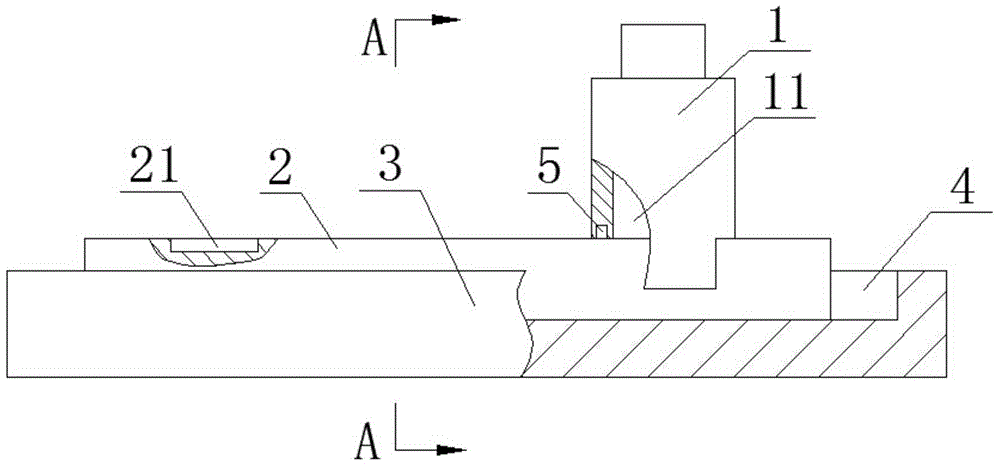
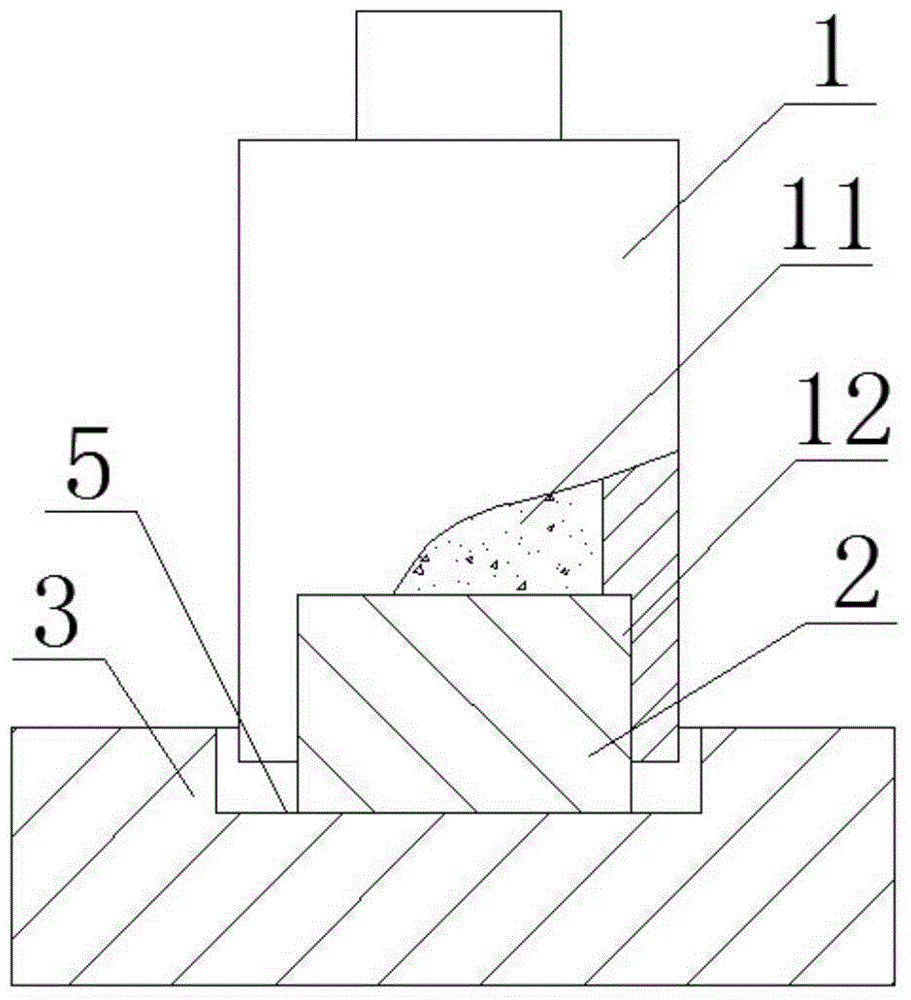
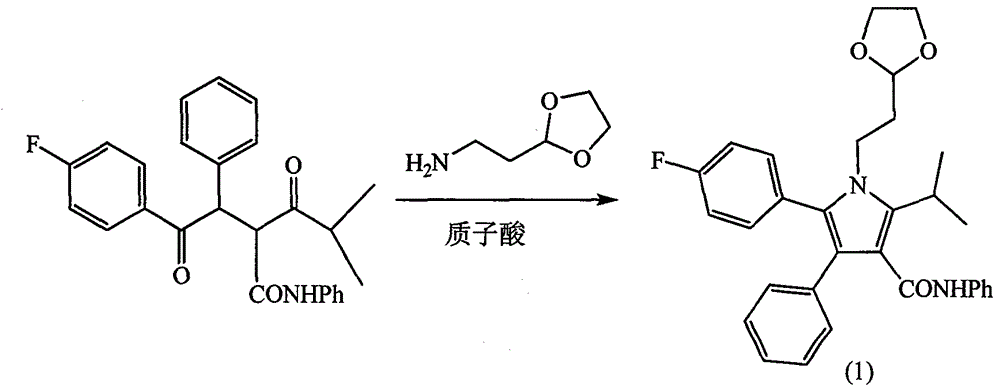
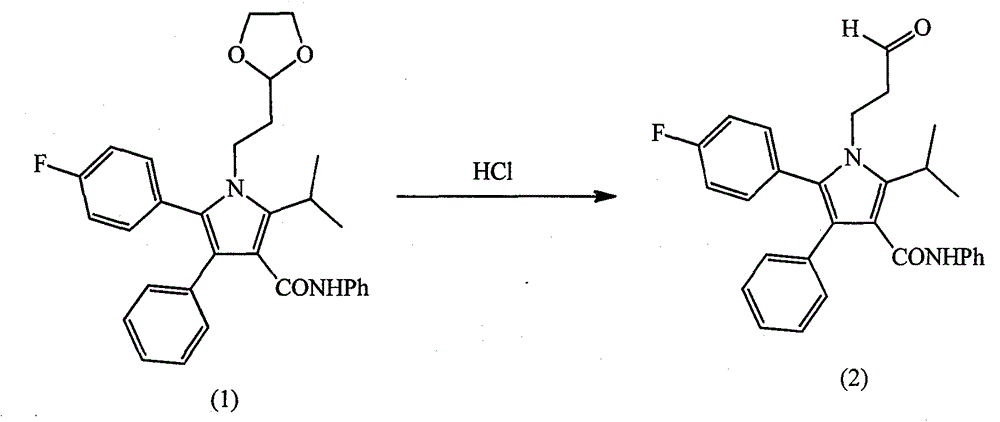
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51results about How to "The process is clean and environmentally friendly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
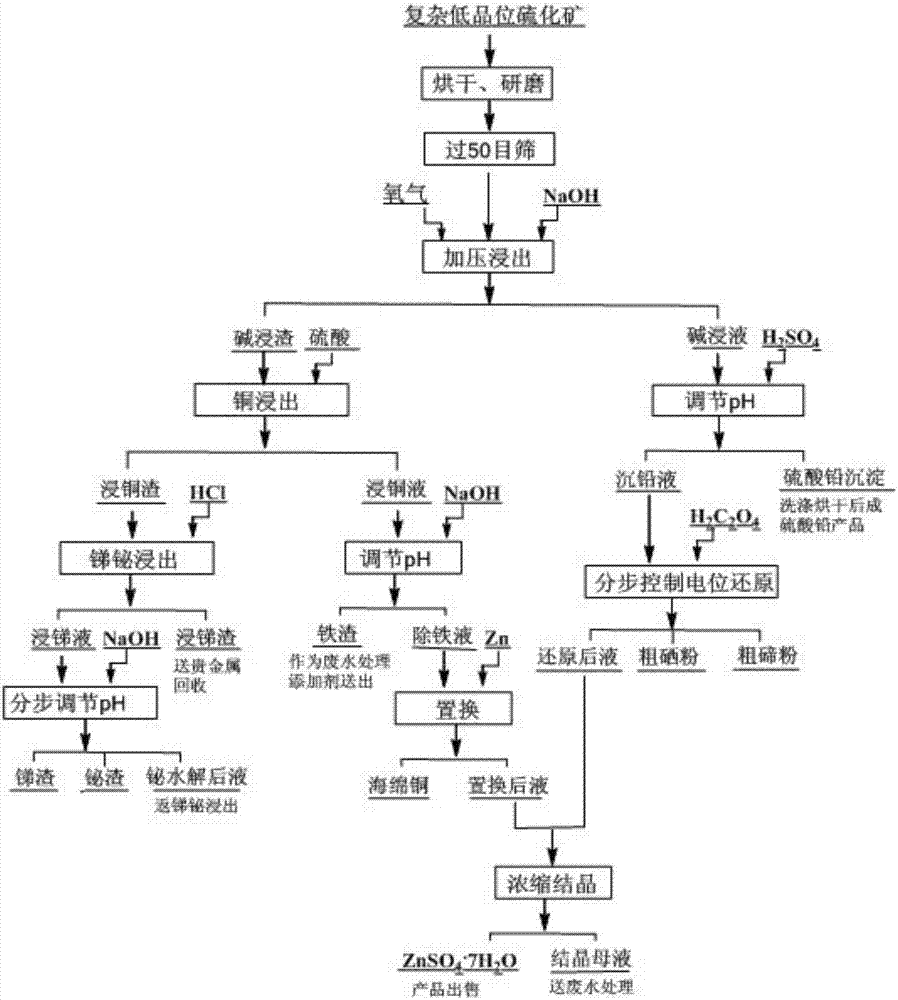
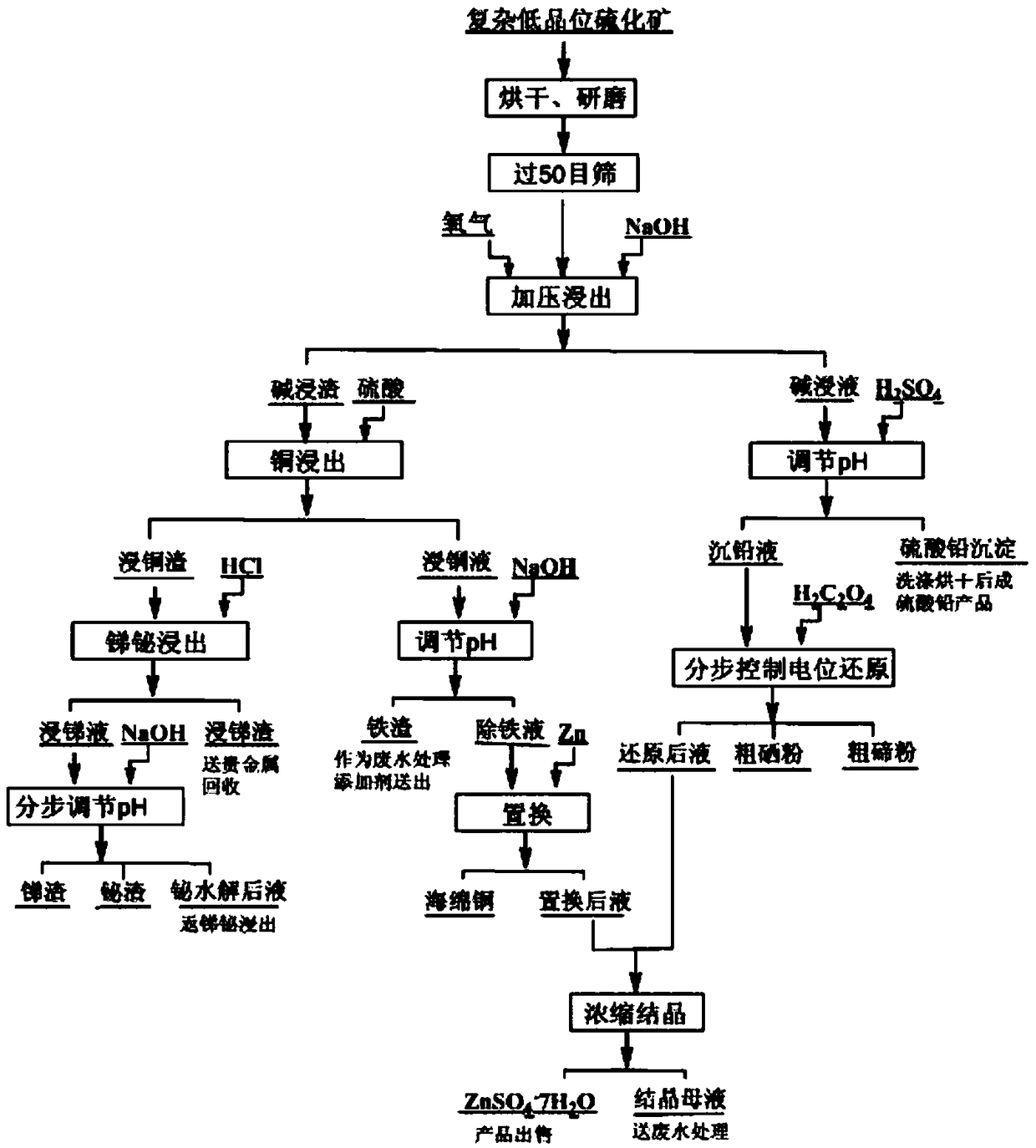
Process for all-wet recovery of valuable elements in complex low-grade sulphide ore
ActiveCN107190143AImprove recycling effectLow investment requirementProcess efficiency improvementSlagLead sulfate
A process for all-wet recovery of valuable elements in a complex low-grade sulphide ore comprises the following steps: crushing and grinding a raw material, sieving the crushed and ground raw material, performing size mixing by use of a sodium hydroxide solution, and after that, performing pressurized oxidizing leaching to obtain an alkaline-leached solution and alkaline-leached residues; acidizing the alkaline-leached solution to obtain a lead sulfate product and a heavy lead solution, and performing potential reduction control step by step on the heavy lead solution to obtain crude selenium powder, crude tellurium powder and a post-reduction solution; performing copper-soaking on the alkaline-leached residues by use of a sulfuric acid solution to obtain copper-leached residues and a copper-leached solution; performing antimony and bismuth soaking on the copper-leached residues by use of an acid liquor containing chloride ions to obtain an antimony-leached solution and antimony-leached residues, feeding the antimony-leached slags to noble metals for recovery, and adjusting the pH of the antimony-leached solution step by step to obtain antimony residues and bismuth residues; adjusting the pH of the copper-leached solution to obtain iron residues and an iron-removed solution, wherein the iron residues serve as a waste water treatment additive, and the iron-removed solution is subjected to zinc dust replacement to obtain copper powder and a post-replacement solution; and merging the post-reduction solution and the post-replacement solution, and performing concentration and crystallization to obtain heptahydrate. The process is good in comprehensive recovery effect, strong in adaptation to the raw material, clean and environment-friendly in process, low in demand on equipment, adjustable in implementation scale and high in practicability.
Owner:JIANGXI COPPER CORP
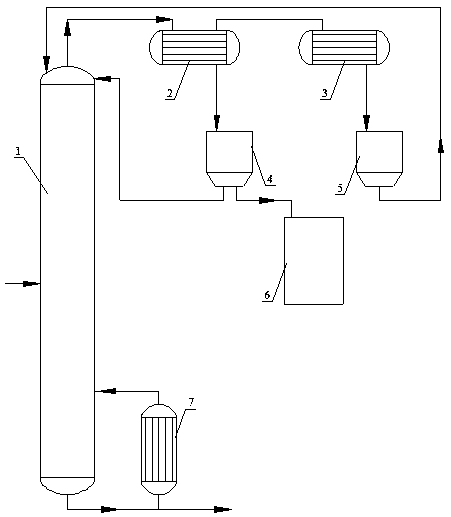
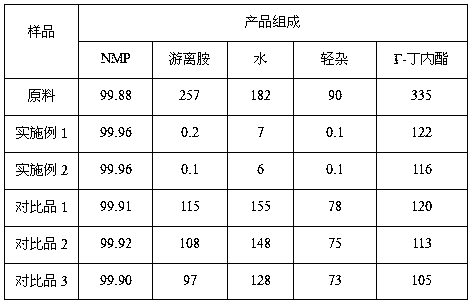
Refining method of N-methyl pyrrolidone product
ActiveCN108794371AReduce light impurity contentThe process is clean and environmentally friendlyOrganic chemistryEngineeringEnergy consumption
The invention discloses a refining method of an N-methyl pyrrolidone product. A classifying and condensing system is arranged on the upper portion of an N-methyl pyrrolidone product rectifying tower,and comprises two or more temperature-controllable heat exchangers and corresponding refluxing tanks. The refining method comprises the following specific steps: feeding N-methyl pyrrolidone which requires to be purified into a rectifying tower for distilling; carrying out primary condensing on an overhead product of the rectifying tower; feeding a gas-phase product subjected to primary condensinginto a secondary heat exchanger for condensing; feeding a liquid-phase product subjected to primary condensing into a primary refluxing tank; discharging a gas product subjected to secondary condensing; refluxing a liquid-phase product subjected to secondary condensing to the rectifying tower; and refluxing part of the products in the primary refluxing tank to the rectifying tower, and meanwhileextracting the other part of the products as an N-methyl pyrrolidone refined product. The refining method of an N-methyl pyrrolidone product is low in investment, low in energy consumption and easy tooperate, and free amines and light impurities are removed simply and effectively to obtain the high-quality N-methyl pyrrolidone product.
Owner:MAIQI CHEM CO LTD
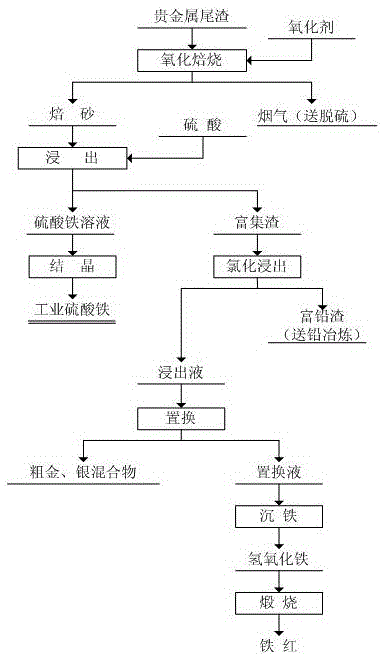
Comprehensive recovering method for tailings containing precious metal
ActiveCN105132694AEffective recyclingThe process is clean and environmentally friendlyProcess efficiency improvementBrown iron oxideLead smelting
The invention discloses a comprehensive recovering method for tailings containing precious metal. The comprehensive recovering method specifically includes the following steps that the tailings are led into oxygen to be subjected to oxidizing roasting; oxidation leaching is conducted on zinc calcine through sulfuric acid, most of iron is removed, a leaching agent is evaporated and crystallized, and industrial iron sulfate is obtained; after chloridizing leaching is conducted on leaching slag through sulfuric acid and sodium chloride, the leaching slag serves as lead-rich slag to be sent to a lead smelting enterprise; replacement is conducted on the leaching agent through iron powder, and crude gold and silver mixtures are obtained and sent to a precious metal deep processing system; and PH of replacement fluid is adjusted through ammonium water or sodium hydroxide, iron is deposited, obtained ferric hydroxide is roasted to obtain iron oxide red, and the iron oxide red serves as the raw material of the coating industry. According to the technology method, the influences on the environment due to long-term stacking of the kind of slag are eliminated, the process is clean and environmentally friendly, and secondary pollution is avoided.
Owner:甘肃高能中色环保科技有限公司
Pretreatment leaching process of flotation zinc oxide concentrates
A pretreatment leaching process of flotation zinc oxide concentrates includes the following steps that a small amount of concentrated sulfuric acid is added into the flotation zinc oxide concentrates and stirred evenly; heat-preservation curing is carried out on obtained mineral powder at a low temperature to partially decompose flotation chemicals on the surface of the flotation zinc oxide concentrates; the obtained clinkers are leached out with waste electrolytes, filtered and separated; and finally, a small amount of active carbon is added into the zinc sulfate solution obtained after filtering and separation for adsorption, filtering and separation are carried out again, and zinc oxide concentrate leachate is obtained. The pretreatment leaching process of flotation zinc oxide concentrates has the advantages of being simple, environmentally friendly and the like, can optimize the leaching conditions of the concentrates, and resolves the problems of residual floatation chemicals and the like.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
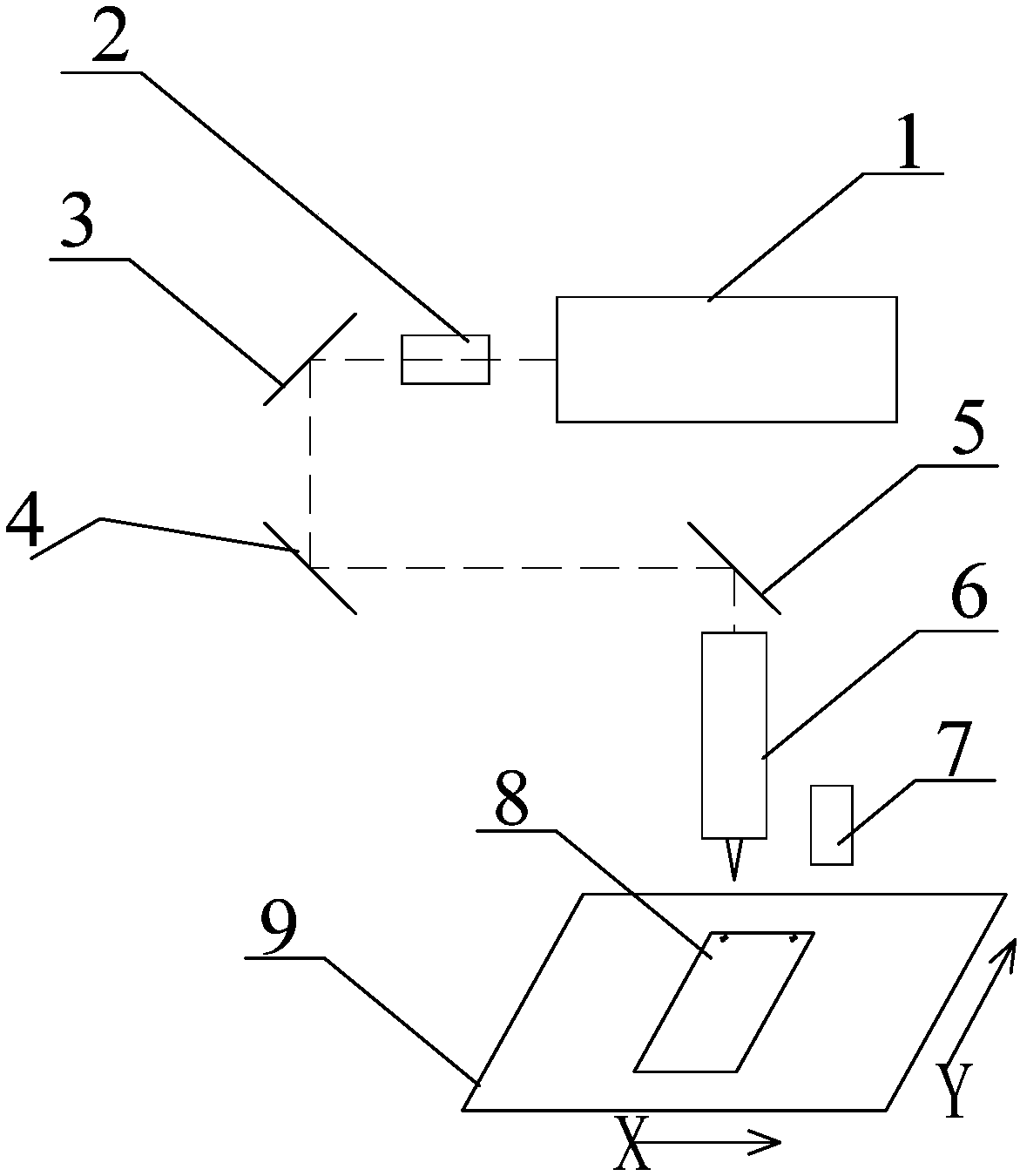
Laser machining system and laser machining method for chamfering glass screen body
PendingCN108127270AGood collimationIncreased beam diameterLaser beam welding apparatusPicosecond laserBeam expander
The invention relates to a laser machining system and a laser machining method for chamfering a glass screen body. A beam expander is arranged at an optical path output end of a picosecond laser; a reflecting mirror is arranged on an output optical path of the beam expander; a laser cutting head is arranged on a reflecting optical path of the reflecting mirror; the optical path output end of the laser cutting head directly faces to a moving platform which is used for bearing the glass screen body; and a CCD alignment system is arranged above the moving platform. According to the laser machining method, the picosecond laser is used for emitting laser; the beam expander is used for collimating the laser and expanding the laser beams so as to improve the collimation degree of the laser and increase the diameter of the emitted laser beams; the reflecting mirror is used for reflecting the laser beams into the laser cutting head and focusing the laser into a beam of laser filament; the laserfilament is focused to the glass screen body on the moving platform; and the CCD alignment system and the moving platform cooperate to realize penetration type scribing at a specified position of theglass screen body. The laser machining system and the laser machining method provided by the invention are suitable for rapidly and efficiently cutting the glass screen body; glass can be penetratedat a time by virtue of a suitable filament length structure; the glass can be cut into pieces after being cut once; and a cut section is smooth and free of taper.
Owner:SUZHOU DELPHI LASER +1
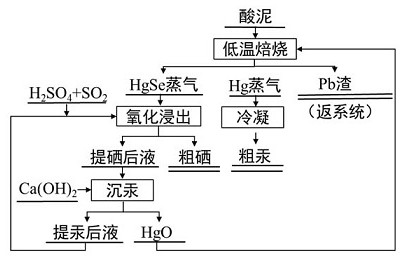
Comprehensive recovery method for valuable metals in lead-zinc smelting acid mud
PendingCN113088694AHigh puritySimple processProcess efficiency improvementElemental selenium/telluriumEnvironmental engineeringMercury pollution
The invention relates to a comprehensive recovery method for valuable metals in lead-zinc smelting acid mud, and belongs to the technical field of hazardous waste resource development and utilization. The method is mainly divided into two steps according to the form of a mercury-containing phase, the mercury-containing phase easy to treat is directly subjected to pyrogenic roasting and volatilization recovery, the selenium-containing and mercury-containing phase difficult to treat is converted into the phase easy to treat through wet leaching, and then the easy-to-treat phase is further treated and recycled; and mercury in the acid mud is recovered in the form of elemental mercury, selenium is recovered in the form of crude selenium, and the lead and silver residues are returned to a Isa furnace for smelting and recovery. According to the method, deep removal and separation of mercury and selenium and efficient recovery of lead, silver, selenium and mercury can be achieved, the recovery rate of mercury and selenium can reach 96% or above, the recovery rate of lead and silver can reach 99%, high-valued utilization of the acid mud can be achieved through the technology, the problem of mercury pollution in production is well solved, the acid mud treatment process is closed, and direct contact with people is avoided in the production process, so that the production safety is ensured, and good popularization and application prospects are achieved.
Owner:YUNNAN CHIHONG ZINC & GERMANIUM
Generator provided with switching catalytic combustion gas turbine
InactiveCN101545403AReduce pollutionCatalytic combustion achievedGas turbine plantsLow speedCombustion chamber
The invention discloses a generator provided with a switching catalytic combustion gas turbine in the technical field of dynamic power generation, wherein the outlet and the inlet of an air filter are connected with low calorific value fuel gas, the outlet of the air filter is connected with the inlet of a centrifugal compressor, the outlet of the centrifugal compressor is connected with the inlet of a four-way valve, the outlet of the four-way valve is connected with one end of a catalytic combustion chamber, a heat accumulator at the other end of the catalytic combustion chamber enters a centripetal turbine, the outlet of the centripetal turbine is connected with the atmosphere directly, a flam ignitor is started and inserted in a cavity of the catalytic combustion chamber, the centrifugal compressor is coaxial with the centripetal turbine, an output shaft at the centrifugal compressor end is connected with a high-speed shaft of a reduction gear box through a shaft coupling, and a low-speed shaft of the reduction gear box is connected with a generator shaft through the shaft coupling. The fuel net content of the low calorific value fuel gas adopted by the generator is less than 5 percent and the heat value of the low calorific value fuel gas is lower than 2 MJ / NM3, and the generator adopts catalytic combustion so that the fuel gas is usually exhausted into the atmosphere directly, and not only the energy is used but also the discharge of greenhouse gas is reduced.
Owner:SHANGHAI JIAO TONG UNIV
Environment-friendly regenerated activated carbon production process
ActiveCN112456491APlay the role of expanding the holeReduce energy consumptionCarbon compoundsActivated carbonPhysical chemistry
The invention provides an environment-friendly regenerated activated carbon production process, and belongs to the technical field of activated carbon recovery. The production process comprises the following steps: carrying out micro-oxidation on the surface of hot air, grinding, carrying out vacuum extraction, sequentially carrying out immersion cleaning with an acid-base solution to obtain a recovered material, heating the recovered material, quickly immersing in low-temperature liquid nitrogen, loading potassium ferrate, carrying out high-temperature activation, then mixing with weakly viscous pulverized coal, pressing, molding, and carrying out secondary activation to obtain activated carbon. According to the method, adsorbate on the activated carbon is separated and desorbed through grinding refining and vacuum extraction, low-temperature liquid nitrogen and pore channel loaded potassium ferrate are decomposed to recover a pore structure of the activated carbon, recycling of the waste activated carbon is achieved, and the process has important significance on the comprehensive utilization efficiency of resources.
Owner:JIANGSU PURESTAR EP TECH CO LTD
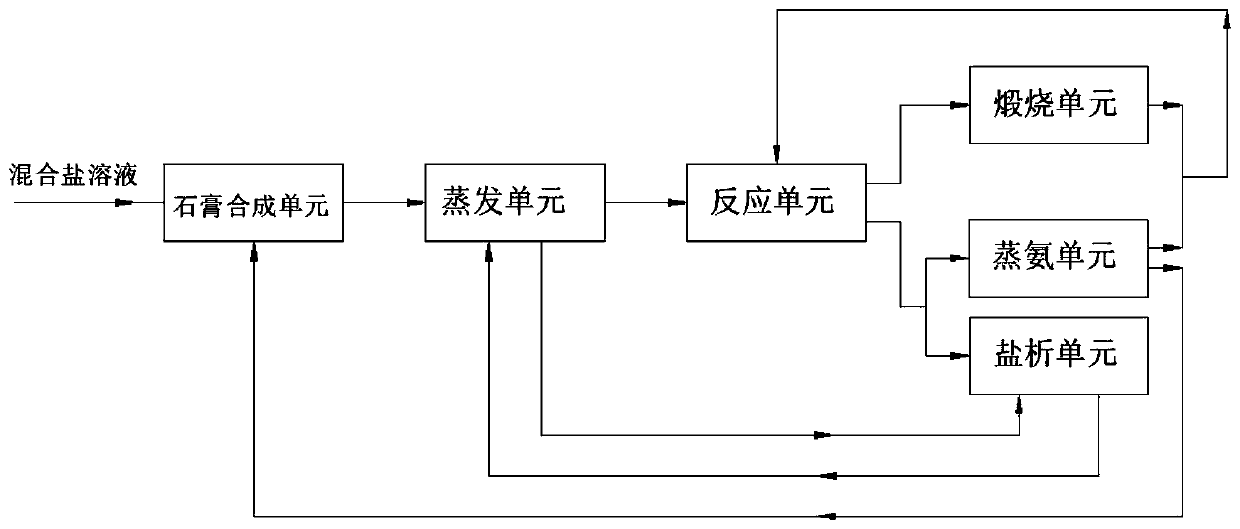
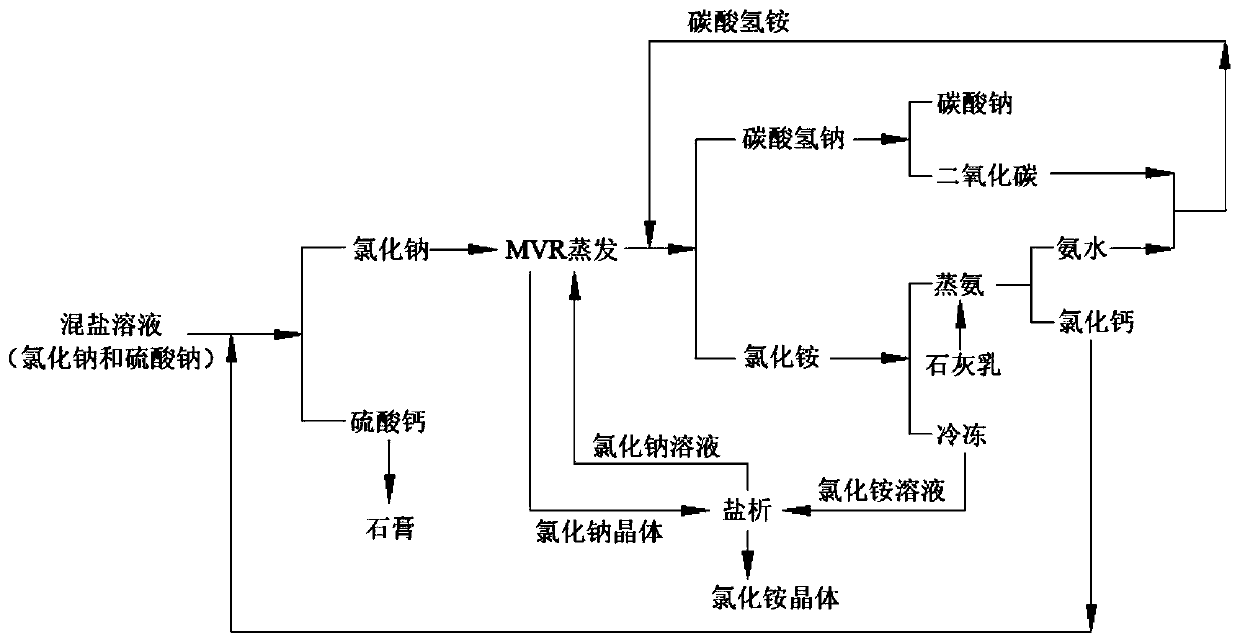
Resourceful treatment system for mixed salt solution and resourceful treatment method thereof
ActiveCN111392746AThe process is clean and environmentally friendlyCalcium/strontium/barium sulfatesAlkali metal chloridesDistillationSodium sulfate
The invention provides a resourceful treatment system for a mixed salt solution and a resourceful treatment method. The resourceful treatment system comprises a gypsum synthesis unit, an evaporation unit and a reaction unit which are sequentially connected in the material flow direction, the resourceful treatment system further comprises a calcining unit, an ammonia distillation unit and a salting-out unit which are respectively connected with the outlet of the reaction unit, wherein an outlet of the calcining unit and an outlet of the ammonia distillation unit are combined into one path and then are connected into the reaction unit, the ammonia distillation unit is further connected with the gypsum synthesis unit, and the salting-out unit is circularly connected with the evaporation unit.The method aims at the characteristics of a mixed salt solution of sodium chloride and sodium sulfate; sodium sulfate and calcium chloride in a normal-pressure sodium chloride solution are synthesized into alpha-semi-hydrated high-strength gypsum, sodium chloride and ammonium bicarbonate react to prepare sodium carbonate, ammonium chloride is prepared through freezing salting-out in a combined-soda method, and other technologies are organically coupled, so that the sodium chloride and sodium sulfate mixed salt solution recycling system and the method are provided.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Regeneration method for waste mercuric chloride contact agent
InactiveCN109046403AEffective disengagementPromote regenerationCatalyst regeneration/reactivationActivated carbonMicrowave
The invention discloses a regeneration method for a waste mercuric chloride contact agent and belongs to a recycling technique of the waste mercuric chloride contact agent. The regeneration method comprises the following steps: firstly, performing wet-process leaching on the waste mercuric chloride contact agent, performing leaching reaction under an ultrasonic effect, and filtering, thereby acquiring a mercury-containing filtrate; controlling temperature, adding a neutralizer for regulating pH value, completely converting mercury ions in the solution into mercuric oxide sediments, and keepingtoxic phosphor and sulfur in the mercury contact agent still in the solution; dissolving mercuric oxide with hydrochloric acid, thereby acquiring a mercuric chloride solution used for preparing the mercury contact agent; performing microwave high-temperature activating on filter residue (waste contact agent for mercury removal) under the condition of activating gas, thereby acquiring a regenerated activated carbon for waste mercury contact agent; soaking the activated carbon for waste mercury contact agent in a mercuric chloride solution for waste mercury contact agent, thereby acquiring a new mercuric chloride contact agent. According to the invention, efficient recycling of mercuric chloride and regeneration of activated carbon can be simultaneously realized in the manner of combining ultrasonic acidizing leaching with microwave high-temperature activating treatment.
Owner:KUNMING UNIV OF SCI & TECH
Collaborative extraction system of magnesium and lithium in salt lake brine and treatment method thereof
ActiveCN107828960AHigh yieldRealize high-value utilizationProcess efficiency improvementSlagGreen cleaning
The invention provides a collaborative extraction system of magnesium and lithium in salt lake brine and a treatment method thereof. The collaborative extraction system mainly comprises a spraying roasting system, a dust removing system, a pulping washing device, a solid-liquid separation device, a magnesium extracting unit, a lithium extracting unit and a smoke treatment system, wherein productsof the magnesium extracting unit comprise magnesium oxide; products of the lithium extracting unit comprise magnesium hydroxide precipitates and lithium carbonate; and the magnesium hydroxide precipitates are fed in the magnesium extracting unit for magnesium extraction. Wet reaction separation is organically coupled with pyrogenic purification; a traditional technological process for extracting salt lake brine elements is integrated to realize high-value collaborative extraction of valuable elements-magnesium and lithium in the salt lake brine; meanwhile, smoke afterheat is fully used in theextracting process to realize gradient utilization of heat; no waste water and no waste slag are generated; the tail gas emission is qualified; and the collaborative extraction system belongs to a green cleaning technological process.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
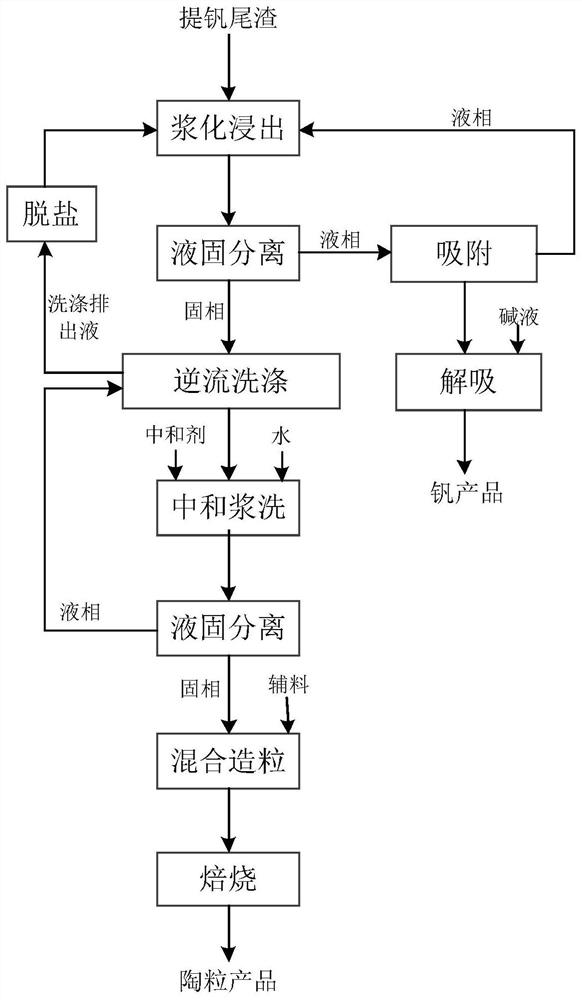
Method for preparing ceramsite from acidic vanadium extraction tailings
ActiveCN112430067AHarmlessImplement resourcesProcess efficiency improvementCeramic materials productionPregnant leach solutionEnvironmental engineering
The invention provides a method for preparing ceramsite from acidic vanadium extraction tailings, and the method comprises the following steps of: carrying out slurrying leaching and liquid-solid separation on the acidic vanadium extraction tailings to obtain a leaching solution and leaching residue, and carrying out adsorption treatment on the obtained leaching solution; and washing and neutralizing the leaching residue, mixing the obtained neutralized residue with auxiliary materials, and conducting granulating and roasting to obtain a ceramsite product. According to the method, high-efficiency recovery of valuable metal vanadium is realized through slurrying leaching of the acidic vanadium extraction tailings and adsorption; the leaching residue is washed and neutralized and then prepared into ceramsite together with the auxiliary materials, so that harmlessness and recycling of various solid wastes are realized; the method is simple to operate and low in cost, the technological process is clean and environmentally friendly, and good economic benefits and application prospects are achieved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Oil cup type pad printing machine ink filling structure
The invention provides an oil cup type pad printing machine ink filling structure. The purpose is to solve the problems that in the process of using an existing pad printing machine, when an oil cup is accidentally broken or upset, the flowing-out position of ink flowing out from the oil cup can not be controlled, the ink is difficult to clear away, and therefore severe local air pollution will be generated. The oil cup type pad printing machine ink filling structure comprises an oil cup and a pad printing steel plate, wherein the oil cup is provided with an ink cavity, and the pad printing steel plate is provided with a concave pattern. A rectangular notch is formed in the middle of the lower end of the oil cup and is throughout the front and back walls of the oil cup, the width of the rectangular notch is equal to that of the pad printing steel plate, the pad printing steel plate is arranged inside the rectangular notch in a matched mode and seals the ink cavity, and the oil cup is arranged on the pad printing steel plate in a sliding fit mode through the rectangular notch in the oil cup. An ink leaking groove is formed below the pad printing steel plate fixed to the middle of the bottom surface of the inner side of the ink leaking groove, and an ink guiding gap is formed between the outer side face of the pad printing steel plate and the inner wall of the ink leaking groove. A magnet is arranged at the lower end of the oil cup. The oil cup type pad printing machine ink filling structure has the advantages that the ink leaking from the oil cup is guided into the ink leaking groove, in this way, the ink is convenient to clear away or reuse, and the treatment process is clean and environmentally friendly.
Owner:长兴艾飞特科技股份有限公司
Method for preparing mercury-containing catalyst by combining microwave with ultrasonic regenerated coal
InactiveCN109092284AHigh recovery rateSimple processCatalyst regeneration/reactivationProcess efficiency improvementActivated carbonMicrowave
The invention relates to a method for preparing a mercury-containing catalyst by combining microwave with ultrasonic regenerated coal, and belongs to the technical field of recycling of coal preparedwaste mercury catalysts. The method comprises the following steps: uniformly mixing a coal prepared waste mercury catalyst with ammonia water to obtain a mixture A; under ultrasonic conditions, carrying out leaching reaction on the mixture A for 20 to 240 minutes, filtering and drying; carrying out microwave drying on the obtained product under the conditions of 50 to 150 DEG C for 5 to 30 minutes; carrying out microwave heating under the conditions of 150 to 400 DEG C for 5 to 120 minutes to obtain a mercury demercurated waste catalyst and Hg vapor; condensing and recycling the obtained Hg vapor to obtain liquid pure mercury; introducing activated gas and carrying out microwave activation on the obtained mercury demercurated waste catalyst under the conditions of 500 to 800 DEG C for 10 to 100 minutes to obtain activated carbon for a regenerated mercury waste catalyst. The method disclosed by the invention has the advantages of simple overall process flow, low cost of raw materials, capability of achieving the regeneration of the activated carbon and efficient recovery of mercury, economic value and actual production significance.
Owner:KUNMING UNIV OF SCI & TECH
Preparation method of atorvastatin calcium
InactiveCN103012240BLow priceImprove reaction efficiencyOrganic chemistryBenzeneAsymmetric hydrogenation
The invention relates to a preparation method of atorvastatin calcium. The method comprises the steps that 5-methyl--2-benzene-1-(4-fluorophenyl)-3-(phenyl amine formyl)-1,4-hexanedione as a starting material, and subjected to cyclization, hydrolysis, aldol condensation, asymmetric hydrogenation, resolution and salifying reaction, and atorvastatin calcium is obtained. The method is short in synthetic route, rich in raw material source, easy and simple to operate and mild in reaction condition and has a good industrialization prospect.
Owner:BAODING LONGRUI PHARMA TECH
Comprehensive utilization device for inferior coal, biomass and solid waste and working method
InactiveCN104263418AIncrease profitEfficient processGasification processes detailsGranular/pulverulent flues gasificationFlueBiomass
The invention discloses a comprehensive utilization device for inferior coal, biomass and solid waste. The comprehensive utilization device comprises a circulating fluidized bed gasifier, a first cyclone separator, a second cyclone separator, a first discharge pipe, a second discharge pipe, a U-shaped return feeder, an M-shaped return feeder, a slagging pipe, a circulating fluidized bed boiler, a third cyclone separator, a second return pipe and a flue, wherein the upper part of the circulating fluidized bed gasifier is connected with the upper part of the first cyclone separator; the lower part of the first cyclone separator is connected with the top of the M-shaped return feeder; the top of the first cyclone separator is connected with the upper part of the second cyclone separator; the lower part of the second cyclone separator is connected with the lower part of the first discharge pipe; the M-shaped return feeder is connected with the lower part of the circulating fluidized bed gasifier; and the upper part of the circulating fluidized bed boiler is connected with the upper part of the third cyclone separator. The device increases the waste utilization rate. Meanwhile, the invention further discloses a working method of the device, the waste can be efficiently utilized, and the rate of conversion is high.
Owner:SOUTHEAST UNIV
Method for producing high-purity selenium through ultrasonic enhanced reduction
ActiveCN112897475AStrong agitationEnhanced diffusion processElemental selenium/telluriumDistillationSelenium Oxide
The invention relates to a method for producing high-purity selenium through ultrasonic enhanced reduction, and belongs to the technical field of materials. The method comprises the following steps: carrying out cascade inert distillation on selenium oxide to obtain high-purity selenium oxide, dissolving the high-purity selenium oxide in dilute sulfuric acid to obtain a seleninic acid solution, carrying out ultrasonic enhanced reduction reaction on the seleninic acid solution, carrying out solid-liquid separation, and washing and drying the solid to obtain high-purity selenium. According to the method, local stirring is enhanced by utilizing reducibility. H generated by ultrasound and a high-temperature and high-pressure environment, a larger reduction potential is achieved, seleninic acid is reduced to generate high-purity selenium, the production cost of the high-purity selenium is low, the purity of the obtained selenium is high, and operation conditions are friendly.
Owner:KUNMING UNIV OF SCI & TECH
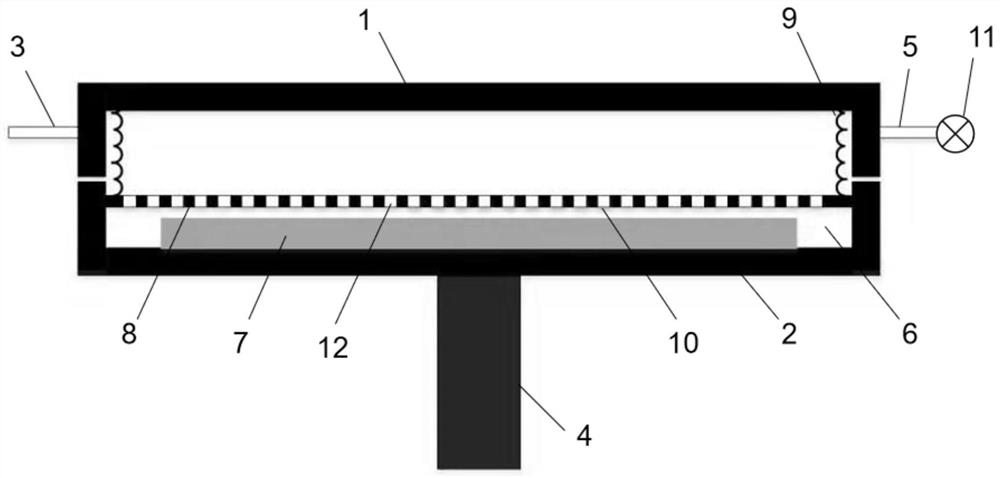
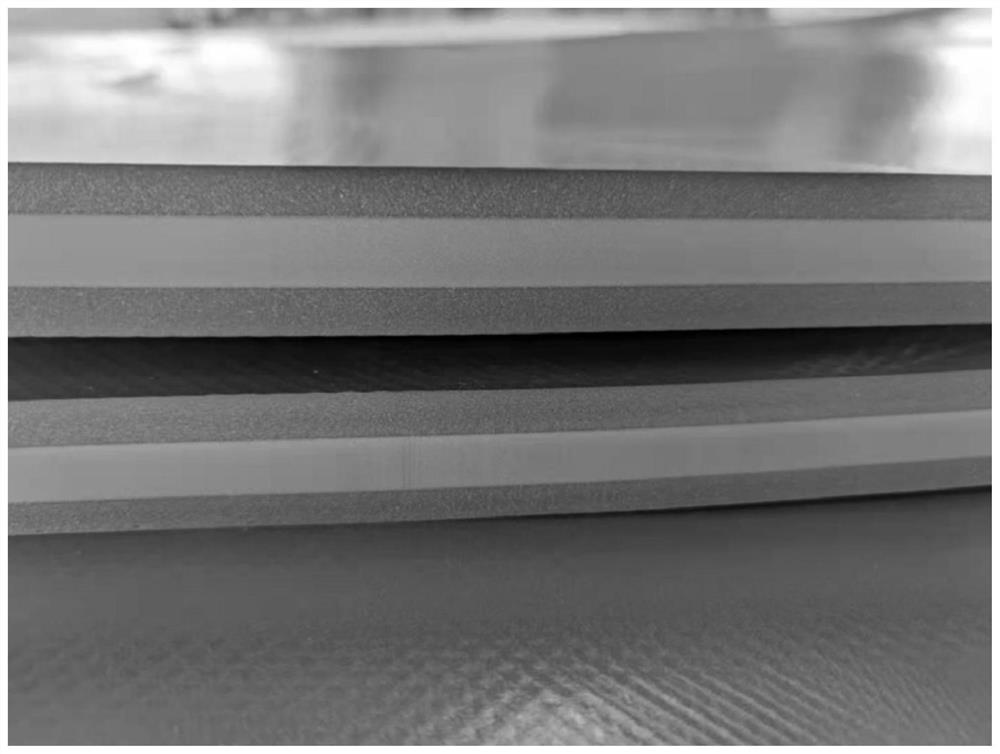
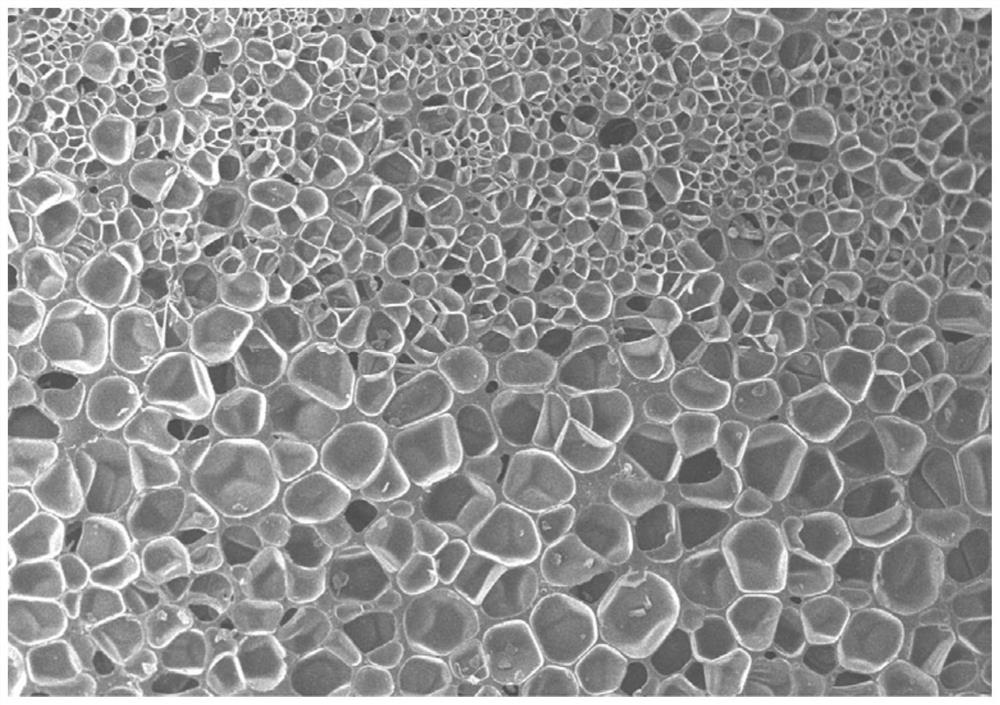
Method for preparing double-color foamed sheet based on temperature control
The invention relates to a method for preparing a double-color foamed board based on temperature control, and belongs to the technical field of foaming. The invention provides a method for preparing a double-color foamed sheet based on temperature control, which comprises the following steps of: putting a foamed mother board into a mold cavity of a foaming mold, raising the temperature in the mold cavity to a first saturation temperature, injecting a foaming agent into the mold cavity, and dissolving the foaming agent into the foamed mother board, and after the foaming agent reaches a saturation state at the first saturation temperature in the foaming mother board, raising the temperature in the mold cavity to a second saturation temperature and keeping the second saturation temperature for a preset length of time, so that the foaming mother board forms a temperature gradient in the thickness direction, and after the temperature gradient is formed, releasing the pressure in the mold cavity to the environmental pressure, so that the foaming mother board expands and foams. According to the method, a temperature gradient is formed in the thickness direction of a foaming mother board by controlling the temperature in a mold cavity, so that the foaming mother board forms a melt strength difference in a foaming environment, and finally chromatic aberration is formed.
Owner:SHINCELL NEW MATERIAL CO LTD
Continuous production system and method for producing low-water-content calcium sulfate from waste sulfuric acid
ActiveCN111362292ALow free moisture contentGuaranteed continuous responseCalcium/strontium/barium sulfatesContinuous reactorCalcium crystals
The invention provides a continuous production system and method for producing low-water-content calcium sulfate from waste sulfuric acid. The continuous production system for producing the low-water-content calcium sulfate from the waste sulfuric acid comprises a limestone slurry supply unit, a waste sulfuric acid supply unit, a quick lime slurry supply unit, a four-stage continuous reactor, a solid-liquid separation unit and a tail gas absorption unit. The reaction process of the waste sulfuric acid, limestone and lime is accurately controlled, and a crystal modifier and a calcium sulfate seed crystal are added to regulate and control the particle size of calcium sulfate particles to obtain large-particle calcium sulfate crystals, so that the free water content of the gypsum product is lower than 10%, and the continuous reaction is ensured by using instrument control means such as an electromagnetic flowmeter, a pH online instrument and the like in the reaction process. The method has the advantages of no waste residue or waste liquid, up-to-standard emission of waste gas, clean and environment-friendly process, and provision of a new approach for resource utilization of waste sulfuric acid.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method and equipment for producing hydrogen through solar photothermal chemical water decomposition
ActiveCN107758614AThe process is clean and environmentally friendlyNo energy consumptionEnergy inputFerroso-ferric oxidesDecompositionMicro nanoparticles
The invention relates to a method and equipment for producing hydrogen through solar photothermal chemical water decomposition. A solar concentrator collector is used as a heat source, and water and carbonyl (CO) or carbonyl metal complexes are used as raw materials; water and CO or one or more carbonyl metal complexes are put into a solar vacuum high temperature heat-absorbing tube (tank), the solar vacuum high temperature heat-absorbing tube (tank) absorbs solar energy concentrated by the solar concentrator collector, the temperature rises to the critical temperature of water, and the pressure also rises to the critical pressure of water along with the temperature rise; and in the solar vacuum high temperature heat-absorbing tube (tank), a reduction reaction is performed on water in thecritical state and CO or the carbonyl metal complexes to produce hydrogen, metal or oxide micronanoparticles thereof, carbon dioxide or carbonate compounds (calcium carbonate, sodium carbonate, ammonium bicarbonate and the like). The method and equipment are low-cost solar hydrogen-producing polygeneration technology and equipment respectively.
Owner:JIANGSU TIANYI ULTRA FINE METAL POWDER
A full-wet recovery process for valuable elements in complex low-grade sulfide ores
Owner:JIANGXI COPPER CORP
Method for deeply extracting vanadium by utilizing vanadium extraction tailings
PendingCN114410988AGuaranteed RecoveryAvoid introducingProcess efficiency improvementProcess equipmentCoke
The invention relates to the field of vanadium metallurgy, in particular to a method for deeply extracting vanadium by utilizing vanadium extraction tailings, which specifically comprises the following steps: grinding the vanadium extraction tailings, adding a carbonaceous reducing agent and a sodium salt additive, reacting at 600-750 DEG C for 1-5 hours, leaching after the reaction is finished, and carrying out solid-liquid separation to obtain a vanadium-containing solution; the carbonaceous reducing agent is at least one of semi-coke or coke, and the sodium salt additive is at least one of sodium vanadate or sodium chloride. According to the method, deep vanadium extraction of the vanadium extraction tailings is achieved at the temperature of 600-750 DEG C, valuable elements in the vanadium extraction tailings are recycled, the obtained leaching residues can be directly used for blast furnace ore blending ironmaking, and the obtained vanadium-containing solution can be used for vanadium crystallization. Meanwhile, process equipment is simple and easy to operate, the whole process flow is in closed cycle, clean and environmentally friendly, and good economic benefits and application prospects are achieved.
Owner:承德钒钛新材料有限公司 +2
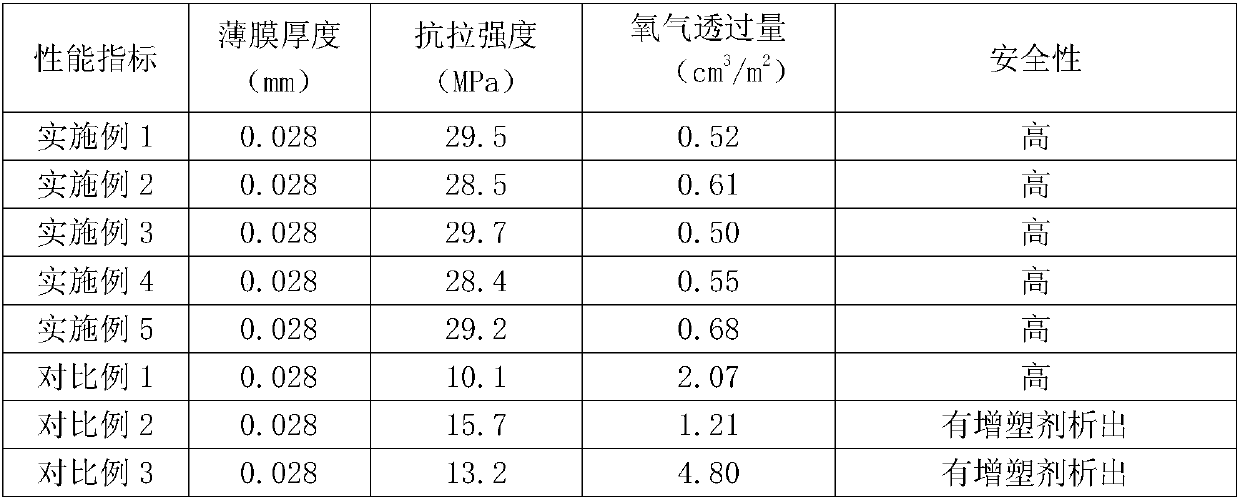
Method for cleanly preparing bioplastic food packaging film at low cost
InactiveCN107903421AGood thermoplastic processabilityImprove tensile propertiesFlat articlesCoatingsHigh energyGlycerol
The invention belongs to the technical field of preparation of packaging films and provides a method for cleanly preparing a bioplastic food packaging film at low cost. The method comprises the following steps: preparing modified cellulose by utilizing hydrogen bonds of ultra ultraviolet radiation high-energy bombardment destroyed cellulose in a vacuum environment, wherein the modified cellulose has a good thermoplastic property; matching polylactic acid, gallic acid and glycerol for calendaring to obtain a prototype film; further melting polyadipic acid-butylene terephthalate and polycarbonate into slurry and coating the surface of the prototype film with the slurry; carrying out rolling, stretching and shaping, thus obtaining a food packaging film. The packaging film prepared by the method has the advantages of stretch resistance, barrier property and good contacting safety with food; in addition, the method has the advantages of simple technological operation in the whole process, high production efficiency, low production cost and capability of realizing batch production.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Method for simultaneously removing various impurity ions in zinc hydrometallurgy waste electrolyte
ActiveCN112030003AImprove removal efficiencyShort processElectrolysis componentsPhotography auxillary processesElectrolytic agentNonferrous metal
The invention relates to a method for simultaneously removing various impurity ions in a zinc hydrometallurgy waste electrolyte, and belongs to the field of nonferrous metallurgy. The zinc hydrometallurgy waste electrolyte is precooled through a heat exchanger, then the precooled solution is added to an impurity removal reaction kettle provided with a stirring device to be subjected to heat exchange and cooled to subzero 15 DEG C-0 DEG C, seed crystals are added in the cooling process for reaction for 20-60 min, impurity components such as lead, calcium, silicon, fluorine, antimony and the like in the solution are precipitated out in the form of a solid crystal product, after the reaction ends, the solid product containing the impurity components is quickly separated, and the electrolyte without impurities is obtained. According to the method, the impurities such as lead, calcium, silicon, fluorine, antimony and the like are simultaneously and directly separated from the waste electrolyte containing high-concentration sulfuric acid on the premise that main components of the zinc hydrometallurgy waste electrolyte are not changed, the electrolyte without the impurities meets the requirement of zinc electrolytic deposition impurity concentration, and the method has the advantages of short technological process, simplicity in operation, low reagent consumption, high impurity ion removal efficiency and the like.
Owner:KUNMING UNIV OF SCI & TECH
Process for separating and recovering zinc magnesium sulfate double salt from sulfate solution and application thereof
ActiveCN111392763AAvoid harmTo achieve the purpose of comprehensive recovery of zinc and magnesium sulfateZinc compoundsFreezing Point TemperatureSulfate zinc
The invention relates to a process for separating and recovering zinc magnesium sulfate double salt from a sulfate solution and an application thereof, which belongs to the technical field of wastewater treatment. The method comprises the following steps: adding a sulfate solution into a precooler, carrying out heat exchange precooling by using a crystallization mother solution, adding into a crystallization reactor, cooling at a cooling rate of 0.2-0.5 DEG C / min in the first stage, stopping cooling when the solution begins to become turbid, and carrying out heat preservation stirring for 15-30 minutes; and in the second stage, cooling at the cooling rate of 0.5-1 DEG C / min, controlling the final temperature to be lower than the temperature in the first stage by 10 DEG C or above and higher than the freezing point temperature of the solution, and performing heat preservation and stirring for 20-60 min. After the reaction is finished, filtering is carried out by adopting a centrifugal filter to obtain a zinc-magnesium sulfate double-salt product, and the crystallized mother liquor obtained by filtering is returned to the precooler to serve as a precooling medium.
Owner:KUNMING UNIV OF SCI & TECH
A kind of preparation method and application of iron oxide, ferrous sulfide and sulfur composite material
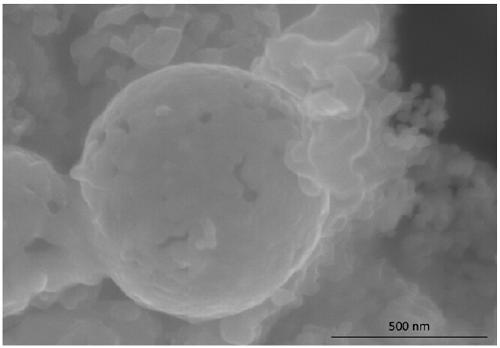
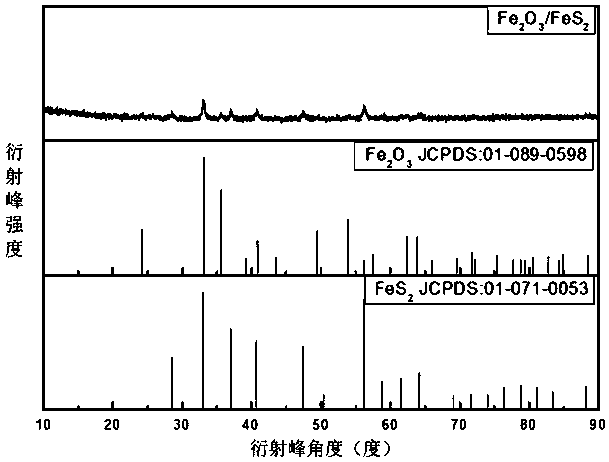
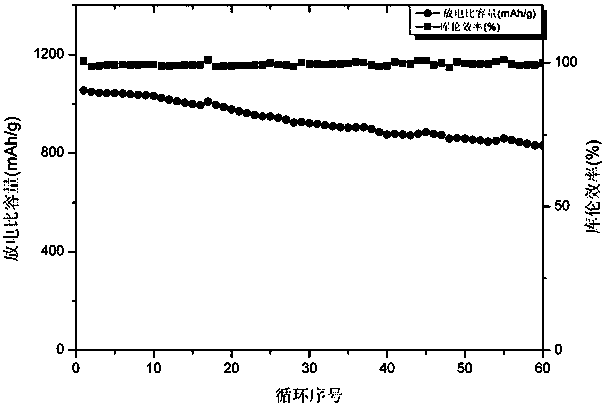
ActiveCN107658459BImprove stabilityImprove adsorption capacityCell electrodesElectrical batteryMetal-organic framework
Belonging to the technical field of energy materials, the invention provides a preparation method and application of an iron oxide, ferrous disulfide and sulfur composite material. The method includesthe steps of: (1) using a solution method to prepare an Fe-metal organic framework material; (2) calcining the dried Fe-metal organic framework material in the air to obtain iron oxide hollow spheres; (3) mixing the iron oxide hollow spheres obtained in step (2) with elemental sulfur, in an inert gas-reducing gas, carrying out certain programmed heating steps to obtain iron oxide and ferrous disulfide materials; and (4) mixing the materials prepared in step (3) with elemental sulfur, performing heating to melting, and then conducting cooling to room temperature, thus obtaining the iron oxide,ferrous disulfide and sulfur composite material. According to the invention, the used raw materials are easily available and low in price, the preparation method is simple, and the process is clean and environment-friendly. And the porous structure of the composite material can buffer the volume change of elemental sulfur in the charging-discharging process, thereby improving the battery performance.
Owner:HARBIN INST OF TECH
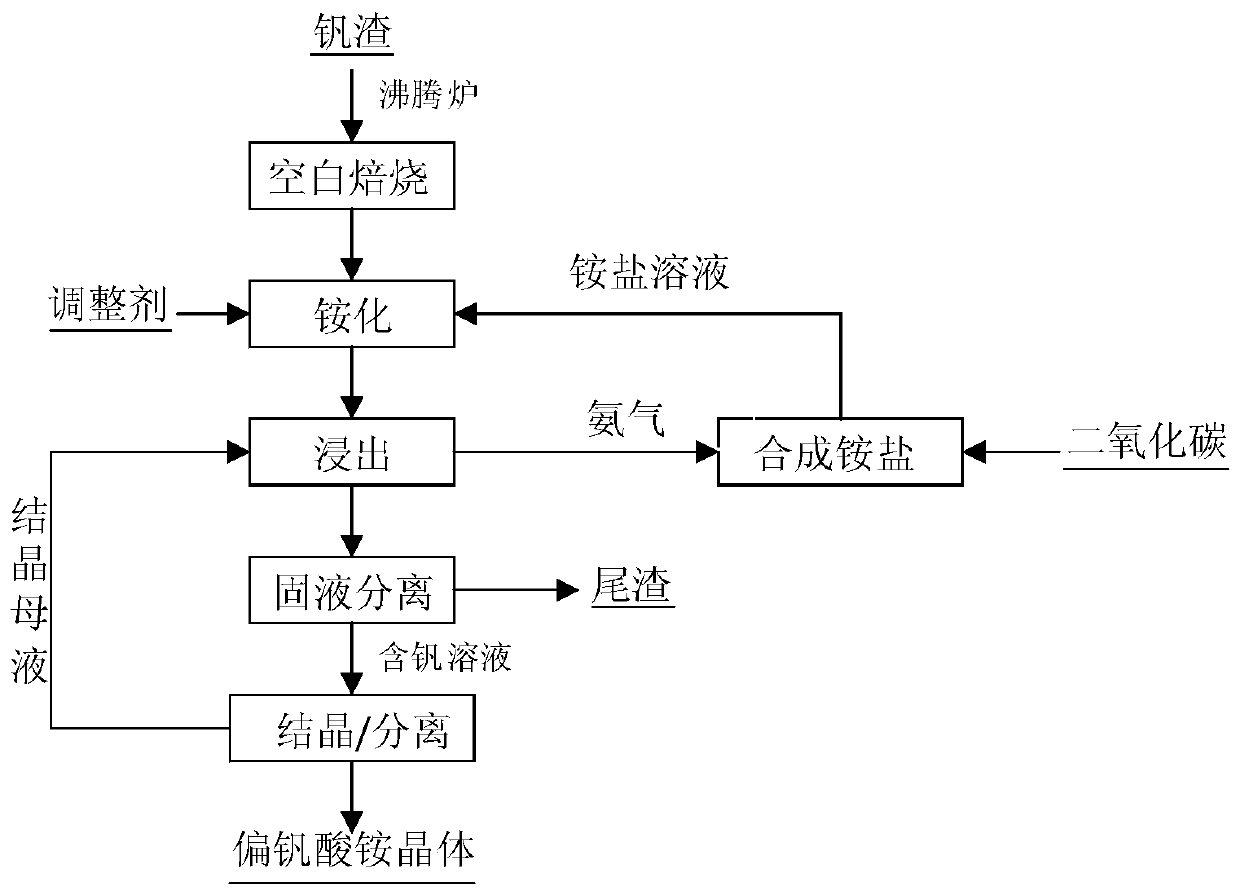
A method for extracting vanadium by ammonification of vanadium slag blank roasting
ActiveCN108149022BImprove heat transfer efficiencyImprove mass transfer effectProcess efficiency improvementWater dischargeSlag
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A continuous production method of copper algae mesoporous activated carbon
ActiveCN108298535BSimple processLow equipment requirementsCarbon compoundsActivated carbonPtru catalyst
The invention discloses a continuous production method of copper algae mesoporous activated carbon. The method uses copper algae as a raw material and adopts a physical activation method to continuously produce activated carbon with well-developed mesopore structure. The activated carbon has the advantages of environmental friendliness and easy industrialization. The activated carbon produced has developed mesopores, narrow pore size distribution, and large specific surface area. It can be widely used in catalysts, supercapacitors, energy storage and other fields, and has a good prospect.
Owner:ZHEJIANG UNIV OF TECH
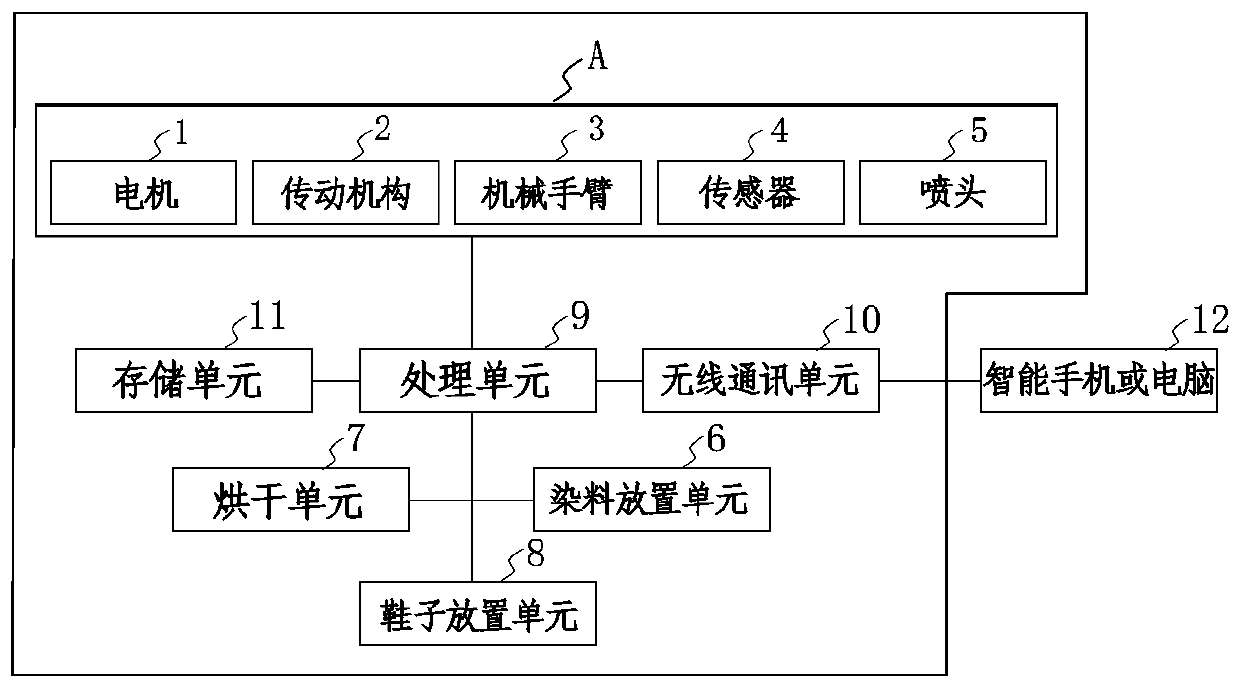
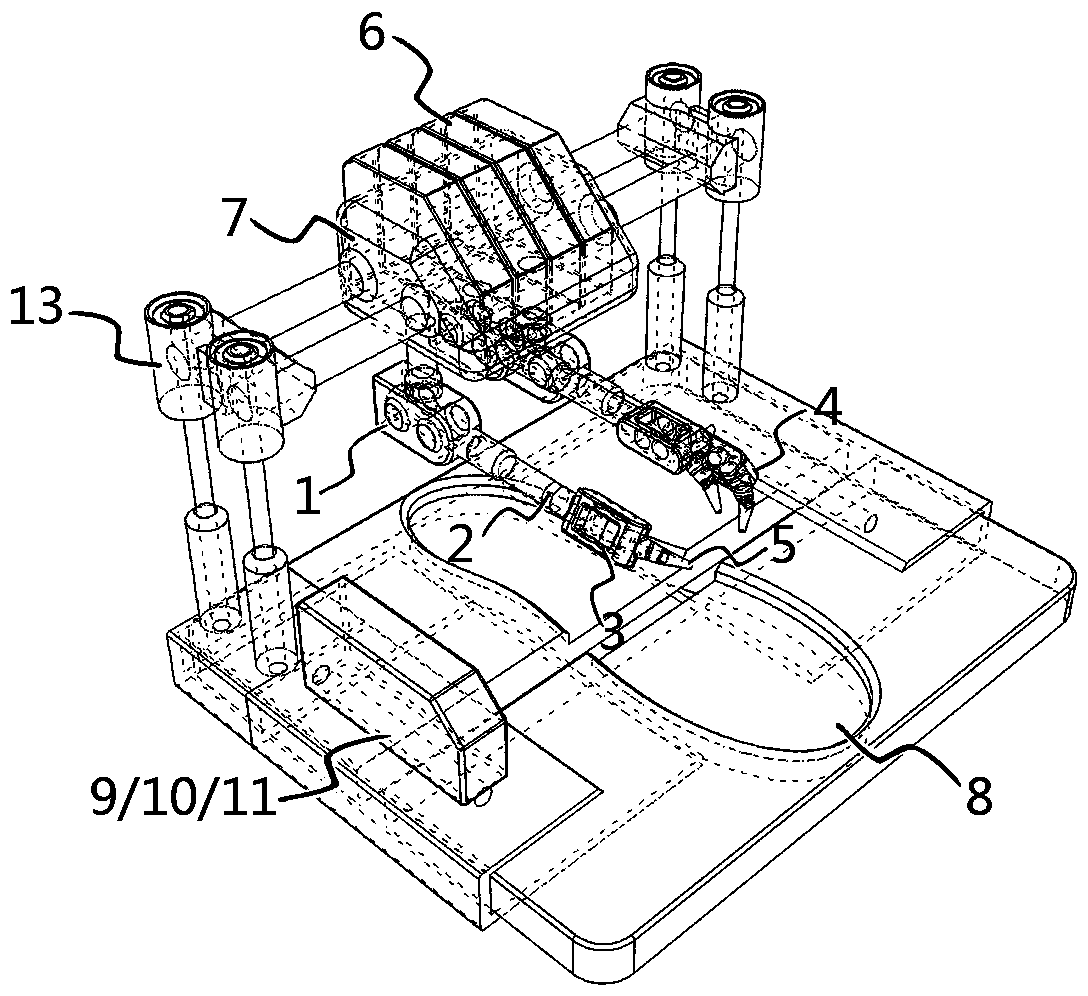
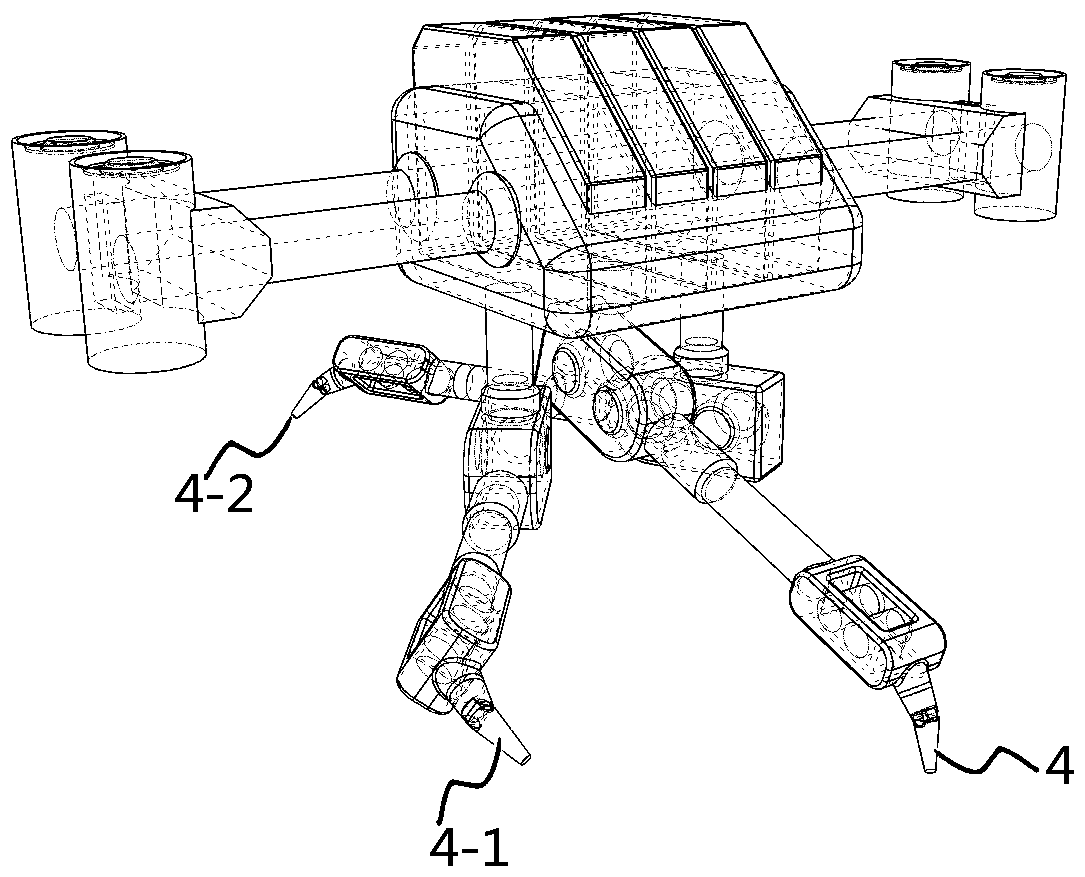
Intelligent 3D shoe customizing machine and working method thereof
InactiveCN107616582ARealize individual needsFast customization speedShoemaking devicesGraphicsPersonalization
The invention discloses an intelligent 3D shoe customizing machine and a working method thereof. A user freely selects all kinds of pictures or graphics drawn by the user, the pictures or graphics canbe rapidly printed on shoes in a sprayed mode, and therefore personalized requirements can be met better for the user. In addition, the user can create or edit favorable patterns through a mobile phone or a computer to achieve the customizing function for the shoe patterns remotely; the patterns can be displayed on the shoes in a three-dimensional mode. The user can user existing shoes or adopt shoes matched with a system for personalized customization, in this way, selective space is larger, and the speed is higher during pattern customization. Then, the intelligent 3D shoe customizing machine is short in customizing time and safe, and clean and environmentally friendly in the whole process, and the user further can watch the whole working process through a vision window, so that the experiencing effect is better.
Owner:DET IND DESIGN
A kind of technology and application of separating and recovering zinc-magnesium sulfate double salt from sulfate solution
ActiveCN111392763BAvoid harmTo achieve the purpose of comprehensive recovery of zinc and magnesium sulfateZinc compoundsSulfate zincFiltration
The invention relates to a process and application for separating and recovering zinc-magnesium sulfate double salt from sulfate solution, and belongs to the technical field of wastewater treatment. First, the sulfate solution is added to the precooler and the crystallization mother liquor is used for heat exchange and precooling, and then added to the crystallization reactor. The first stage is cooled at a cooling rate of 0.2 to 0.5 °C / min, and the cooling is stopped when the solution begins to become turbid. , keep stirring for 15-30min; in the second stage, cool down at a cooling rate of 0.5-1 ℃ / min, control the end point temperature to be more than 10 ℃ lower than the temperature of the first stage, and at the same time be higher than the freezing point temperature of the solution, keep stirring for 20-60min. After the reaction, centrifugal filter was adopted to obtain the zinc-magnesium sulfate double salt product. The crystallization mother liquor obtained by filtration is returned to the precooler as a precooling medium.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com