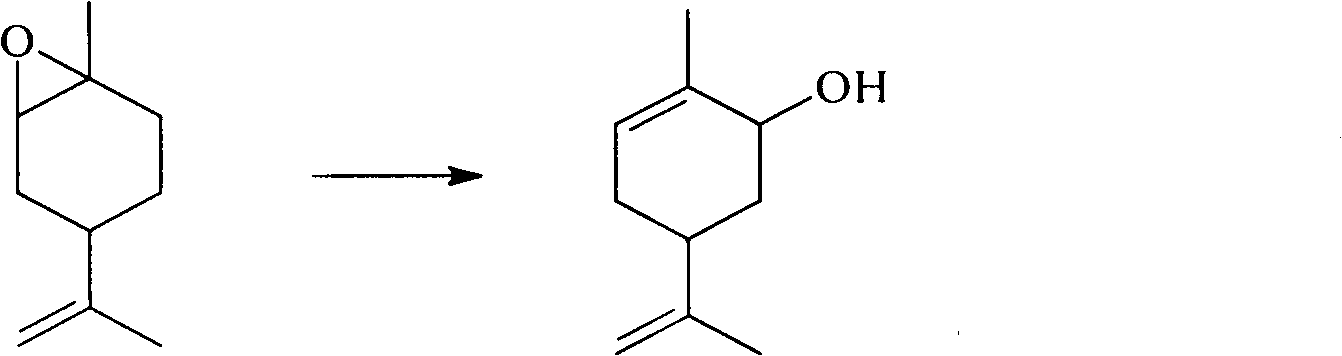
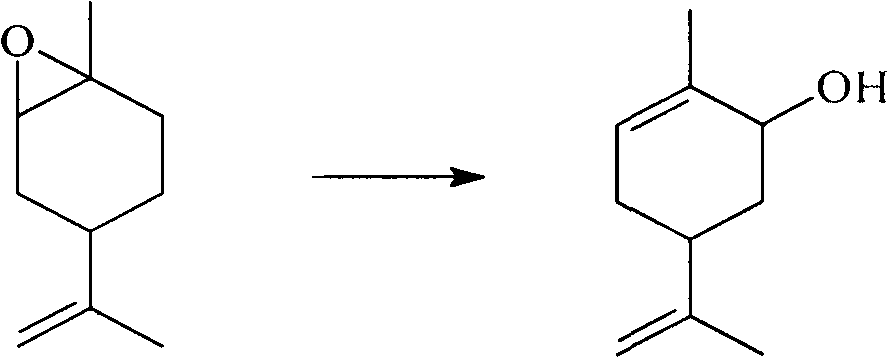
Method for synthesizing carvone
InactiveCN101891602ANo generationRaw materials are easy to obtainCarbonyl compound preparation by oxidationChemical recyclingEpoxyChemical industry
The invention discloses a perfume synthesis method in the technical field of chemical industry, in particular relates to a method for synthesizing carvone by taking epoxy limonene as a raw material. The method comprises the following steps of: taking the epoxy limonene, zinc caprylate and 2-aminophenol as raw materials; performing an isomerization reaction on the epoxy limonene under the action of a catalyst to obtain carveol; and oxidizing the carveol to obtain the carvone. The method has the advantages of economic and environmental-friendly synthesis technology, no production of sewage, capability of avoiding the production of a large amount of waste water which has high organic matter content and is difficult for biodegradation and oxygenolysis in the conventional process, capability of recycling the catalyst, simple and readily available raw materials, short reaction time, high conversion rate, high selectivity and suitability for industrial production.
Owner:ZHEJIANG XINHUA CHEM
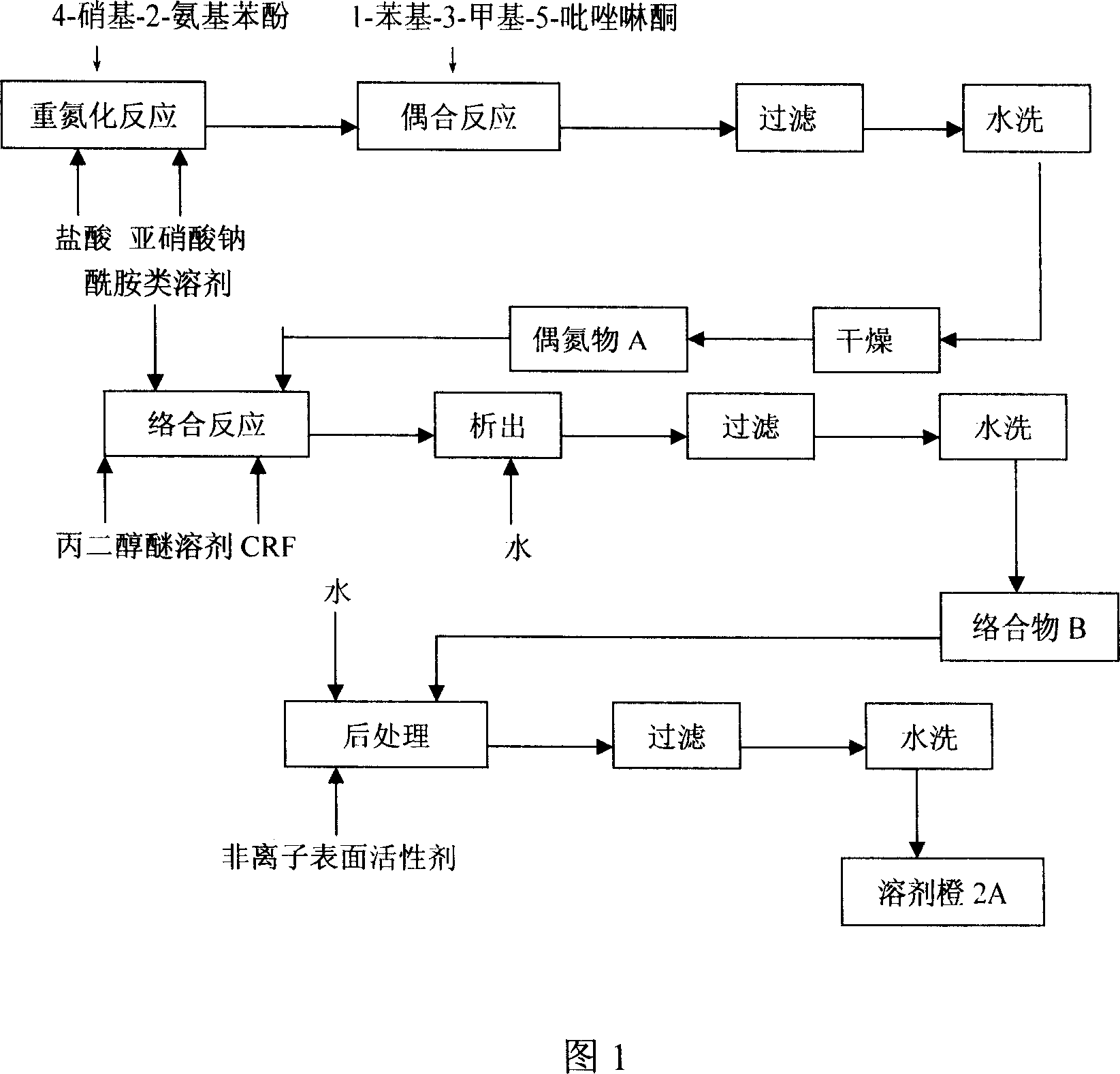
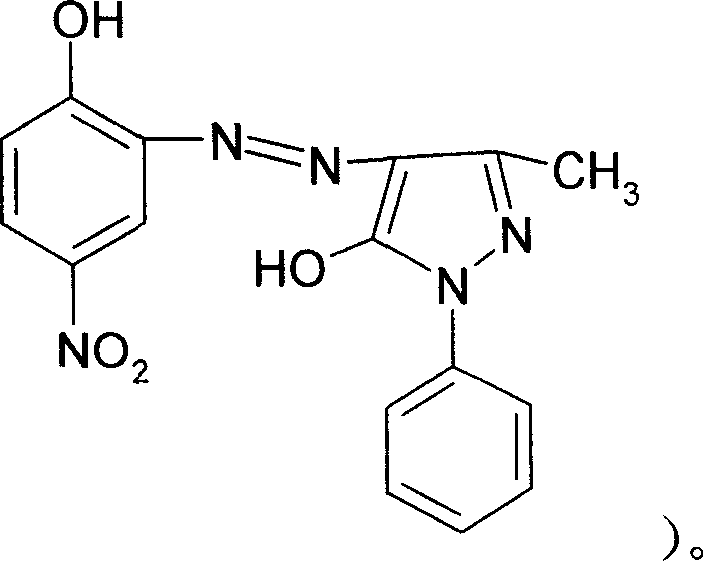
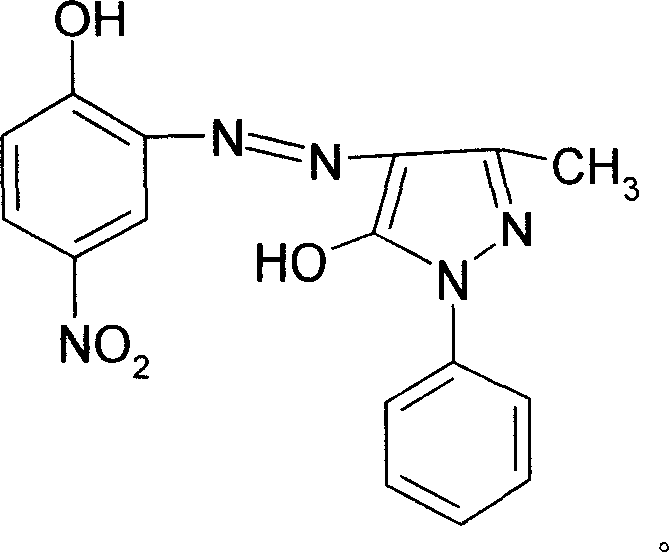
Process for preparing solvent orange 2A
InactiveCN1952016AImprove solubilityIncrease polarityComplex metal azo dyesAlcoholSubstitution reaction
The invention discloses a solvent orange 2A processing technique in the chemical engineering domain, which comprises the following steps: adopting 4-nitro-2-aminophenol and 1-phenyl-3-methyl-5-pyrazolone as basic raw material; coupling; complexing; substituting; adding composite solvent of amide in the alcohol ether solvent and complexing agent CRF; heating to 100 deg.c; adding azo A; heating to 138-150 deg.c directly for 2.5-3.5h; cooling to evolve in the water; filtering; washing through water to obtain the complex B.
Owner:南通市争妍新材料科技有限公司
Norbornene group capping benzoxazine oligomer and preparation method thereof
ActiveCN106699748AImprove machinabilitySimple preparation processOrganic chemistryMannich condensationOrtho position
The invention belongs to the technical field of heat convertible resins and preparation thereof, and discloses a norbornene group capping benzoxazine oligomer and a preparation method thereof. The preparation method comprises the concrete steps of carrying out condensation reaction on 2-aminophenol and carbic anhydride to prepare norbornene functionalized phenol containing a carbon-carbon double bond, and utilizing the norbornene functionalized phenol containing the carbon-carbon double bond as a capping group of the benzoxazine oligomer; utilizing the norbornene functionalized phenol containing the carbon-carbon double bond, bisphenol, diphenacylamine and paraformaldehyde to be subjected to Mannich condensation reaction in an organic solvent; preparing high-performance thermosetting resin through the benzoxazine oligomer. Compared with the prior art, the norbornene group capping benzoxazine oligomer and the preparation method thereof provided by the invention has the advantage that the cross-linkable capping group is introduced through ortho-position benzoxazine chemical so as to prepare the benzoxazine oligomer. The ortho-position benzoxazine chemical is utilized to enable a benzoxazine synthesis process to be simplified, the introduced cross-linkable capping group fills a performance gap of the benzoxazine oligomer caused by low molecular weight, and the machinability of abenzoxazine resin is improved.
Owner:成都科宜高分子科技有限公司
Method used for rapid preparation of benzo-heterocycle compound with physical grinding under solvent-free room temperature conditions
InactiveCN109180590ASimple and fast operationOperational securityOrganic chemistryBenzeneEnvironmental resistance
The invention discloses a method used for rapid preparation of benzo-heterocycle compound with physical grinding under solvent-free room temperature conditions. According to the method, glacial aceticacid is taken as a catalyst; at solvent-free room temperature conditions, physical grinding is adopted, reaction of 2-substituted arylamines (2-mercapto arylamine, 2-aminophenol, and o-phenylenediamine) and aromatic aldehydes is carried out using physical grinding. The method is friendly to the environment, is simple in operation, is safe, is low in cost, and is high in efficiency. Compared withthe prior art, the advantages are that: the method is suitable for a large amount of functional groups, yield is high, less by-product is generated, operation is simple, the method is safe, cost is low, and the method is friendly to the environment.
Owner:FUJIAN MEDICAL UNIV
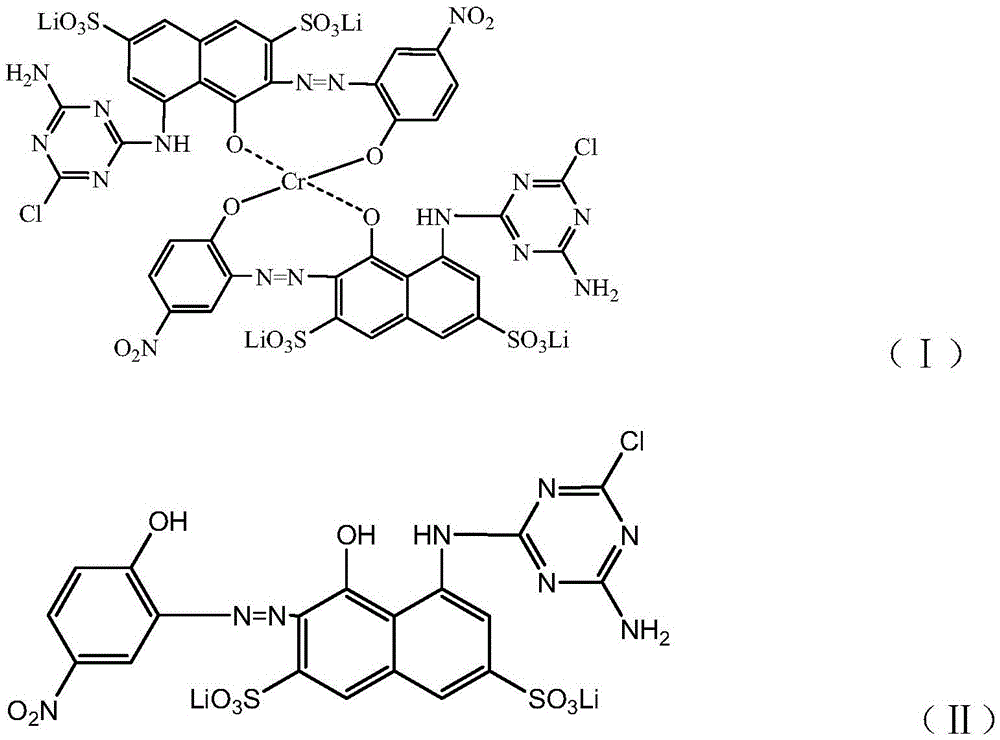
Gray reactive dye and preparation method thereof
The invention discloses a preparation method of gray reactive dye. 4-nitro-2-aminophenol diazonium and H acid have an alkaline coupling reaction, the pH value is controlled to be larger than 6.5, and the mixture reacts for 10 h at the temperature of 0-5 DEG C; a coupling body and chromium trichloride have a metal complex reaction under the action of a catalyst at the temperature of 95-100 DEG C, and the residual amount of free chromium metal is controlled; tricyanogen chloride and 3 times of ammonia water have a primary condensation reaction, ice water is added to a condensation product, washing is performed twice, and ammonium chloride is removed; the primary condensation product and a complexing solution have a secondary condensation reaction; finally, a dispersant NNO and a dispersant MF are added for standardization, and a gray reactive dye product is obtained through filtering, impurity removal and spray drying.
Owner:ZHEJIANG JINGGUANG IND
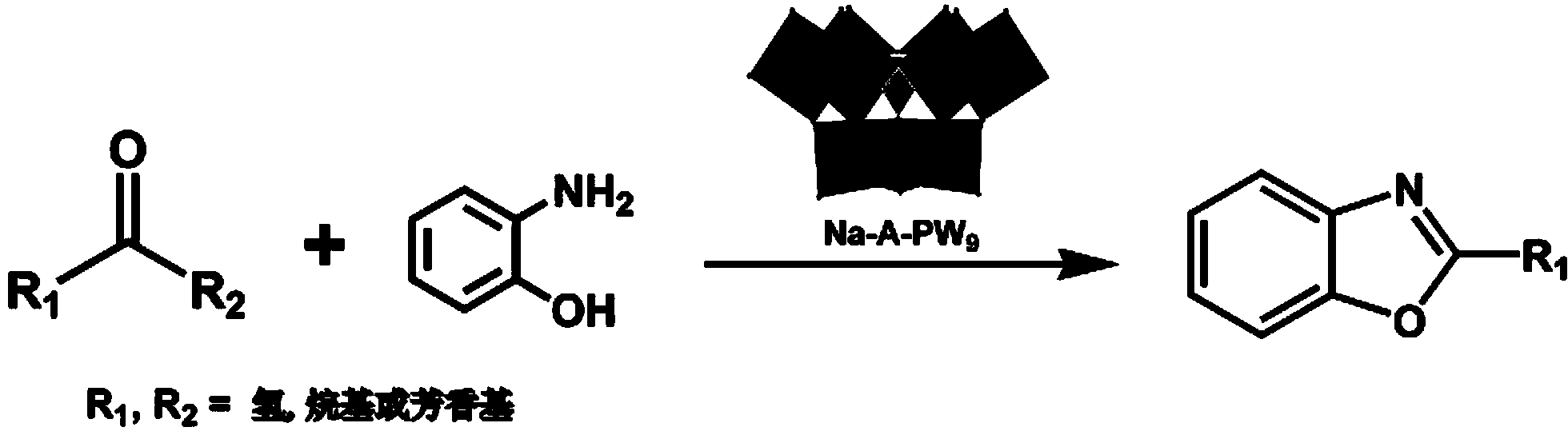
Keggin type vacancy polyacid and application thereof in catalytic synthesis of benzoxazole derivative
ActiveCN103657724ASolve various problems in compositingHigh catalytic activityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoxazoleKetone
The invention belongs to the technical field of application of inorganic functional materials, and discloses Keggin type vacancy polyacid and application thereof in a reaction for catalytic synthesis of a benzoxazole derivative. The chemical formula of the Keggin type trivacancy polyacid is Na8H [A-PW9O34]*7H2O, wherein A represents a structure type of the Keggin type trivacancy polyacid. The Keggin type trivacancy polyacid is taken as an environment-friendly catalyst and applied to a reaction in which aldehydes and 2-aminophenol are taken as raw materials for catalytic synthesis of the benzoxazole derivative in a liquid phase, catalysis for oximation reaction of most aldehyde or ketone under a mild condition is achieved, high catalytic activity and principal product selectivity are provided, and various problems in the conventional synthesis of the benzoxazole derivative are solved.
Owner:BEIJING UNIV OF CHEM TECH
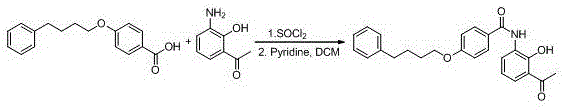
Method for preparing pranlukast key intermediate 3-amino-2-hydroxyacetophenone
ActiveCN107098822ACheap and easy to getRich choiceOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideBromine
The invention provides a method for preparing a pranlukast key intermediate 3-amino-2-hydroxyacetophenone. The method takes 2-aminophenol as an initial raw material, 2-aminophenol and acetic anhydride are subjected to a synthesis reaction in water to obtain 2-acetaminophenol, then 2-acetamido-4-bromophenol is prepared by 2-ace-taminophenol and NBS under room temperature, 2-hydroxy-3-amino-5-bromoacetophenone is prepared by a Hoesch reaction, finally 2-hydroxy-3-amino-5-bromoacetophenone is dissolved in ethanol, and a Pd / C is added for catalytic hydrogenation and bromine removal to obtain the 3-amino-2-hydroxyacetophenone. The raw materials have the advantages of low cost, easy acquisition, diversified raw material selection, easy realization of production technology, and easy control, the purity of the final product is high, no dangerous process is generated, the equipment is simple, the synthesis route is novel, the synthesis route is short, the production power is increased, and production processing cost is reduced.
Owner:上海微巨实业有限公司
Flexible electromagnetic-wave shielding material and preparation method thereof
InactiveCN105647080AExcellent electromagnetic wave shielding performanceMechanical properties softEpoxyPolymethyl methacrylate
The invention relates to a flexible electromagnetic-wave shielding material which comprises the following components in parts by weight: 30-40 parts of fluorinated ethylene propylene resin, 25-35 parts of epoxy resin, 6-9 parts of silver methanesulfonate, 3-5 parts of titanium silicide, 5-8 parts of nano silicon carbide, 4-9 parts of nano aluminum oxide, 8-12 parts of 2-aminophenol-4-sulfuryl aniline, 7-13 parts of 4-phenyl-2-pyrrolidone, 10-15 parts of polymethyl methacrylate, 15-20 parts of ethambutal, 10-15 parts of propanediol, 9-13 parts of poly(neopentylene glycol sebacate) and 6-10 parts of methylprednisolone acetate. The material has the advantages of obvious shielding effects on electromagnetic waves, high flexibility and high plasticity, and can satisfy the specific demands in the actual application process.
Owner:SUZHOU KEMAO ELECTRONICS MATERIALS TECH
Synthesis technology of nitrophenol and intermediate thereof
InactiveCN106866432AStrong operability in seriesOrganic compound preparationAmino-hyroxy compound preparationChemical reactionSynthesis methods
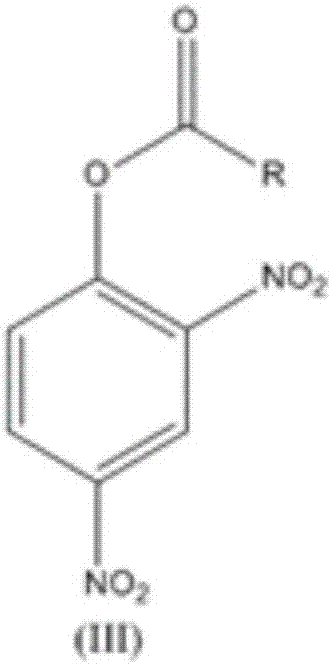
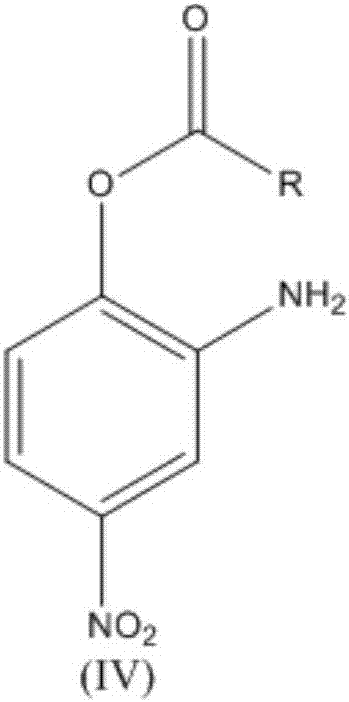
The invention relates to a synthesis technology of nitrophenol and an intermediate thereof, and discloses a synthesis method of 2-amino-4-nitrophenol. The synthesis method of 2-amino-4-nitrophenol comprises the following steps: 1, reacting 2-aminophenol (I) with hydrochloric acid or sulfuric acid in an organic solvent to synthesize a compound (II), and directly carrying out a next step reaction without recovering the organic solvent; 2, carrying out low temperature nitric acid nitration on the compound (II) in the organic solvent to obtain a compound (III), and ending the reaction; and 3, adding a liquid alkali to the above obtained system, distilling the system to recover the organic solvent, mixing the obtained distillation residue with an inorganic acid, carrying out solid-liquid separation to obtain a compound (IV), carrying out distilling concentration on the obtained mother liquor to obtain a commercial byproduct sodium chloride or sodium sulfate, and recovering distillation cooling water to use the distillation cooling water in the reaction. The chemical equation is shown in the description, and R in chemical equation is a chloride ion or a bisulfate ion. The technology has the advantages of high continuous operability, high safety, almost no wastes, realization of needed production devices being routine reaction devices, and easiness in realization of industrialization.
Owner:JIUJIANG SHANSHUI TECH
New preparation method of 3-amino-2-hydroxyphenylacetone
ActiveCN106831457ACheap and easy to getHigh purityOrganic compound preparationSulfonic acid preparationState of artFries rearrangement
The invention discloses a new preparation method of a key intermediate, namely 3-amino-2-hydroxyphenylacetone, for preparation of Pranlukast. The new preparation method comprises the following main steps: taking 2-aminophenol-4-sulfonic acid as a starting raw material, and carrying out acylation, Fries rearrangement, hydrolysis and deprotection, so that 3-amino-2-hydroxyphenylacetone is obtained. Compared with the prior art, the new preparation method disclosed by the invention has the advantages that the used raw materials are cheap and easily available, technology can easily realize industrialization, and the obtained final product is high in purity; no danger technology is adopted, and equipment is simple; and route is novel, and synthesis route is short.
Owner:上海微巨实业有限公司
Animophenoxy phthalonitrile resin monomer and synthetic method thereof
InactiveCN106631893AHigh reactivityLow costCarboxylic acid nitrile preparationOrganic compound preparationHigh resistancePolymer science
The invention discloses an animophenoxy phthalonitrile resin monomer and a synthetic method thereof. The molecular structural formula of the animophenoxy phthalonitrile resin monomer is shown as formula (I). The synthetic method comprises the following steps: (1) 2-aminophenol, a catalyst and a solvent are mixed, and the mixture is heated to 90-100 DEG C under nitrogen protection and subjected to heat preservation; (2) 3-nitrophthalonitrile is added to the solution subjected to heat preservation, the materials react at the temperature of 80-90 DEG C, and the animophenoxy phthalonitrile resin monomer is obtained. The animophenoxy phthalonitrile resin monomer has the advantages of high reaction activity, high heat resistance, low curing temperature and short curing time, and the synthetic method is simple, convenient and low in cost.
Owner:NAT UNIV OF DEFENSE TECH
Preparation method and application of composite nano nickel catalyst
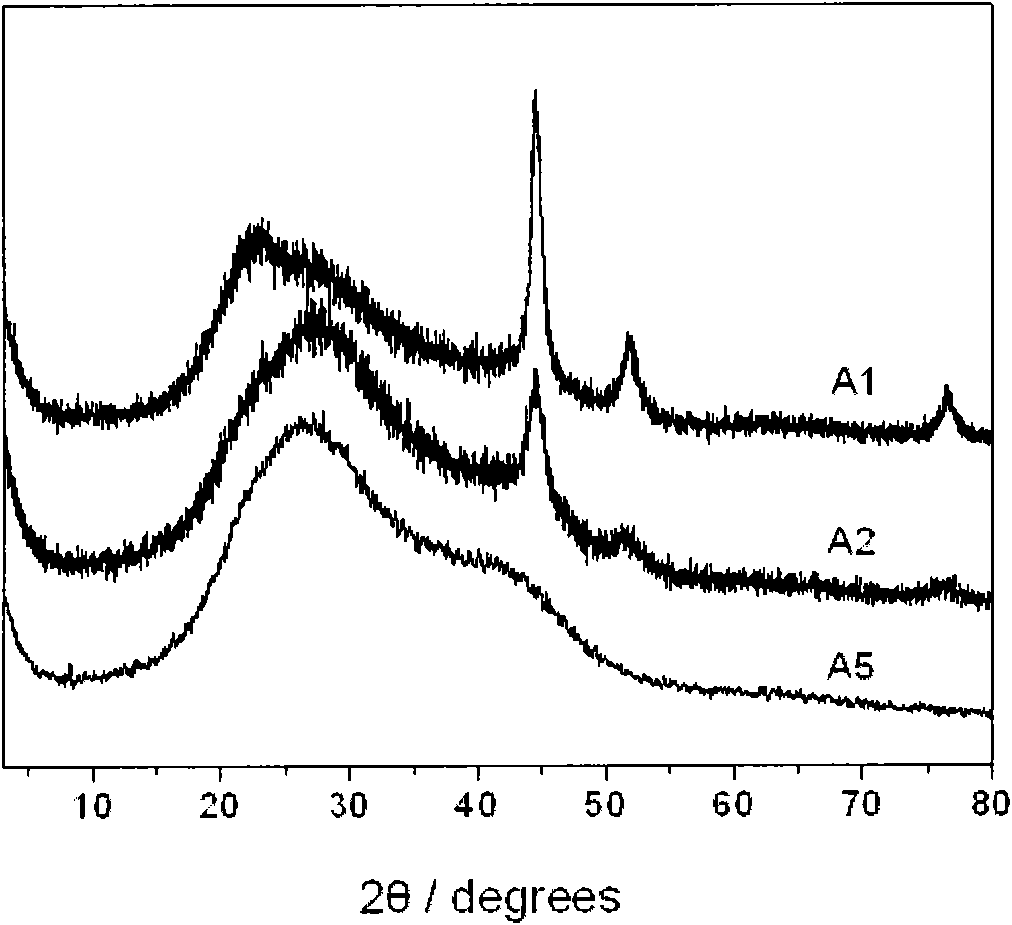
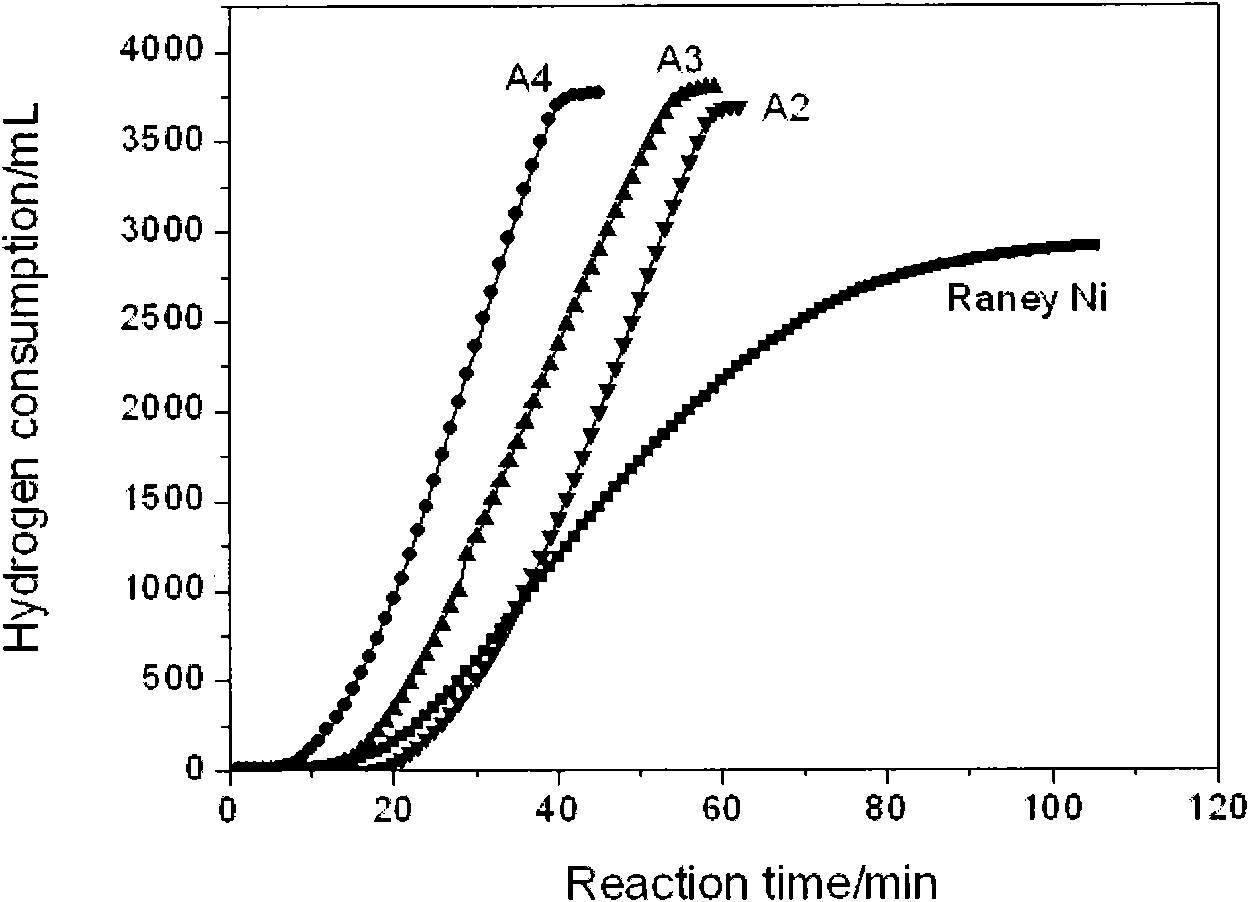
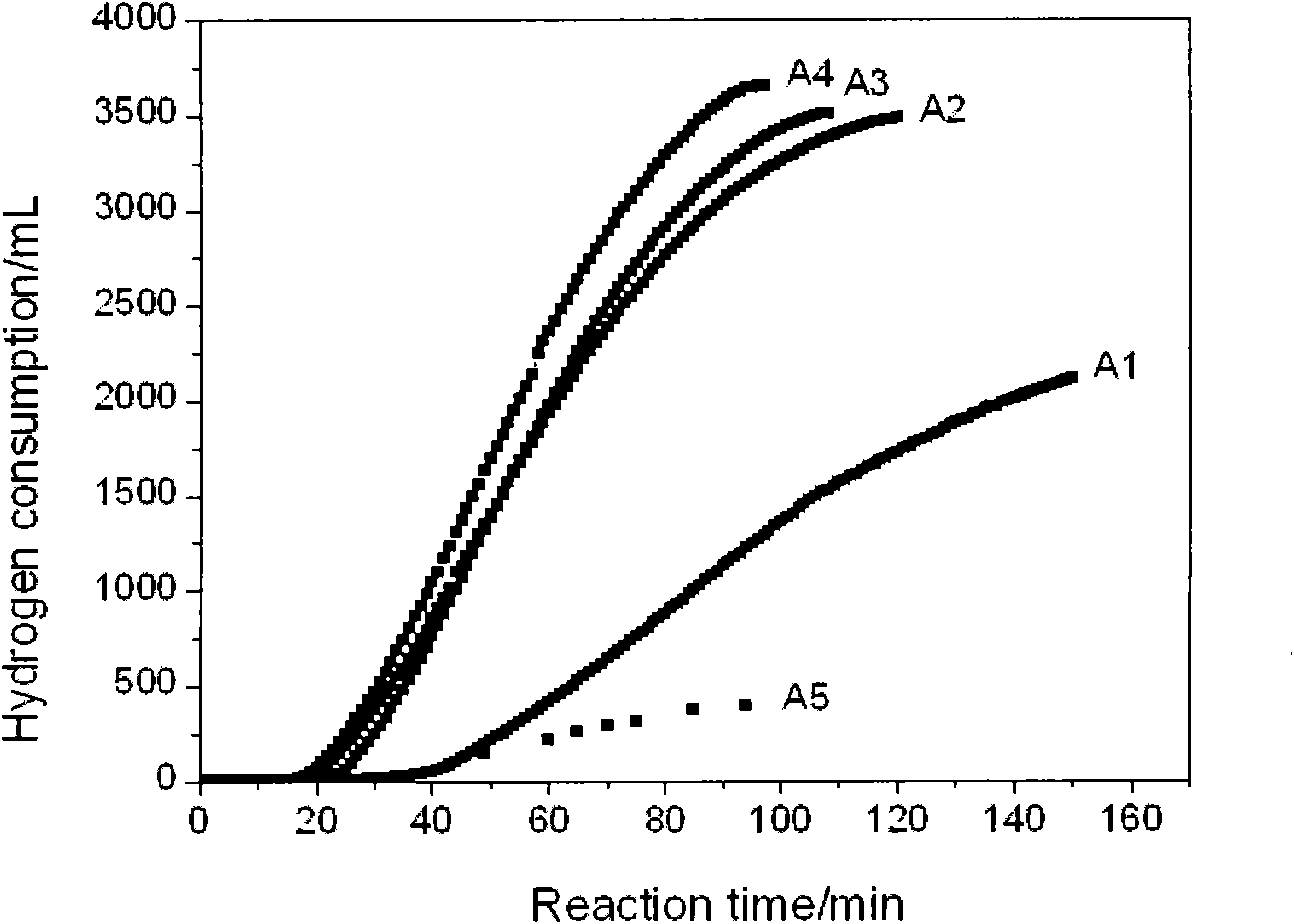
The invention discloses a preparation method of a composite nano nickel catalyst. The preparation method comprises the steps of: preparing amorphous-state nickel with small nanoscale and highly dispersed active components by using a chemical reduction method in the presence of ludox as soft load material; reducing and depositing crystalline-state nano nickel on a carrier by using the amorphous-state nickel as an inductive agent; and adjusting the proportion of amorphous-state nickel and crystalline-state nickel to prepare the ludox loaded composite nano nickel catalyst. Compared with an industrial Raney Ni catalyst and single amorphous-state nickel or crystalline-state nickel, the composite catalyst shows excellent catalysis performance in hydrogenation reaction of 4-chloro-2-nitrophenol for preparing 4-chloro-2-amino phenol.
Owner:TIANJIN ANKAITE CATALYST
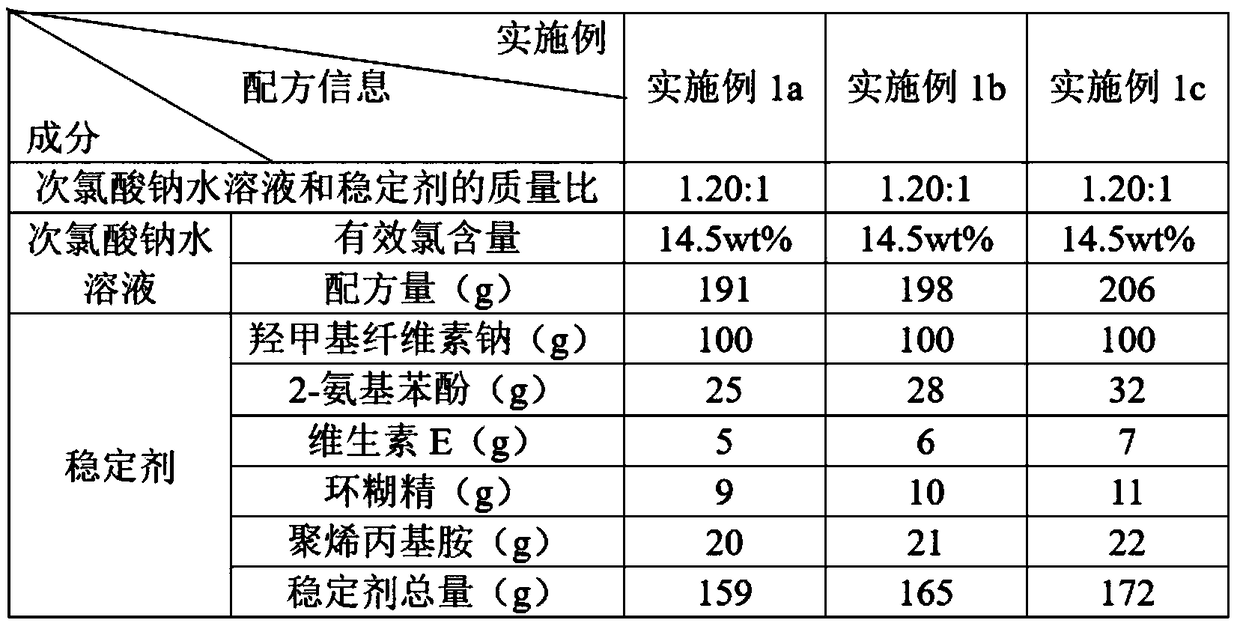
Water treatment disinfecting agent and preparation method thereof
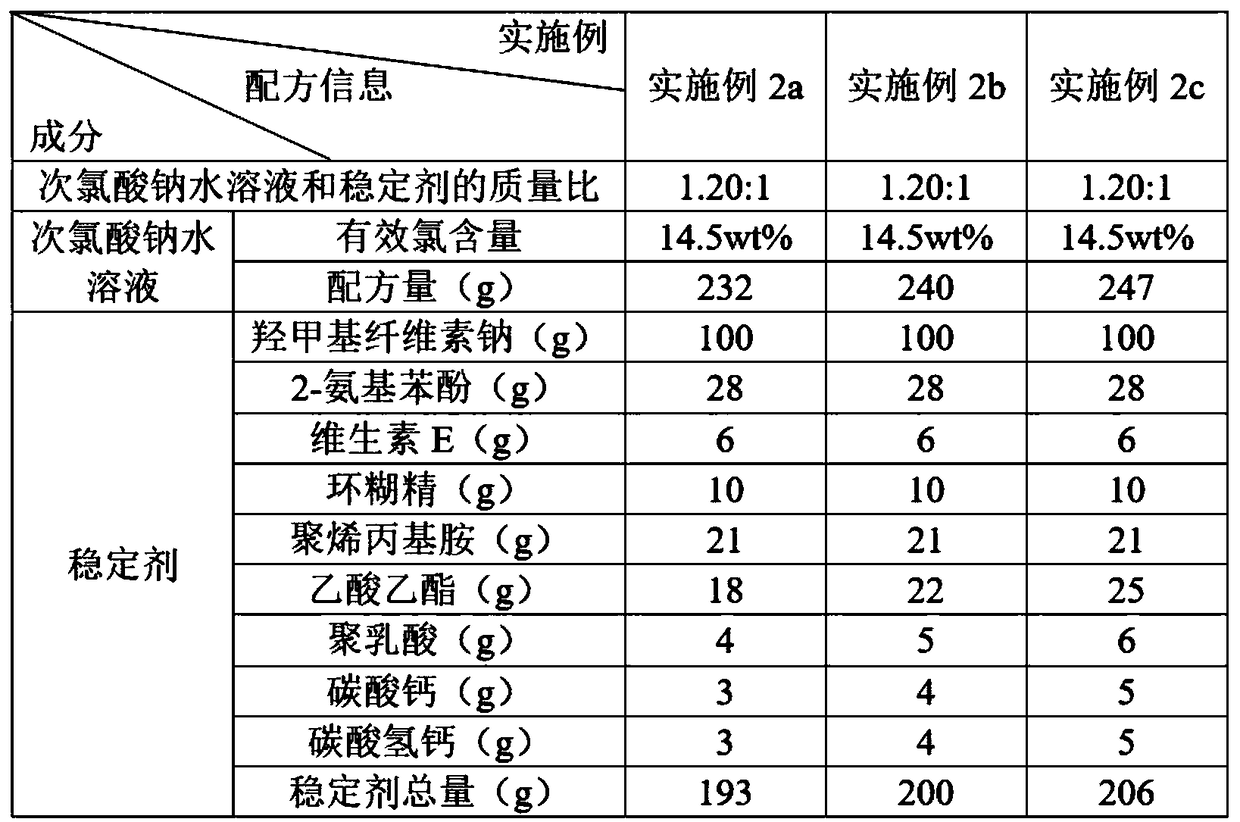
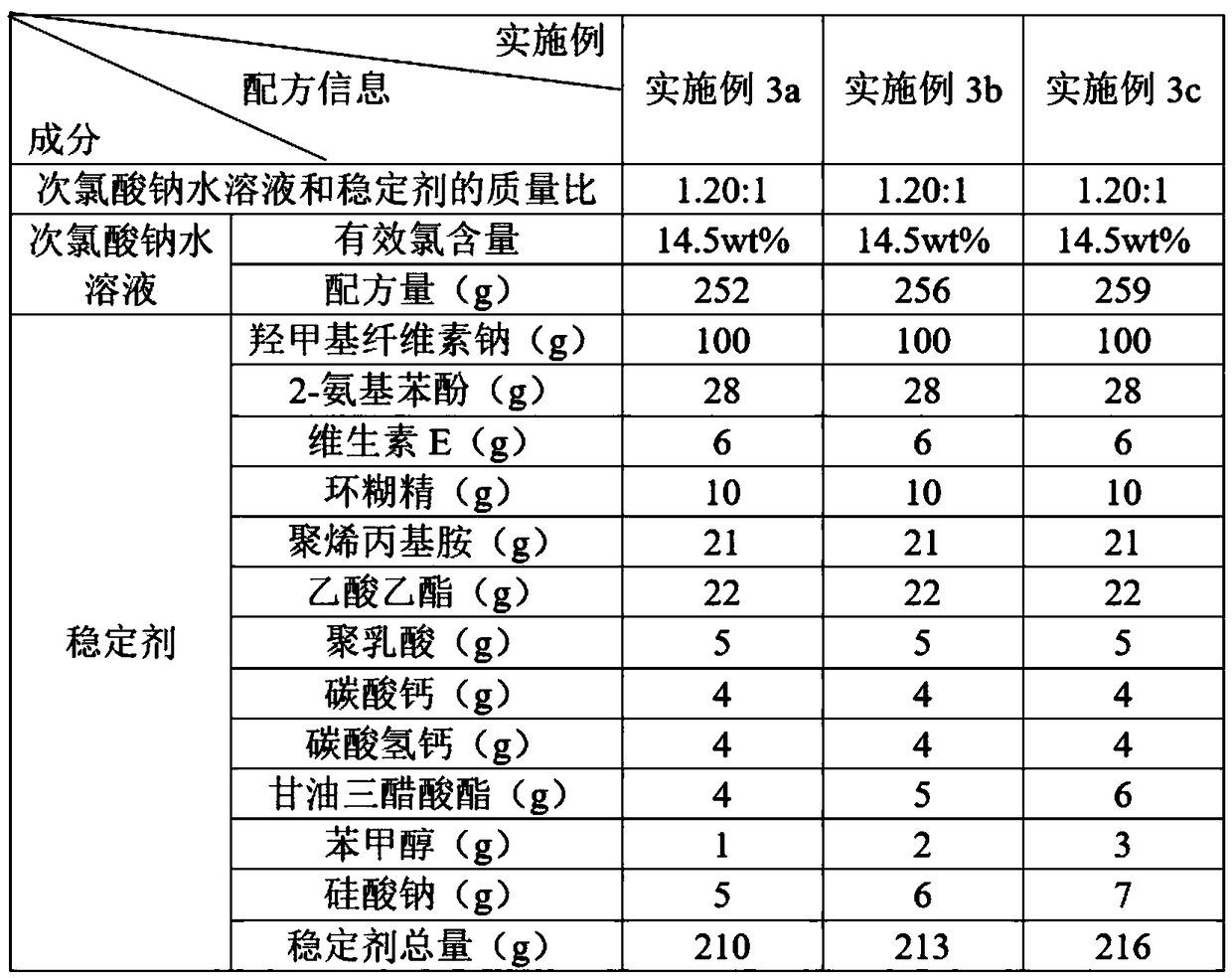
The invention discloses a water treatment disinfecting agent and a preparation method thereof. The water treatment disinfecting agent is prepared from a sodium hypochlorite aqueous solution and a , wherein the stabilizer is prepared from carboxymethyl cellulose sodium, 2-aminophenol, vitamin E, cyclodextrin and polyallylamine; and a mass ratio of sodium hypochlorite aqueous solution, carboxymethylcellulose sodium, 2-amino phenol, vitamin E, cyclodextrin and polyallylamine is (0.85 to 1.40): 1.00: (0.25 to 0.32): (0.05 to 0.07): (0.09 to 0.11): (0.20 to 0.22), and the active chlorine content of the sodium hypochlorite aqueous solution is 5.0 to 20.0 weight percent, and the water treatment disinfecting agent has the advantage of high stability.
Owner:陈邦国
Novel adsorption composite material and preparation method thereof
ActiveCN106669627AImprove adsorption capacityAvoid defects that cannot be deeply purifiedOther chemical processesWater contaminantsDiethylene glycol monobutyl etherEpoxy
The invention discloses a novel adsorption composite material, prepared from the following substances in parts by weight: 30-45 parts of activated carbon, 25-35 parts of ion exchange resin, 8-12 parts of aliphatic epoxy resin, 7-13 parts of glycerophosphate, 10-15 parts of phosphopyridoxal, 11-16 parts of 4-chloro-2-aminophenol-6-sulfonic acid, 4-7 parts of 2,6-dimethoxyphenol, 8-12 parts of 3,4-dimethoxybenzyl alcohol and 15-20 parts of diethylene glycol monobutyl ether. Through mixing the modified activated carbon with the modified ion exchange resin, the composite material with good adsorption effects on heavy metal ions as well as chemical organic matters is prepared.
Owner:SUZHOU HUANYAN ELECTRIC
Antiinflammatory agent comprising 2-aminophenol or derivative thereof as active ingredient
InactiveCN101534810ALittle side effectsExcellent for alleviating inflammation symptomsCosmetic preparationsOrganic active ingredientsSide effectBULK ACTIVE INGREDIENT
Disclosed is an anti-inflammatory agent having an excellent effect and having little adverse side-effects. The anti-inflammatory agent comprises 2-aminophenol or a derivative thereof as an active ingredient.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Antiinflammatory agent comprising 2-aminophenol or derivative thereof as active ingredient
InactiveUS20100324285A1Effect melanin productionHigh living cell levelCosmetic preparationsOrganic active ingredientsSide effectAdditive ingredient
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
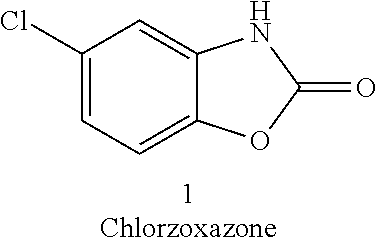
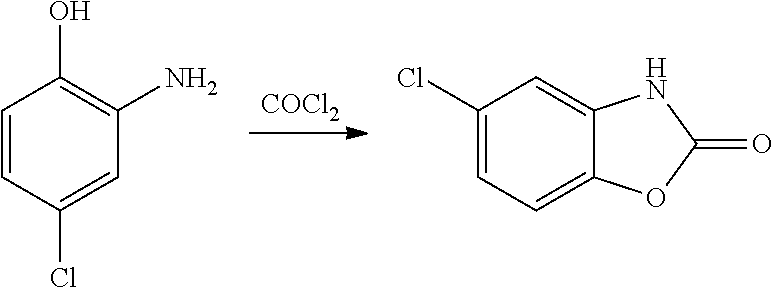
Process for the synthesis of chlorzoxazone
Disclosed is a process for the synthesis of chlorzoxazone (1) from 4-chloro-2-aminophenol and ethyl chloroformate in the presence of a base.The process is particularly advantageous because it uses ethyl chloroformate instead of triphosgene, a highly dangerous reagent that releases phosgene and must be handled with extremely strict procedures to guarantee the safety of operators in industrial facilities.Ethyl chloroformate allows the possibility of working with a number of solvents, including water.The yield and purity of the product obtained are very high.
Owner:PROCOS
Solvent-based yellow dye for environment-friendly fluorescent pigment
The invention relates to a solvent-based yellow dye for an environment-friendly fluorescent pigment. The ratio of raw materials is as follows: ethyl cyanoacetate:ethanol:hydrogen chloride:4-chlorine-2-aminophenol:4-N,N-diethylaminosalicylaldehyde:catalyst=1:(0.5-0.6):(0.9-1.05):(0.95-1.0):(0.05-0.2). A solvent-based yellow dye preparation method comprises the following steps: adding the ethyl cyanoacetate and absolute alcohol to a solvent, wherein the solvent is benzene, toluene or xylene, and the temperature is controlled in the range from 5 DEG C and 25 DEG C; introducing the hydrogen chloride for 4-8 hours, keeping the constant temperature for 8-24 hours, and then filtering; and with a C1-C8 alcohol as a solvent, reacting the filtered material with 4-chlorine-2-aminophenol, the 4-N,N-diethylaminosalicylaldehyde and the catalyst for 0.5-2 hours at a room temperature, then raising the temperature to a backflow temperature, and filtering and drying after heat preservation for 2-8 hours to obtain the solvent-based yellow dye (160:1). Compared with the conventional process, the method is relatively simplified in operation, less in three wastes, high in product yield, high in quality, simple in solvent recovery and high in recovery ratio.
Owner:WANLONG CHEM
Preparation method of 2-aryl benzoxazole and 2-aryl benzothiazole compounds
The invention belongs to the technical field of organic synthesis, and in particular relates to a preparation method of 2-aryl benzoxazole and 2-aryl benzothiazole compounds. According to the invention, 2-aminophenol(2-aminothiophenol) and aromatic aldehydes are stirred at the temperature of 100 to 130 DEG C, imidazolium salt of which the molar equivalent amount is 10% to 20% and K2CO3 of which the molar equivalent amount is 25% to 50% are added, air is used as an oxidant, and then 2-aryl benzoxazole and 2-aryl benzothiazole compounds are synthesized by a reaction. According to the invention, the cheap and easily prepared imidazolium salt is taken as a catalyst and the cheap air is taken as the oxidant to synthesize target products in a high yield, so that the preparation method of the 2-aryl benzoxazole and 2-aryl benzothiazole compounds is greatly lowered in production cost and capable of adapting to the industrial application to a greater extent.
Owner:FUDAN UNIV
Preparation method of 5-chloro-8-hydroxyquinoline and purification method thereof
ActiveCN108610288AReaction control is easyEasy to industrializeOrganic chemistryPurification methodsWater insoluble
The invention discloses a preparation method of 5-chloro-8-hydroxyquinoline and a purification method thereof, which aims at solving the problem when 4-chloro-2-aminophenol, 4-chloro-2-nitrophenol andglycerol ring are used to obtain 5-chloro-8-hydroxyquinoline, the reaction process is difficult to control, so that a large amount of tar can be generated, the reaction yield is low, the reaction operation is difficult, and a large amount of waste acid is generated after the post treatment. Boric acid and insoluble organic solvents being added into the reaction solution is the innovation of the preparation method, by the method, the acuteness of direct production of acrolein from sulphuric acid and glycerol is mitigated by boric acid, the speed of the acrolein polymerization to generate the tar is reduced, the yield and the safety are effectively improved, and a small amount of an water-insoluble organic solvents can effectively prevent 4-chloro-2-nitrophenol from coagulating on the reactor wall, the problem that it is difficult to control in the process of production operation is solved, which shortens the reaction cycle and improves production efficiency. The preparation method of 5-chloro-8-hydroxyquinoline and purification method thereof has the advantages of less tar, high yield and safe reaction, and can effectively reduce waste acid production and is easy to industrialize.
Owner:LIER CHEM CO LTD +1
Novel chelate fiber, preparation method thereof, and application of novel chelate fiber for detecting Pb(II) in preserved eggs
PendingCN109701502AWide variety of sourcesLow priceOther chemical processesColor/spectral properties measurementsFiberRepeatability
The invention provides a novel chelate fiber, a preparation method thereof, and an application of the novel chelate fiber for detecting Pb(II) in preserved eggs. The novel chelate fiber is prepared through chelation by taking polyacrylonitrile fibers as a matrix and 2-aminophenol-4-sulfonic acid as a ligand. The novel chelate fiber disclosed by the invention is stable in performance, large in adsorption capacity and specific in selectivity, can be used to carrying out separation and enrichment on heavy metal Pb (II) in the preserved eggs, and then can be combined with ultraviolet spectrophotometry to detect the content of Pb (II) in the preserved eggs. The detection method is green and is free of pollution, operation is convenient and simple, cost is low, universality is high, repeatability is high, and accuracy and precision can meet requirements of sample detection.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a 2-aminophenol and derivatives thereof
An oxidative dyeing composition comprising a 1-hexyl-4,5-diaminopyrazole compound or a 1-heptyl-4,5-diaminopyrazole compound of formula (I) in combination with a 2-aminophenol compound of formula (II): wherein R1, R2, R3, R4, R5, R6 and R7 are as defined herein and a=1 or 2. The composition provides good hair color intensity together with good wash and bleeding fastness.
Owner:WELLA OPERATIONS US LLC
3-(2-benzoxazole)coumarin formamide compound, and preparation method and application thereof
ActiveCN108752327AShort routeRaw materials are easy to getOrganic chemistryNon-linear opticsBenzoxazoleSalicylaldehyde
The invention discloses a 3-(2-benzoxazole)coumarin formamide compound, a preparation method thereof, and an application thereof as a third-order nonlinear optical material. The preparation method comprises the following steps: reacting substituted salicylaldehyde, ethyl cyanoacetate and 4-carboxy-2-aminophenol in an alcohol solvent A under the catalysis of an organic acid to obtain a 3-(2-benzoxazole)coumarin carboxylic acid compound; reacting the 3-(2-benzoxazole)coumarin carboxylic acid compound with oxalyl chloride in chlorination solvent B under the catalysis of DMF to obtain a 3-(2-benzoxazole)coumarin chloride compound; and reacting the 3-(2-benzoxazole)coumarin chloride compound with amine in an organic solvent C under the action of an inorganic alkali to obtain the target compoundhaving a structure represented by formula (I). The preparation method has the advantages of short route, easily available raw materials, simple process, reaction conditions, realization of the development of the application of the 3-(2-benzoxazole)coumarin formamide compound in third-order nonlinear optics, great enforcement values and good social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
8-hydroxyquinoline type compounds and preparation method thereof
8-hydroxyquinoline type compounds and a preparation method thereof are disclosed. The general structure formula of the compounds is shown in the description, wherein R1 and R2 are each independently selected from alkanes, cycloalkanes, phenyl, substituted phenyl or heterocyclic aromatic hydrocarbons, and a substitute of the substituted phenyl is one or a combination of two or more selected from alkyl, alkoxy, hydroxyl, halogen and an ester group. The method includes reacting a uniformly mixed system of 2-aminophenol, an aldehyde, an acetylene, a catalyst and an organic solvent in an air atmosphere at 60-110 DEG C for 0.5-4 h; and subjecting a reaction solution after the reaction is finished to after-treatment to obtain a target product that is a 8-hydroxyquinoline type compound. A multi-component one-pot process is adopted, raw materials are simple in source and rapid to prepare, the dosage of the catalyst is low, and the environment cost is greatly reduced. The method is simple to operate, high in yield and wide in substrate universality, and establishment of a molecular library of the 8-hydroxyquinoline type compounds is completed and enriched.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
S protein of SARS virus, its vaccine and method for screening medicament using it
The present invention discloses a S protein of SARS virus, which has SEQ ID NO.2 aminophenol sequence, and has mutation of (a) 778D to Y; (b) 77D to G; (c) 244T to I; (d) 1182K to Q; (e) 360F to S; (f) 479N to R or K; (g) 480D to G; (h) 609A to L. the invention also discloses nucleic acid encoding the S protein and SARS virus containing the mutations. The invention further discloses medicament for screening the SARS and its vaccine of the S protein aiming at SARS virus.
Owner:SOUTH RES CENT NAT HUMAN GENE GROUP +3
Process for producing solvent yellow GR
The invention discloses a process for producing solvent yellow GR. The process is characterized by comprising a coupling reaction step, a complexation reaction step, a neutralization reaction step and an acid precipitation reaction step, wherein the coupling reaction step comprises the following substeps of diazotizing 4-nitro-2-aminophenol-6-sulfonic acid, and then, reacting the diazotized 4-nitro-2-aminophenol-6-sulfonic acid with acetoacetate aniline for 1-3 hours at the temperature of 50-80 DEG C under an alkaline condition, so as to produce azo A and water; the complexation reaction step comprises the following substeps of adding the azo A, obtained in the coupling reaction step, into a reaction kettle, adding water, a complexation agent and a PH regulator, controlling the temperature to 90-100 DEG C, and reacting for 5-15 hours, so as to produce a sodium sulfonate containing complex and an acidic compound; and the acid precipitation reaction step comprises the following substeps of reacting the sodium sulfonate containing complex, obtained in the complexation reaction step, with sulfuric acid for 1-5 hours at the temperature of 80-95 DEG C, so as to produce a sulfonic group containing complex and sodium sulfate, and then, filtering and washing, thereby obtaining the solvent yellow GR. The process has the advantages that the production of acetic acid during complexation reaction is avoided and the process steps are simple and friendly to the environment.
Owner:南通市争妍新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com