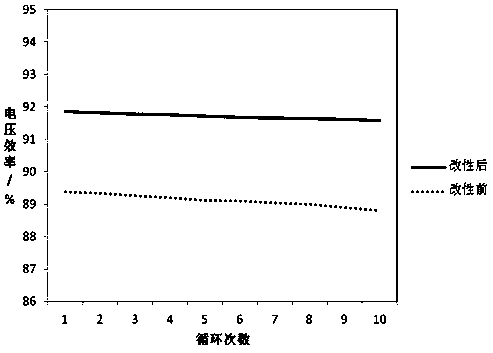
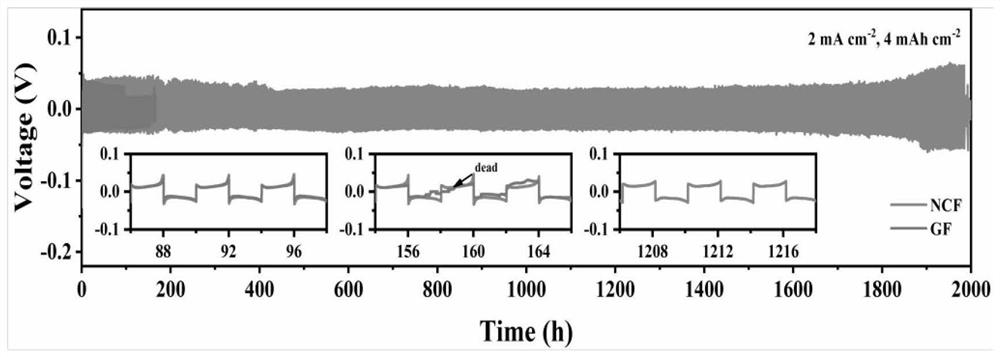
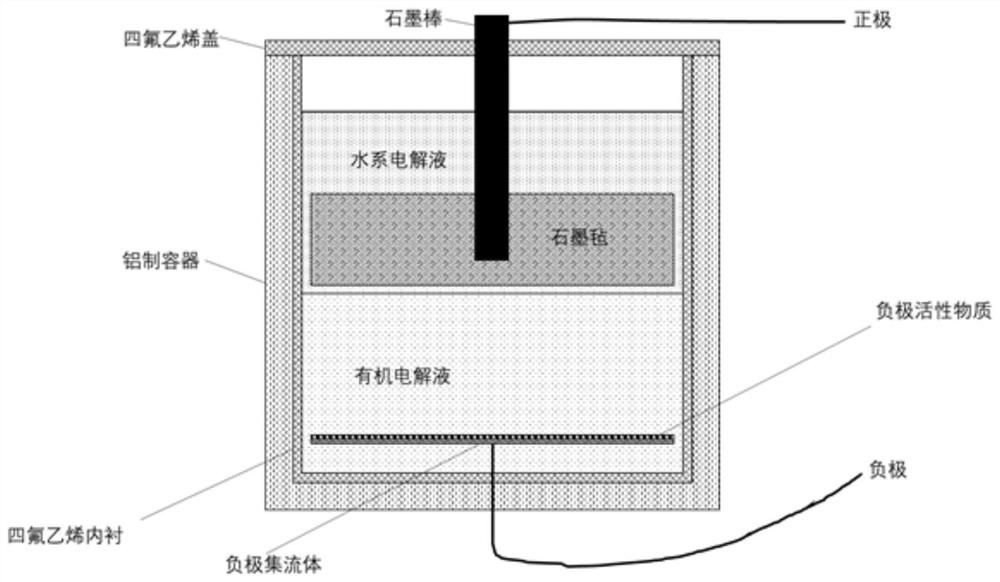
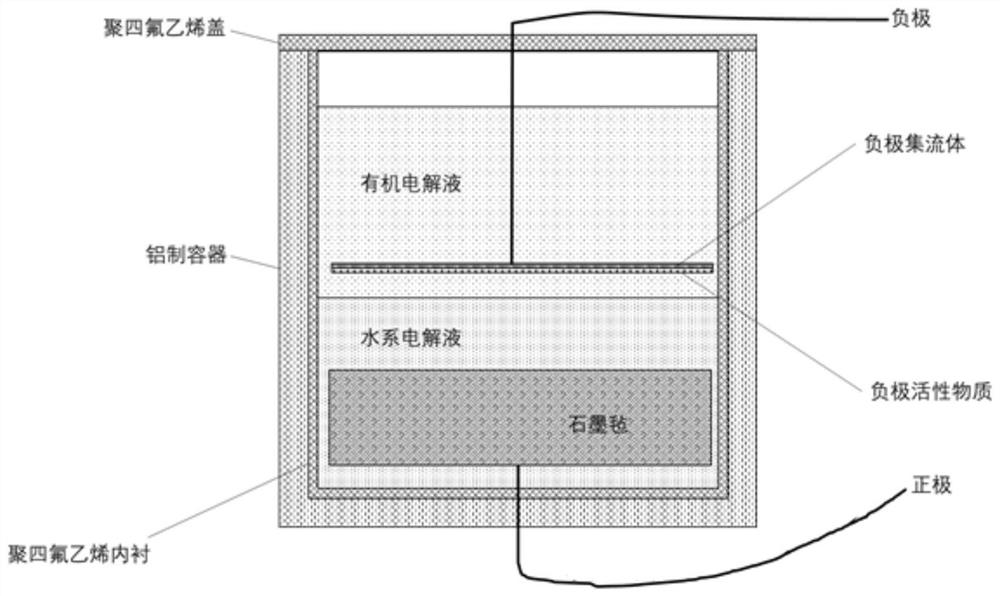
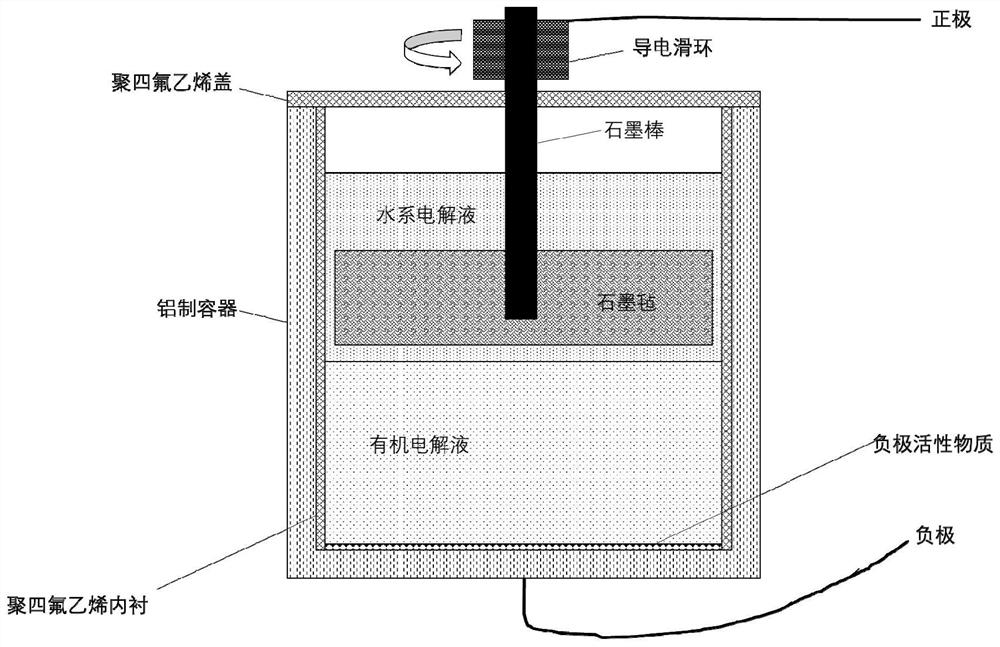
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "Inhibition of hydrogen evolution side reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
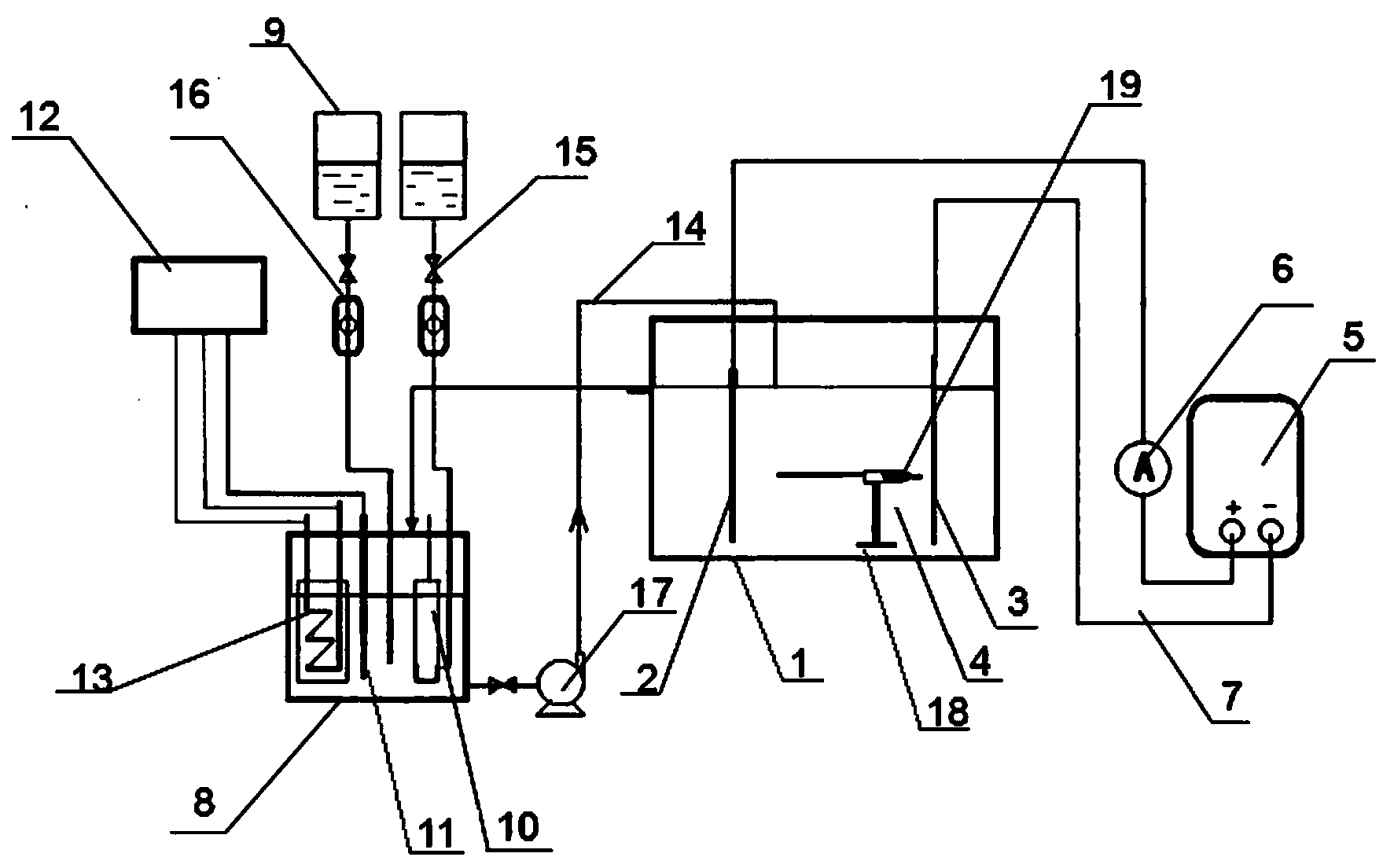
Device and method for synthesizing ammonia through photoelectrocatalysis nitrogen fixation
ActiveCN110079816AInhibition of hydrogen evolution side reactionAvoid hydrogen evolution reactionCellsMetal/metal-oxides/metal-hydroxide catalystsElectrolysisNitrogen gas
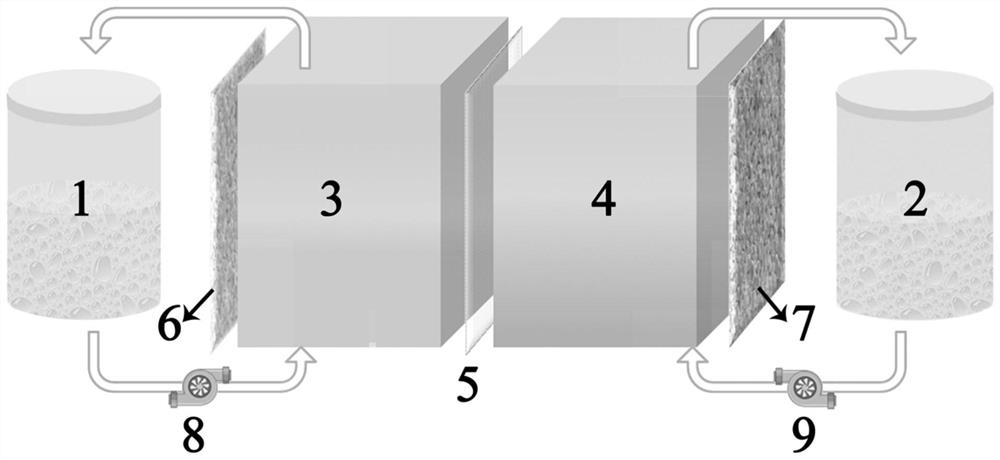
The invention provides a device and a method for synthesizing ammonia through photoelectrocatalysis nitrogen fixation and relates to the technical field of electro-catalysis electrolysis. The device for synthesizing ammonia through photoelectrocatalysis nitrogen fixation comprises a photoelectric composite membrane, wherein the photoelectric composite membrane consists of a bipolar membrane and anano nitrogen fixation catalysis membrane and is used for dividing an anode chamber and a cathode chamber into a double-chamber electrolytic bath; the bipolar membrane comprises an anion exchange membrane and a cation exchange membrane; the nano nitrogen fixation catalysis membrane is carried on the surface of the cation exchange membrane; an electrolyte solution is put into the anode chamber; thecathode chamber is filled with nitrogen; a BiVO4 or Pt electrode is adopted as an anode; a nitrogen fixation catalyst membrane is used as a cathode; a container filled with dilute sulfuric acid is arranged in the cathode chamber and is used for absorbing generated ammonia gas; a xenon lamp is adopted as a light source; an external direct-current power supply is adopted to provide electric field force; a positive pole and a negative pole of the external direct-current power supply are respectively connected with the anode and the cathode. The device is adopted for photoelectrocatalysis nitrogen fixation in a non-water solution, and has benefits that hydrogen evolution side reactions can be effectively inhibited, and the photoelectrocatalysis nitrogen fixation efficiency can be improved.
Owner:TAIYUAN NORMAL UNIV
Carbon nanofiber and metal composite electrode and application thereof
ActiveCN110970628ALarge specific surface areaImprove electrocatalytic activityCell electrodesRegenerative fuel cellsSpinningElectrical battery
The invention discloses a carbon nanofiber / metal composite electrode for a flow battery and a preparation method thereof. The electrode material is prepared by the following steps of: taking a mixtureof a high-molecular polymer and a metal salt as a precursor; preparing nanofibers by adopting an electrostatic spinning method; and performing high-temperature carbonization treatment, and performingsurface oxidation and etching to manufacture the electrode material, wherein the diameter of the carbon nanofibers is 100-1000 nm, the diameter of the metal particles is 2-100 nm, the metal particlesare distributed on the surfaces of the carbon nanofibers, one parts of the metal particles are embedded into the carbon nanofibers, and the other parts of the metal particles are exposed out of the surfaces of the carbon nanofibers. The carbon nanofiber / metal composite electrode prepared by adopting the preparation method has good electrocatalytic activity and electrochemical reversibility when used for the flow battery, and has the advantages of simple preparation method, few procedures, easily available raw materials, low price and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
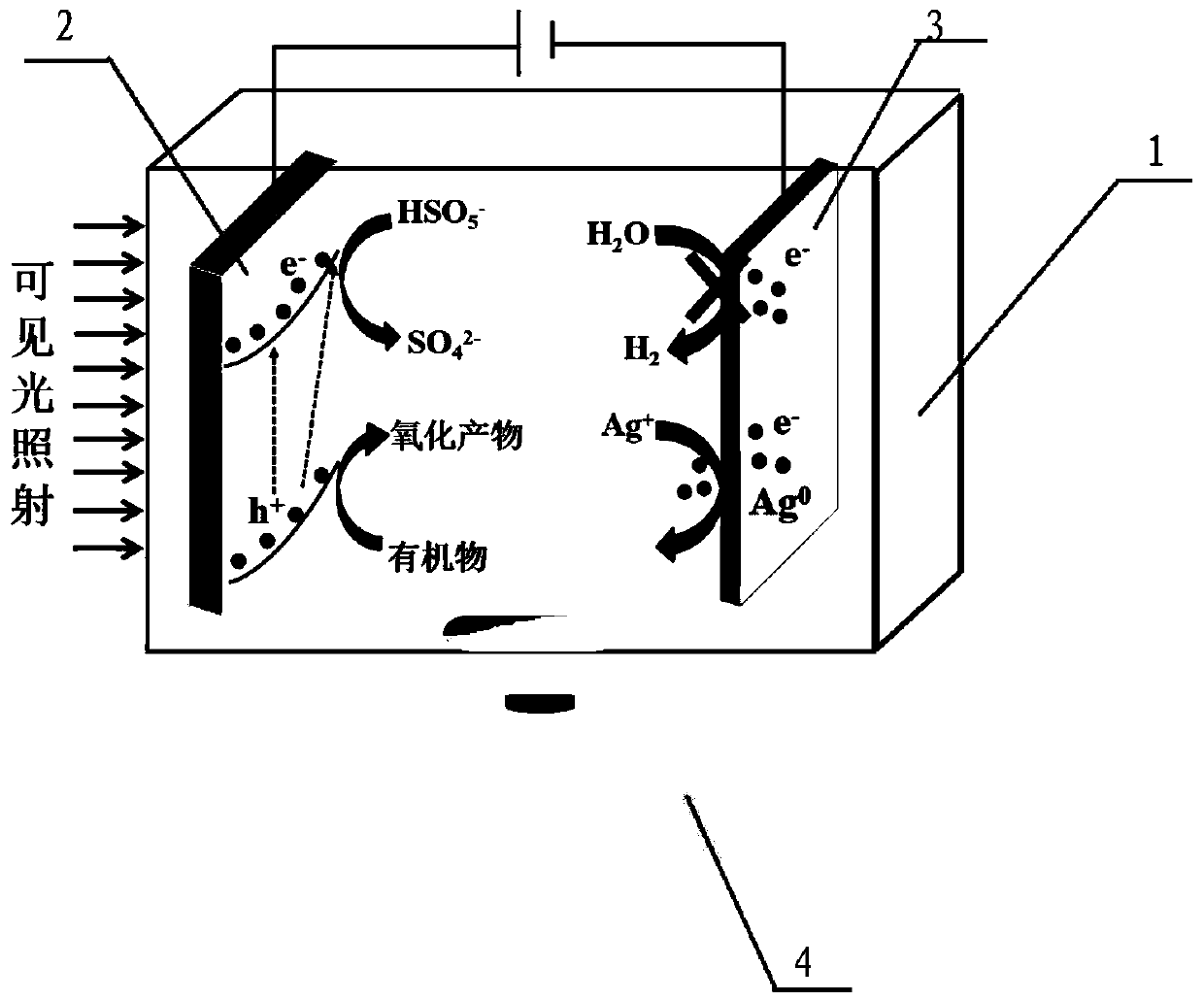
Photoelectrocatalytic system and method for recovering precious metal silver while degrading organic pollutants
InactiveCN110240221AEfficient removalEasy to recycleWater/sewage treatment by irradiationWater contaminantsPersulfateWastewater
The invention discloses a photoelectrocatalytic system and a method for recovering precious metal silver while degrading organic pollutants. The photoelectrocatalytic system comprises a photoelectric reactor, a photo-anode, a cathode, electrolyte and a persulfate solution, wherein the photoelectric reactor is used for containing the electrolyte and the persulfate solution; the photo-anode and the cathode are arranged in the photoelectric reactor; the photo-anode is connected with the cathode by means of an external circuit; the photo-anode is a visible light responsive photocatalytically active semiconductor photoelectrode. The invention also discloses the method for recovering precious metal silver while degrading organic pollutants; wastewater containing silver ions and the organic pollutants is added into the photoelectrocatalytic system, and a photoelectrocatalytic reaction is carried out under the action of visible light. The method provided by the invention can realize efficient removal of the organic pollutants and the recovery of the precious metal silver, the removal rate of the organic pollutants can reach 96.5% or above, and the total recovery rate of the silver ions can reach 94.6% or above.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Aqueous electrolyte and aqueous metal ion battery
ActiveCN110034340AEnhanced electrochemical stability windowStabilizes the electrode/electrolyte interfaceSecondary cellsAqueous electrolytesTriethylamine phosphateAqueous electrolyte
The invention provides an aqueous electrolyte and an aqueous metal ion battery. The aqueous electrolyte provided by the invention comprises a stabilizer, a metal salt and water, wherein the stabilizeris an oxygen-containing non-alcohol organic solvent of C3-C6; the oxygen-containing non-alcohol organic solvent of C3-C6 is selected from acetone and / or triethyl phosphate; and the metal salt is selected from one or more of lithium salt and sodium salt. The aqueous electrolyte is formed by matching the specific stabilizer with water and metal salt, so that high-potential water can be effectivelyinhibited from being oxidized into oxygen to cause an oxygen evolution side reaction, and low-potential water can be effectively inhibited from being oxidized into hydrogen to cause a hydrogen evolution side reaction, and the electrochemical stability window of the aqueous electrolyte is further improved; and the formed aqueous electrolyte has a stable electrode / electrolyte interface, high conductive effect, and relatively high capacity retention rate and coulombic efficiency.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
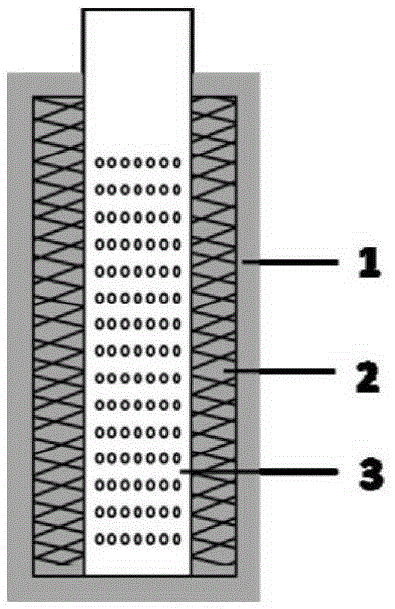
High-uniformity electroplating device for through holes of HDI (high density inverter) printed wiring board
InactiveCN104328465AHigh cathode current efficiencyInhibition of hydrogen evolution side reactionCellsPrinted element electric connection formationSide reactionEngineering
The invention relates to the technical field of through hole processing of HDI (high density inverter) printed wiring boards and particularly relates to a high-uniformity electroplating device for through holes of an HDI (high density inverter) printed wiring board. The device comprises an electroplating bath (1), an anode (2), a cathode (3) and a rectifying power supply (5), wherein the electroplating bath (1) is internally provided with a spraying mechanism (4) which can move in the electroplating bath (1) to directionally spray the surfaces of the through holes of the HDI board at a high speed. The cathode deposition currents on the surfaces of the through holes of the HDI board obtained are uniformly distributed, the copper layers are continuously distributed and are uniform in thickness, compact in organization structure, and good in conductivity, and the plating quality is improved. By using the electroplating device, the cathode current efficiency in the electroplating device is high, the hydrogen evolution side reaction is better inhibited, the efficiency of the electroplating production process is improved and the influence of hydrogen evolution on the quality of the copper layers is reduced, thereby facilitating reduction of the production cost.
Owner:ZHEJIANG ZHENYOU ELECTRONICS CO LTD
Method for coating copper on surface of iron powder
InactiveCN102051606AImprove microstructureImprove surface topographyLiquid/solution decomposition chemical coatingCopper platingThiourea
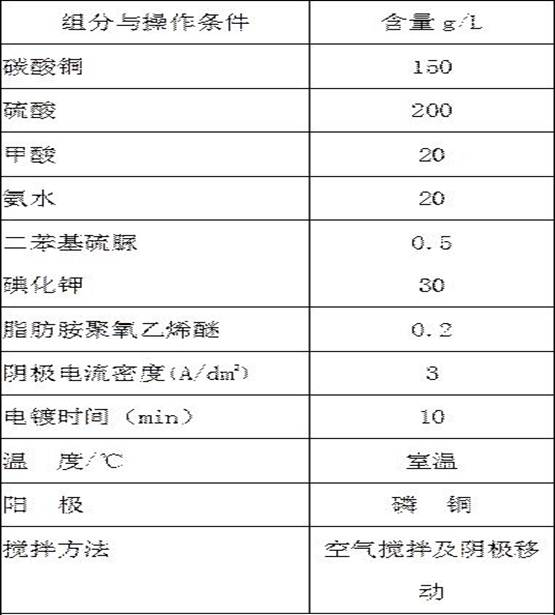
The invention provides a method for coating copper on the surface of iron powder. The method comprise the following steps: under the conditions of room temperature and stirring, taking the iron powder as a core, coating an even, compact and high-bond strength copper layer on the surface of the iron powder by a chemical displacement coppering method, and controlling the thickness of the coppering layer through regulating the composition, acid degree, stirring rate and plating time of a solution. The displacement coppering solution comprises the following components: 7-20g / L of copper phytic acid, 20-80g / L of sulphuric acid (98%) and 3.0-5.0g / L of compound addictive; the plating process parameters are as follows: the temperature is the room temperature, the stirring speed is 30-500rpm, and the time is 10-120 minutes; and the compound addictive comprises the following components by weight: 5% of thiourea, 5% of alylthiourea, 28% of benzotriazole, 12% of 8-oxyquinoline, 15% of sulfosalicylic acid, 1% of po1yoxyethylene octylphenol ether (OP) emulsifier, 2% of sodium dodecyl benzene sulfonate, 8% of saccharin and 24% of polyethylene.
Owner:SHANDONG UNIV
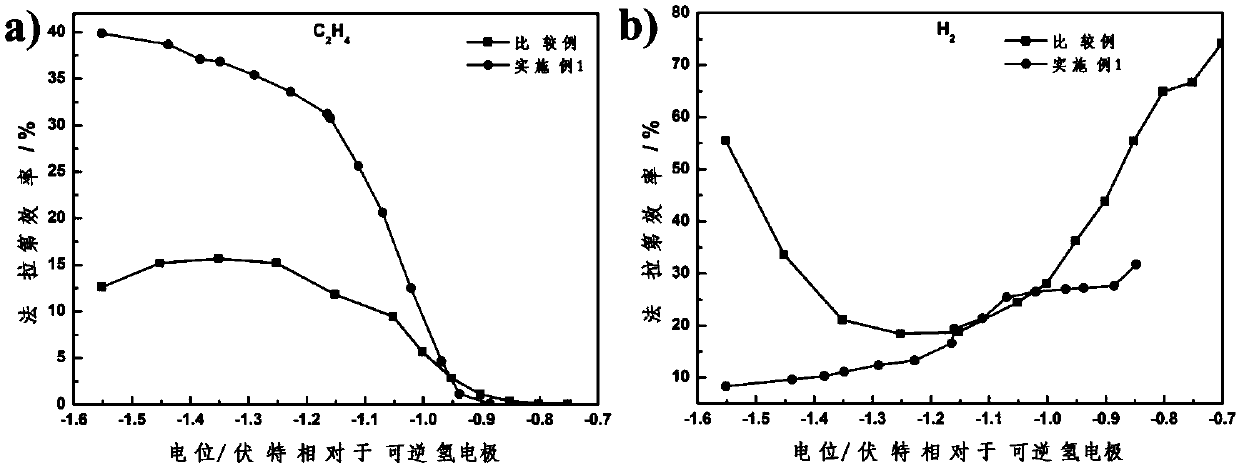
Electrode for CO2 electrochemical reduction and preparation and application thereof
ActiveCN110938846AImprove surface roughnessIncrease the electrochemical reaction areaElectrolytic organic productionElectrodesHydrogenation reactionElectroplating
The invention relates to an electrode for CO2 electrochemical reduction and preparation and application of the electrode for CO2 electrochemical reduction. The preparation process of the electrode includes the steps of soaking a substrate material into an electroplating liquid containing main salt and additives after performing impurity removal on the substrate material, and performing electrochemical deposition under inert atmosphere protection and stirring conditions to obtain the electrode with micrometer Cu particles growing on the surface. The proportion of side atoms of the Cu particlesdeposited on the substrate surface of the prepared electrode is much higher than that of corner atoms, which is beneficial to improving the coverage of CO* on the surface of the electrode and providesa favorable environment for a subsequent CO* dimerization reaction and a hydrogenation reaction; and the electrode has a significant effect of inhibiting the side reaction of hydrogen evolution and meanwhile has high C2H4 selectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Negative electrode of lead-carbon battery and preparation and application thereof
InactiveCN109841833ALow costLower internal resistanceLead-acid accumulatorsLead-acid accumulator electrodesFiberBattery charge
The invention relates to a negative electrode of a lead-carbon battery. The negative electrode contains 0.1-50wt% of modified carbon fibers, and the modified carbon fibers are obtained by activating and then poisoning carbon fibers. The cost of the lead-carbon battery is reduced, the internal resistance of the battery is reduced by a conductive network structure in a negative plate, the sulfationof the battery is effectively inhibited, the water loss during the cycle life process of the lead-carbon battery is reduced, the hydrogen evolution overpotential is improved, the high-rate partial state of charge (HRPSOC) of the lead-carbon battery in some nuclear power states is extended, and the battery charge acceptance ability and low temperature start performance are enhanced.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Application of additive in negative electrode electrolyte of alkaline zinc-nickel flow battery
InactiveCN111244516AImprove charging capacityImprove Coulombic efficiencyRegenerative fuel cellsElectrolytic agentElectrical battery
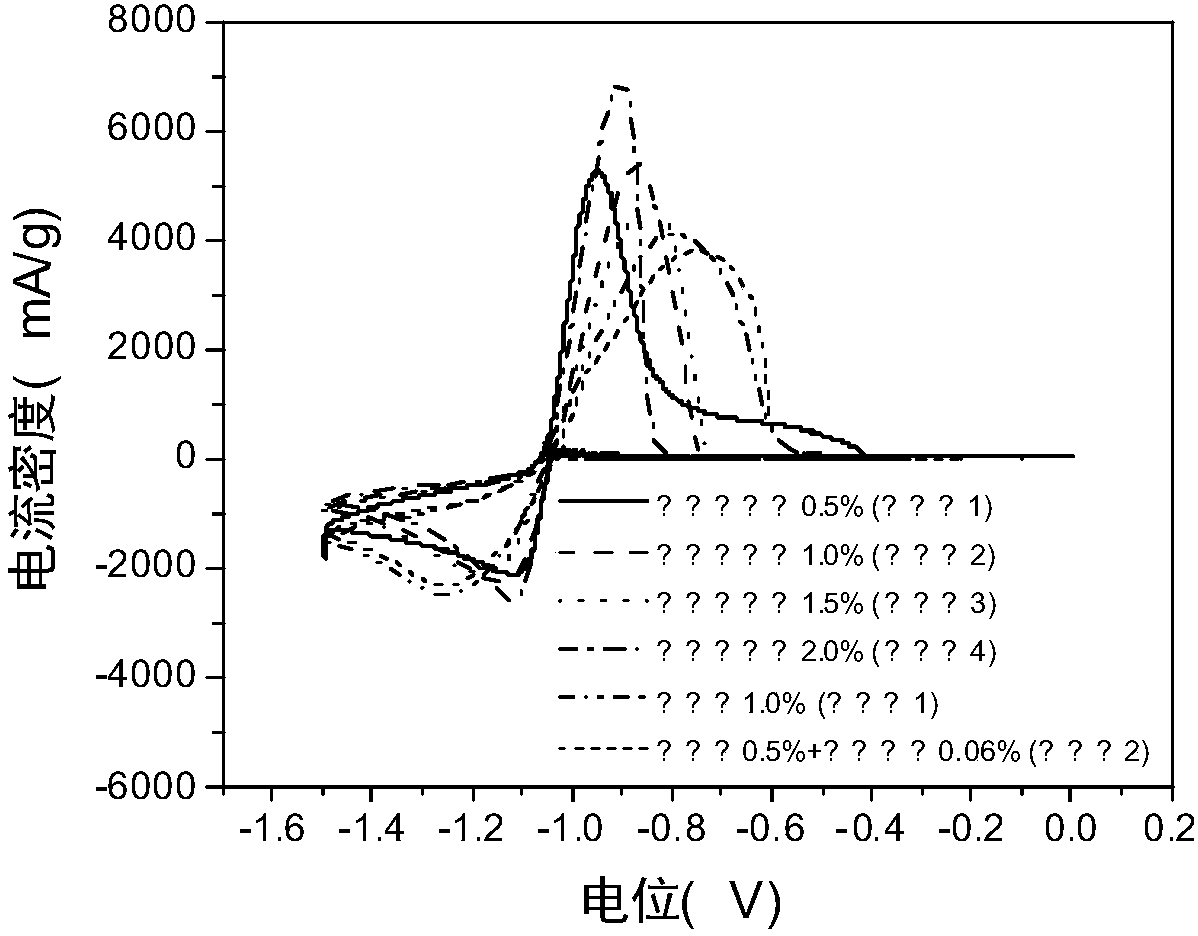
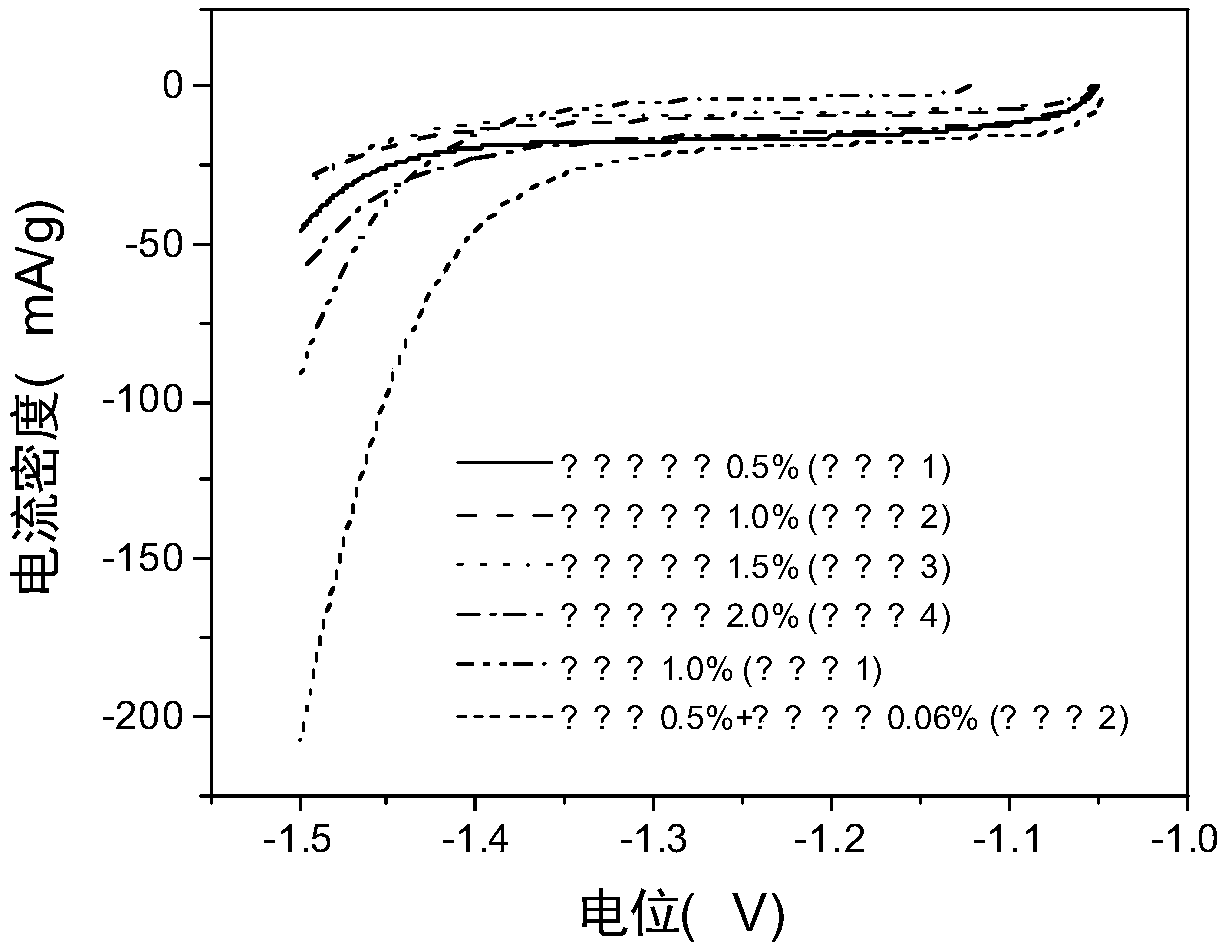
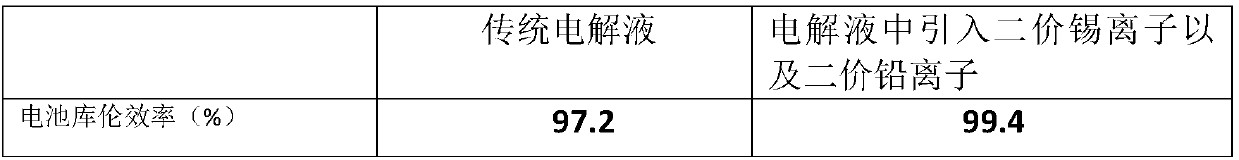
The invention relates to application of a metal ion additive which is stable in an alkaline solution and has high hydrogen evolution overpotential in a zinc-nickel flow battery, which belongs to the field of flow batteries. The additive is one or more of divalent soluble lead salt and divalent soluble tin salt. The additive is stable in property in an alkaline solution and has high hydrogen evolution overpotential, and the deposition potential is higher than that of zinc ions. In the battery charging process, the negative electrode generates a small amount of hydrogen evolution side reaction,battery performance is influenced, after the additive is added, in the initial stage of charging, divalent lead ions and divalent tin ions in a solution can preferentially obtain electrons to become elemental lead to be deposited on a negative electrode, hydrogen evolution side reaction during zinc deposition is inhibited due to high hydrogen evolution overpotential of the elemental lead and the elemental tin, and when the two ions are introduced together, the effect is optimal, so that the coulombic efficiency of the battery is improved by the method.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Polypyrrole/nano-copper composite gas diffusion electrode and preparation and application thereof
InactiveCN105316702AImprove Faraday efficiencyImprove conversion rateElectrolytic organic productionElectrodesPolypyrroleFaraday efficiency
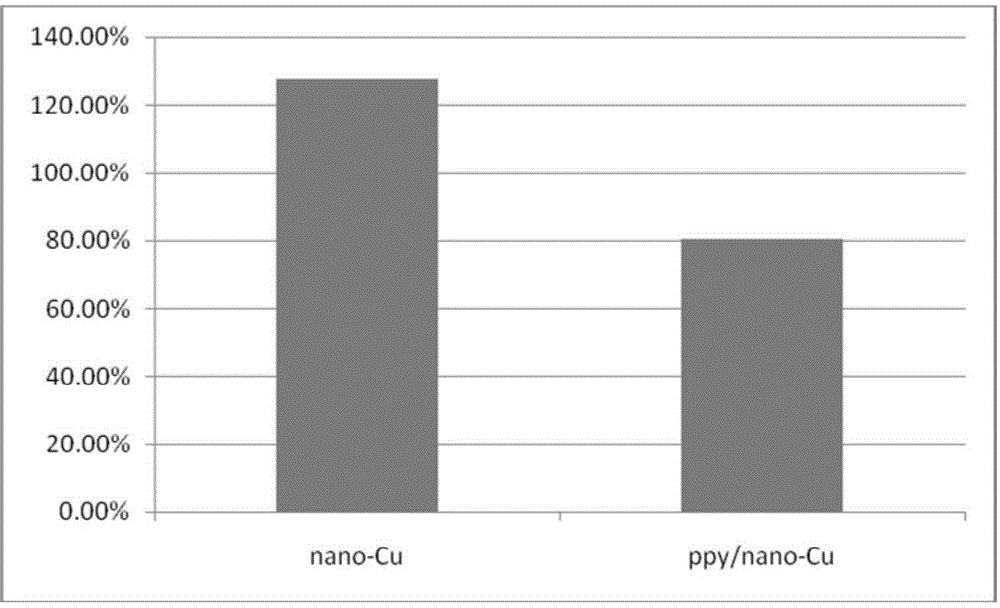
The invention relates to a polypyrrole / nano-copper composite gas diffusion electrode and preparation and an application thereof. Carbon paper or carbon cloth serves as a substrate, a polypyrrole layer with the thickness being 0.05-1.0 [mu]m is attached to the surface of the substrate, and a nano-copper material layer with the thickness being 0.1-1.0 [mu]m is deposited on the polypyrrole layer. Polypyrrole is aggregated and nano-copper is deposited on the carbon-based electrode, the hydrogen evolution side reaction is reduced, and the Faradic efficiency of products is improved and the conversion rate of carbon dioxide is increased.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Production method for modified vanadium battery porous electrode graphite felt
InactiveCN110518260ASteady productionImprove electrochemical activityCell electrodesElectrochemical responseCarbonization
The invention relates to a production method for a modified vanadium battery porous electrode graphite felt, which comprises the following steps: a pre-oxidized felt sequentially enters a low-temperature carbonization zone and a high-temperature graphitization zone of a continuous sintering furnace to be sintered, and then enters a continuous activation furnace to be activated; before the graphitefelt is subjected to activating treatment, a catalyst bismuth nitrate powder is uniformly scattered on the surface of the graphite felt, so that one-catalyst and two-step modification effect can be realized. That is, the added bismuth nitrate not only can oxidize the surface of the felt body to form a nanoscale microporous structure and increase the specific surface area, but also the bismuth nitrate can be decomposed in the activation process, and the decomposed product is deposited on the surface of the carbon felt to further modify the surface of the carbon felt. The production method hasthe advantages that the electrochemical reaction activity can be improved, the side reaction of the electrode can be inhibited, and the service life of the battery is prolonged. The cost is saved in industrial production, and the method is simple, easy to operate and high in environmental protection property.
Owner:LIAONING JINGU CARBON MATERIALS CO LTD
Method for preparing metal rhodium or rhodium alloy by electrodeposition or chemical deposition
InactiveCN109097800AAvoid displacement reactionsImprove bindingPhotography auxillary processesLiquid/solution decomposition chemical coatingPorosityAlloy
The invention provides a method for preparing metal rhodium or rhodium alloy by electrodeposition or chemical deposition. The method comprises an electrodeposition method and a chemical deposition method, adopts a weakly acidic plating solution or a basic plating solution and comprises the following steps for preparing metal rhodium or rhodium alloy: (1) preparing a complex solution of rhodium according to composition and method described in the patent CN105906668B; (2) preparing a complex solution of dissimilar metal ions; (3) preparing a mixed solution containing a reducing agent and an additive; (4) proportionally mixing the solutions obtained in steps (1), (2) and (3) and regulating pH with ammonium hydroxide and an acid solution; (5) preparing a rhodium or rhodium alloy clad layer with the electrodeposition method or the chemical deposition method. A weakly acidic or basic solution system is adopted in the method, a replacement reaction in a strongly acid plating solution is avoided, binding force of the clad layer is good, the problems of low current efficiency and high porosity caused by massive hydrogen evolution of the strongly acid system are solved, and by means of adoption of an improved complexing agent and introduction of the additive, stability of the basic plating solution is improved.
Owner:HARBIN INST OF TECH
Method for preparing 1,3-butadiene through electro-catalytic acetylene coupling
ActiveCN112342562AEasy to separateRelieve great pressureCellsElectrolytic organic productionPtru catalystFaraday efficiency
The invention relates to a method for preparing 1,3-butadiene by electrocatalytic acetylene coupling, and a gas diffusion electrode electrolytic tank is adopted. A gas diffusion electrode is adopted,a catalyst is sprayed on a gas diffusion layer substrate (including conductive carbon paper and metal) to prepare the gas diffusion electrode, and a cathode and an anode are isolated by an ion exchange membrane. A three-electrode or two-electrode system constant-voltage method is adopted to carry out electrochemical performance test, wherein the reaction gas is high-purity acetylene. Experimentalresults show that the Faraday efficiency of the target product 1,3-butadiene reaches 70% or more by regulating and controlling a proper voltage range, and byproducts ethylene and hydrogen are easy toseparate from 1,3-butadiene. Compared with a traditional thermocatalysis technology, the method has the advantages that the production cost of 1,3-butadiene can be effectively reduced, the huge pressure brought by the coal-rich lean oil energy resource structure in China is relieved, the technical requirements of green chemical engineering are met, and the method has great strategic significance.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Method for preparing glass fiber separator for lead carbon battery with double functions of in-situ pore enhancement and negative electrode hydrogen evolution suppression
ActiveCN108666500ASmall self-dischargeImprove oxygen recombination efficiencyLead-acid accumulatorsCell component detailsGlass fiberElectricity
The invention discloses a method for preparing a glass fiber separator for a lead carbon battery with double functions of in-situ pore enhancement and negative electrode hydrogen evolution suppression. The method comprises the following steps of: first preparing glass fiber separator slurry; then adding an additive with double functions of in-situ pore enhancement and negative electrode hydrogen evolution suppression thereto; using dilute sulphuric acid to dilute and adjust a pH value of the slurry; filtering and molding the slurry to obtain a semi-finished product of the glass fiber separator; and drying, trimming, slicing the semi-finished product or the like to prepare the glass fiber separator for the lead carbon battery with double functions of in-situ pore enhancement and negative electrode hydrogen evolution suppression. According to the method for preparing the glass fiber separator for the lead carbon battery, particles of the additive that are acid-soluble and have the doublefunctions of in-situ pore enhancement and negative electrode hydrogen evolution suppression are added and evenly distributed on the glass fiber separator. The glass fiber separator is used between positive and negative electrodes of the lead carbon battery. When acid is added during manufacturing of the lead carbon battery, the double-function micro-scale additive particles in the separator are dissolved and released ions. The released ions are directionally adsorbed, electroreduced, and deposited on a surface of a carbon material during a charging process. Therefore, the negative electrode hydrogen evolution overpotential is improved, the side reaction of the hydrogen evolution is suppressed, and the self-discharge during storage of the battery is reduced.
Owner:ZHEJIANG UNIV OF TECH
Electroplating solution for direct cyanide-free copper plating of steel matrix under strong acidic condition and preparation method of electroplating solution
ActiveCN112899738AImprove adsorption capacityInhibition of displacement reaction rateCopper platingCyanide
The invention discloses an electroplating solution for direct cyanide-free copper plating of a steel matrix under the strong acidic condition. The electroplating solution comprises the following components: 50-250 g / L of main salt, 30-100 g / L of a compound adsorbent, 1-5 g / L of an additive and 160-300 g / L of a strong acid composition. The effect that 1 + 1 is larger than 2 is generated through the synergistic effect of the compound adsorbent, the additive and the strong acid composition, the speed of the iron-copper replacement reaction under the strong acidic condition is effectively restrained, the steel matrix forms a simple substance copper layer with firm binding force at the moment of making contact with an acid plating solution, the content of sulfuric acid is 150-250 g / L, the technical problem that the acidity is higher, a plating layer is looser is solved, and the plating layer is still firm when reaching 300 [mu]m.
Owner:山东夸克电化学科技有限公司
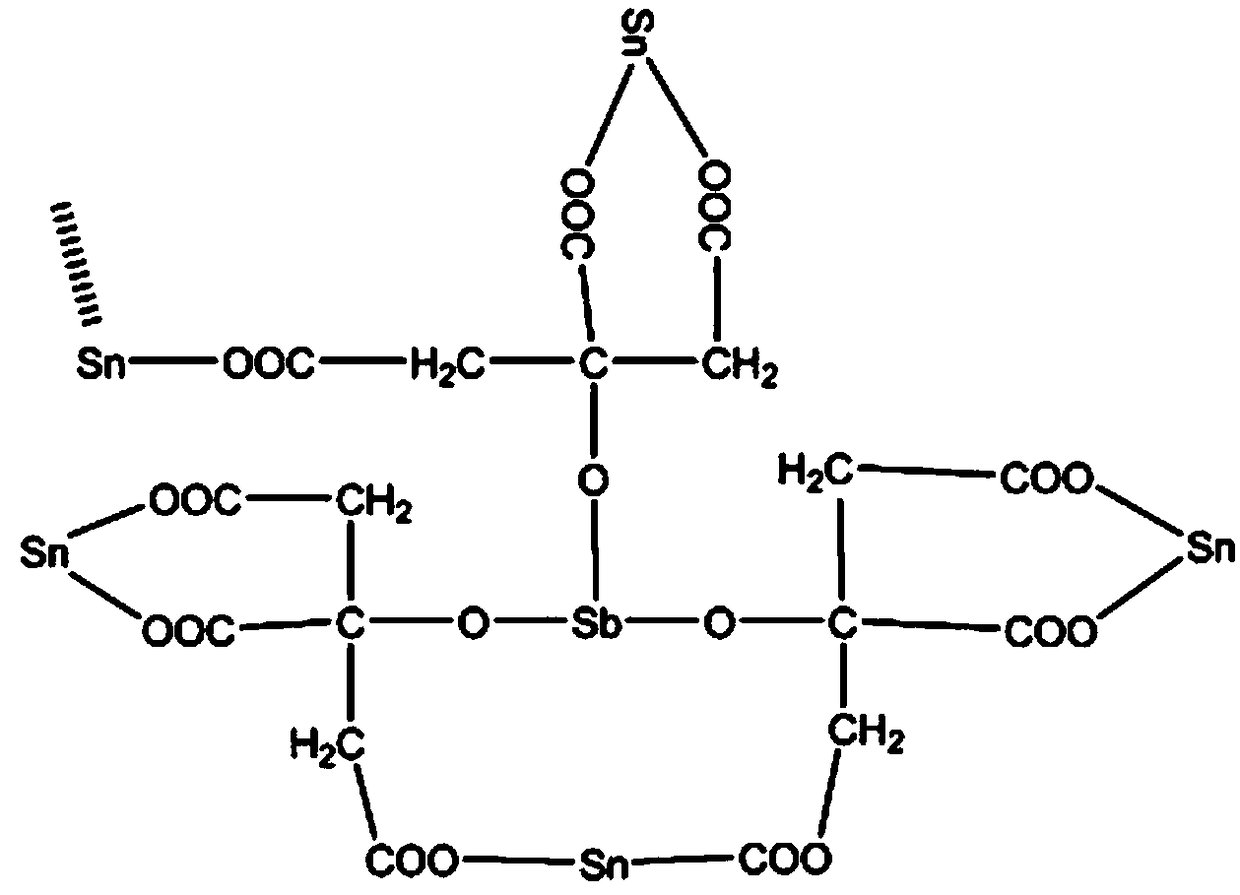
Method for preparing Ti/SnO2-Sb (titanium/tin dioxide-antimony) electrode by tricarboxylic organic acid-Sn(II)/Sb(III) complex
ActiveCN109052574AEfficient and stable performanceAvoid deposition potential effectsWater contaminantsWater/sewage treatment by oxidationTin dioxideAntimony trichloride
The invention relates to a preparation technology of an electrochemical oxidation electrode material, and aims at providing a method for preparing a Ti / SnO2-Sb (titanium / tin dioxide-antimony) electrode by a tricarboxylic organic acid-Sn(II) / Sb(III) complex. The method comprises the following steps of using the pretreated Ti sheet as a working electrode, arranging counter electrodes at both sides in parallel, and respectively soaking the working electrode and the counter electrodes into an electrodeposition solution, wherein the electrodeposition solution is a mixed water solution of stannous dichloride, antimony trichloride, tricarboxylic organic acid and gelatin; connecting a positive pole of a direct current power source and the counter electrodes, and connecting a negative pole and theworking electrode; after the electrodeposition is completed, drying the working electrode at room temperature without cleaning; after drying, warming for 1 to 3h at the temperature of 450 to 650 DEG C, and cooling to room temperature, so as to obtain the electrode product. The preparation technology has the advantages that the operation is simple, the environment-friendly effect is realized, and the cost is low; the electrode surface is uniform and complete, and the covering degree is good; the antimony-doped tin dioxide is uniformly and densely distributed at the electrode surface, the specific surface area is high, and more active sites are provided in the electrochemical oxidation process; the service life of accelerated test of the electrode product is long, and the stability is good.
Owner:ZHEJIANG UNIV
Porous electrode complex for carbon dioxide electrochemical reduction as well as preparation and application thereof
ActiveCN106521544AImprove conversion rateExtended stayElectrode shape/formsReduction rateReaction rate
The invention provides a porous electrode complex structure for carbon dioxide electrochemical reduction and a preparing method thereof. The porous electrode complex structure comprises a gas diffusion base layer and a porous metal electrode layer which are laminated with each other and further comprises two hollow annular edge frames with the same shape and size, wherein the circumferential edges of the two annular edge frames are glued through an adhesive, and the gas diffusion base layer and the porous metal electrode layer are located between the two annular edge frames in the mutually laminated manner, forming a porous electrode complex. The porous electrode complex not only can utilize a porous gas diffusion base to rapidly transmit gas towards an electrode catalysis station and discharge liquid, but also can utilize metal electrode longitudinal holes to increase the area of an electrode reaction interface, so that the carbon dioxide electrochemical reduction rate is increased. The porous electrode complex structure is particularly suitable for a carbon dioxide electrochemical reactor in which reacting gas vertically flows across the electrode reaction interface.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Recyclable and charge-modified mesoporous nanocellulose paper-based zinc ion battery diaphragm
PendingCN114696034APromote circulationReduce corrosionSecondary cellsCell component detailsElectrical batteryEngineering
The invention provides a recyclable charge-modified mesoporous nanocellulose paper-based zinc ion battery diaphragm, which is prepared by the following steps: treating nanocellulose by adopting a simple process, weighing a certain mass of seaweed nanocellulose powder, putting the seaweed nanocellulose powder into water, performing ultrasonic dispersion to obtain a uniform cellulose suspension, performing suction filtration, and drying to obtain the charge-modified mesoporous nanocellulose paper-based zinc ion battery diaphragm. The original cellulose diaphragm is obtained; the seaweed nanocellulose diaphragm has a very small pore size, different substances are used for charge modification on the basis, and compared with a negative charge diaphragm, the obtained positive charge diaphragm is more favorable for improving the cycle performance of the battery; the preparation process is simple, the material source is rich, no pollution is caused to the environment, the battery performance can be greatly improved, compared with an existing common diaphragm, the diaphragm is lighter and thinner and has better mechanical strength, and more space and capacity are reserved for a positive electrode and a negative electrode; according to the invention, the unique effect of the surface charge in the aqueous battery is explored, and the stability and the rate characteristic of the zinc ion battery are greatly improved.
Owner:HUNAN UNIV
Double-electrolyte secondary battery
PendingCN113346147AIncrease profitIncrease speedCell electrodesFinal product manufactureElectrolytic agentAqueous electrolyte
The invention belongs to the field of secondary batteries, and particularly relates to a double-electrolyte secondary battery. The secondary battery comprises a positive current collector, a positive active material, an aqueous electrolyte, an organic electrolyte, a negative active material and a negative current collector; the positive active material is dissolved in the aqueous electrolyte, and the positive current collector is infiltrated in the aqueous electrolyte in which the positive electrode active material is dissolved; the negative active material is attached to the negative current collector and is soaked in the organic electrolyte; and the aqueous electrolyte and the organic electrolyte are in direct contact and are not dissolved with each other. The positive active material exists in the liquid in the form of solute, adverse effects caused by deterioration of a crystal structure of a solid do not need to be considered in the charging and discharging process, and the ion diffusion rate and the conductivity in the liquid are far higher than those of the solid, so that the utilization rate of the positive active material is greatly improved compared with that of a traditional battery.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for coating copper on surface of iron powder
InactiveCN102051606BImprove microstructureImprove surface topographyLiquid/solution decomposition chemical coatingCopper platingThiourea
The invention provides a method for coating copper on the surface of iron powder. The method comprise the following steps: under the conditions of room temperature and stirring, taking the iron powder as a core, coating an even, compact and high-bond strength copper layer on the surface of the iron powder by a chemical displacement coppering method, and controlling the thickness of the coppering layer through regulating the composition, acid degree, stirring rate and plating time of a solution. The displacement coppering solution comprises the following components: 7-20g / L of copper phytic acid, 20-80g / L of sulphuric acid (98%) and 3.0-5.0g / L of compound addictive; the plating process parameters are as follows: the temperature is the room temperature, the stirring speed is 30-500rpm, and the time is 10-120 minutes; and the compound addictive comprises the following components by weight: 5% of thiourea, 5% of alylthiourea, 28% of benzotriazole, 12% of 8-oxyquinoline, 15% of sulfosalicylic acid, 1% of po1yoxyethylene octylphenol ether (OP) emulsifier, 2% of sodium dodecyl benzene sulfonate, 8% of saccharin and 24% of polyethylene.
Owner:SHANDONG UNIV
Device and method for photoelectrocatalytic nitrogen fixation and synthesis of ammonia
ActiveCN110079816BInhibition of hydrogen evolution side reactionAvoid hydrogen evolution reactionCellsMetal/metal-oxides/metal-hydroxide catalystsPtru catalystNitrogen gas
The present invention provides a device and method for photoelectric catalytic nitrogen fixation and synthesis of ammonia, which relate to the technical field of electrocatalysis and electrolysis. A photoelectric catalytic nitrogen fixation and synthesis of ammonia device comprises a photoelectric composite film consisting of a bipolar film and a nanometer nitrogen-fixing catalytic film. The anode chamber and The cathode chamber is divided into a double-chamber electrolytic cell, the bipolar membrane includes an anion exchange membrane and a cation exchange membrane. As the anode, the nitrogen-fixing catalyst film is used as the cathode, and the cathode chamber is equipped with a container filled with dilute sulfuric acid to absorb the generated ammonia gas. A xenon lamp is used as the light source, and a DC power supply is added to provide the electric field force. The positive and negative electrodes of the DC power supply are respectively connected to the anode and Cathode connection; the present invention performs photocatalytic nitrogen fixation in a non-aqueous solution, and has the beneficial effects of effectively inhibiting the occurrence of hydrogen evolution side reactions and improving the efficiency of photocatalytic nitrogen fixation.
Owner:TAIYUAN NORMAL UNIV
A kind of carbon nanofiber and metal composite electrode and its application
ActiveCN110970628BLarge specific surface areaImprove electrocatalytic activityCell electrodesRegenerative fuel cellsFiberCarbon fibers
The invention discloses a nano-carbon fiber / metal composite electrode for a liquid flow battery and a preparation method thereof. The electrode material is made of a mixture of high molecular polymer and metal salt as a precursor, and is made into nanofibers by electrospinning, then carbonized at high temperature, and then surface oxidation and etching. The diameter of carbon nanofibers is 100-1000nm , the diameter of the metal particles is 2-100nm, the metal particles are distributed on the surface of the carbon nanofibers, one part of the metal particles is embedded in the carbon nanofibers, and the other part is exposed on the surface of the carbon nanofibers. The nano-carbon fiber / metal composite electrode prepared by the preparation method has good electrocatalytic activity and electrochemical reversibility when used in a liquid flow battery, and has the advantages of simple preparation method, less process, readily available raw materials, and low price.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Method for preparing glycolic acid by electrocatalytic reduction of CO2 in heteropolyacid ionic liquid-indium dual-catalytic system
ActiveCN112144073ASolve the scarcitySolve the problem of quantity shortageOrganic-compounds/hydrides/coordination-complexes catalystsElectrolytic organic productionElectrolytic agentAcetic acid
The invention relates to a method for preparing glycolic acid by electrocatalytic reduction of CO2 in a heteropolyacid ionic liquid indium dual-catalytic system. The invention belongs to the field ofpreparation of glycolic acid by electrocatalytic reduction of CO2. The invention aims to solve the technical problems that the existing CO2 reduction reaction is generally 2e-, 4e- transfer reaction,the deep reduction of CO2 cannot be realized, and the ionic liquid preparation process of the existing reaction system is complex. The method comprises the following steps: preparing polyoxometallateionic liquid (n-Bu4N) 3SVW11O40, dissolving the polyoxometallate ionic liquid (n-Bu4N) 3SVW11O40 in acetonitrile to obtain a catholyte, and carrying out electrocatalytic reduction on carbon dioxide byusing dilute sulfuric acid as an anolyte to prepare liquid-phase products ethanol and acetic acid which are greater than or equal to C1. The method provided by the invention is simple, effectively solves the problems that the existing polyacid ionic liquid is rare in variety and short in quantity, is mild in reaction condition and low in economic cost, and can improve the energy efficiency to 4-5times, and the Faraday efficiency of the obtained ethanol is 7-16%, and the Faraday efficiency of the obtained acetic acid is 14-67%.
Owner:HARBIN INST OF TECH
Metal zinc negative electrode protected by chemical passivation layer as well as preparation method and application of metal zinc negative electrode
PendingCN114824151AInhibition of hydrogen evolution side reactionEasy to depositElectrode manufacturing processesActive material electrodesTitanateVanadate
The invention discloses a metal zinc negative electrode protected by a chemical passivation layer. The chemical passivation layer is generated on the surface of the zinc metal negative electrode in situ through a chemical passivation method. The active component of the passivation solution used in the chemical passivation method is one or more of vanadate, silicate and titanate, and the solvent is water. The invention further discloses a corresponding preparation method. Therefore, the inventor also develops a corresponding aqueous zinc ion battery, and the passivation layer is uniformly distributed, so that water and oxygen can be effectively isolated, the aqueous zinc ion battery has an anti-corrosion characteristic, and hydrogen evolution side reaction of a zinc negative electrode in charge-discharge circulation is inhibited; meanwhile, the zinc negative electrode can induce uniform deposition of zinc and inhibit growth of dendritic crystals, the cycle performance of the zinc negative electrode is remarkably improved, and the cycle stability of the zinc negative electrode is improved. In short, the zinc metal negative electrode is simple in production process and low in raw material cost, and the obtained battery product is good in effect and suitable for large-scale production.
Owner:GUANGXI UNIV
Negative electrode electrolyte for zinc-iron flow battery
PendingCN114551954AReduce generationInhibit growthRegenerative fuel cellsOrganic electrolytesZinc bromideSupporting electrolyte
The invention is applied to the field of zinc-iron flow batteries, and particularly relates to a negative electrode electrolyte for a zinc-iron flow battery. An electrolyte in a negative electrode electrolyte of the zinc-iron flow battery comprises a zinc salt, an additive and a supporting electrolyte, the zinc salt is one or two of zinc chloride, zinc bromide, zinc sulfate and zinc iodide, the additive is nicotinamide, and the supporting electrolyte is potassium ions or sodium ions. According to the invention, by selecting the additive and utilizing the complexing structure of divalent zinc ions and the additive and the adsorption effect of a zinc deposition layer and the additive, the growth of zinc dendrites and the generation of byproducts are inhibited, the zinc deposition morphology is improved, the zinc deposition is compact and uniform, the performance of the battery is improved, and the cycle life of the battery is prolonged. The invention has the advantages of outstanding performance, safety, environmental protection, low price and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
A photocatalytic reduction of co 2 Method and application of pickering microbubble system for preparing methanol
ActiveCN111701586BIncrease contact areaSolve the problem of difficult accessOrganic compound preparationHydroxy compound preparationPhoto catalysisMethanol
The invention belongs to photocatalytic CO 2 Reduction field, specifically related to a photocatalytic reduction of CO 2 The construction method and application of the Pickering microbubble system for the preparation of methanol. The Pickering microbubble system is composed of an amphiphilic Pickering solid photocatalyst as an emulsifier, which is spontaneously assembled at the air-water interface to form a water-in-CO 2 microbubble system. Using this system for photocatalytic CO 2 For the reduction reaction, the reduction efficiency can be increased by 10-80% compared with the traditional gas-liquid two-phase reaction; the selectivity of the reduction product methanol can be increased by 20-80%.
Owner:SHANXI UNIV
A kind of carbon dioxide electrochemical reduction catalyst and its preparation and application
InactiveCN105680061BIncrease contact areaInhibition of hydrogen evolution side reactionCell electrodesSTANNOUS OXIDEChloride
Owner:DONGHUA UNIV
Construction method and application of Pickering microbubble system for photocatalytic nitrogen fixation ammonia synthesis
ActiveCN111704146AIncrease contact areaSolve the problem of difficult accessOrganic-compounds/hydrides/coordination-complexes catalystsMetal/metal-oxides/metal-hydroxide catalystsPhoto catalysisNitrogen fixation
The invention belongs to the field of photocatalytic nitrogen fixation synthesis ammonia, and particularly relates to a construction method and application of a Pickering microbubble system for photocatalytic nitrogen fixation ammonia synthesis. The Pickering microbubble system disclosed by the invention is an N2-in-water microbubble system formed by taking an amphiphilic Pickering solid photocatalyst as an emulsifier and spontaneously assembling the amphiphilic Pickering solid photocatalyst on a gas-water interface. When the system is used for photocatalytic nitrogen fixation ammonia synthesis reaction, the nitrogen fixation efficiency can be improved by 5-40% in comparison with that of the traditional gas-liquid two-phase reaction, and the selectivity of ammonia can be improved by 10-50%.
Owner:SHANXI UNIV
Construction method and application of Pickering microbubble system for preparing methanol through photocatalytic reduction of CO2
ActiveCN111701586AIncrease contact areaSolve the problem of difficult accessOrganic compound preparationHydroxy compound preparationPhoto catalyticMethanol
The invention belongs to the field of photocatalytic CO2 reduction, and particularly relates to a construction method and application of a Pickering microbubble system for preparing methanol through photocatalytic reduction of CO2. The Pickering microbubble system is a CO2-in-water microbubble system, which is formed by taking an amphiphilic Pickering solid photocatalyst as an emulsifying agent and spontaneously assembling the amphiphilic Pickering solid photocatalyst on a gas-water interface. When the system is used for photocatalytic CO2 reduction reaction, the reduction efficiency can be improved by 10-80% in comparison with that of the traditional gas-liquid two-phase reaction, and the selectivity of the reduction product methanol can be improved by 20-80%.
Owner:SHANXI UNIV
a co 2 Electrode for electrochemical reduction and its preparation and application
ActiveCN110938846BImprove surface roughnessIncrease the electrochemical reaction areaElectrolytic organic productionElectrodesHydrogenation reactionSide reaction
The present invention relates to a CO 2 Electrode for electrochemical reduction and its preparation and application. The electrode is made by soaking the base material in the electroplating solution containing the main salt and additives after removing impurities, and performing electrochemical deposition under the protection of an inert atmosphere and stirring conditions to obtain surface growth. Electrodes with micron Cu particles; the proportion of side atoms of Cu particles deposited on the surface of the prepared electrode substrate is much higher than that of corner atoms, which is conducive to improving the coverage of CO* on the electrode surface, and providing support for the subsequent CO* dimerization reaction and The hydrogenation reaction provides a favorable environment, which has the effect of significantly inhibiting the side reaction of hydrogen evolution, and at the same time has a high C 2 h 4 selective.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com