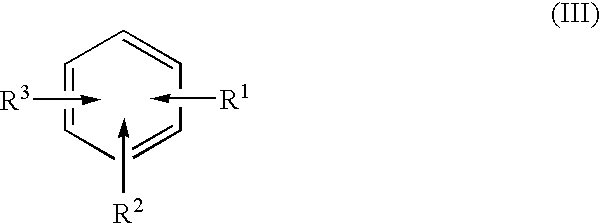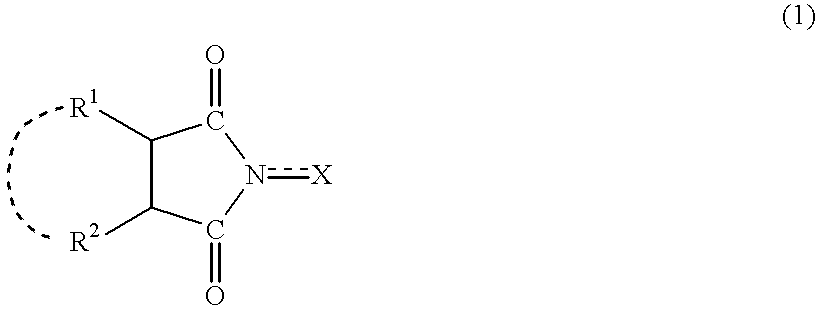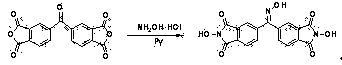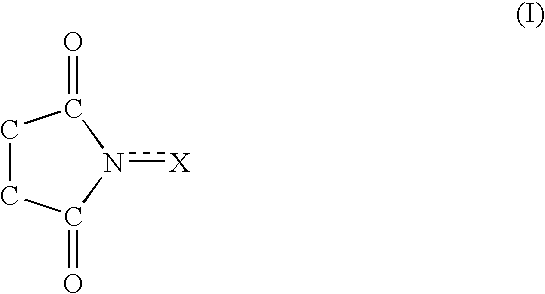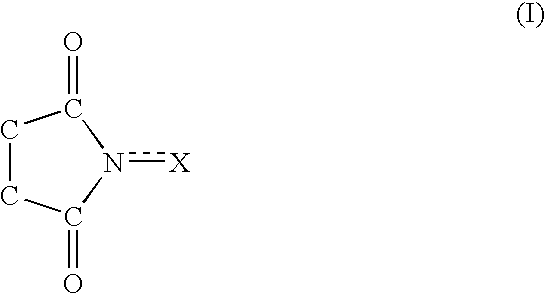Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "N-hydroxyphthalimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
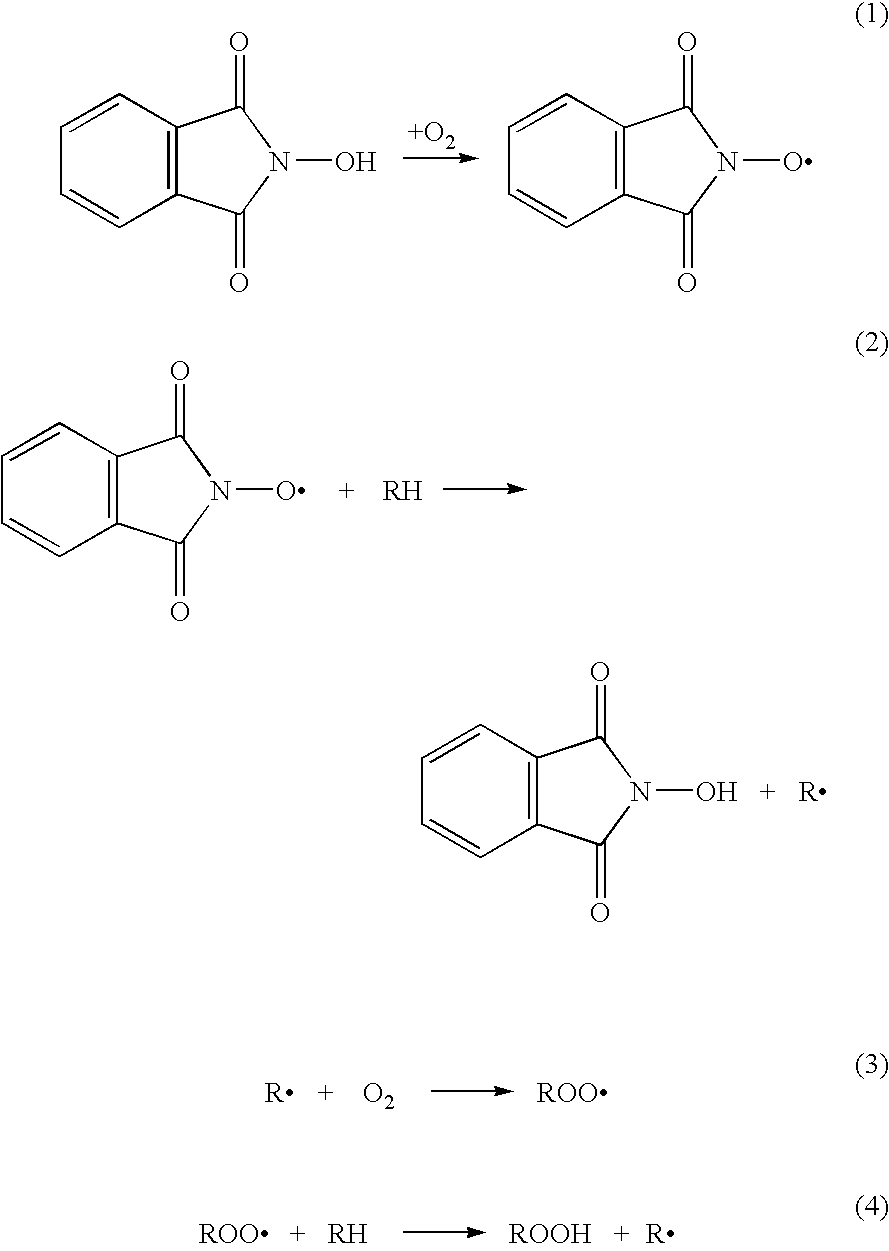
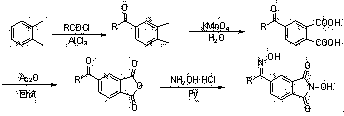
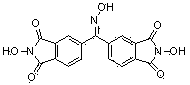
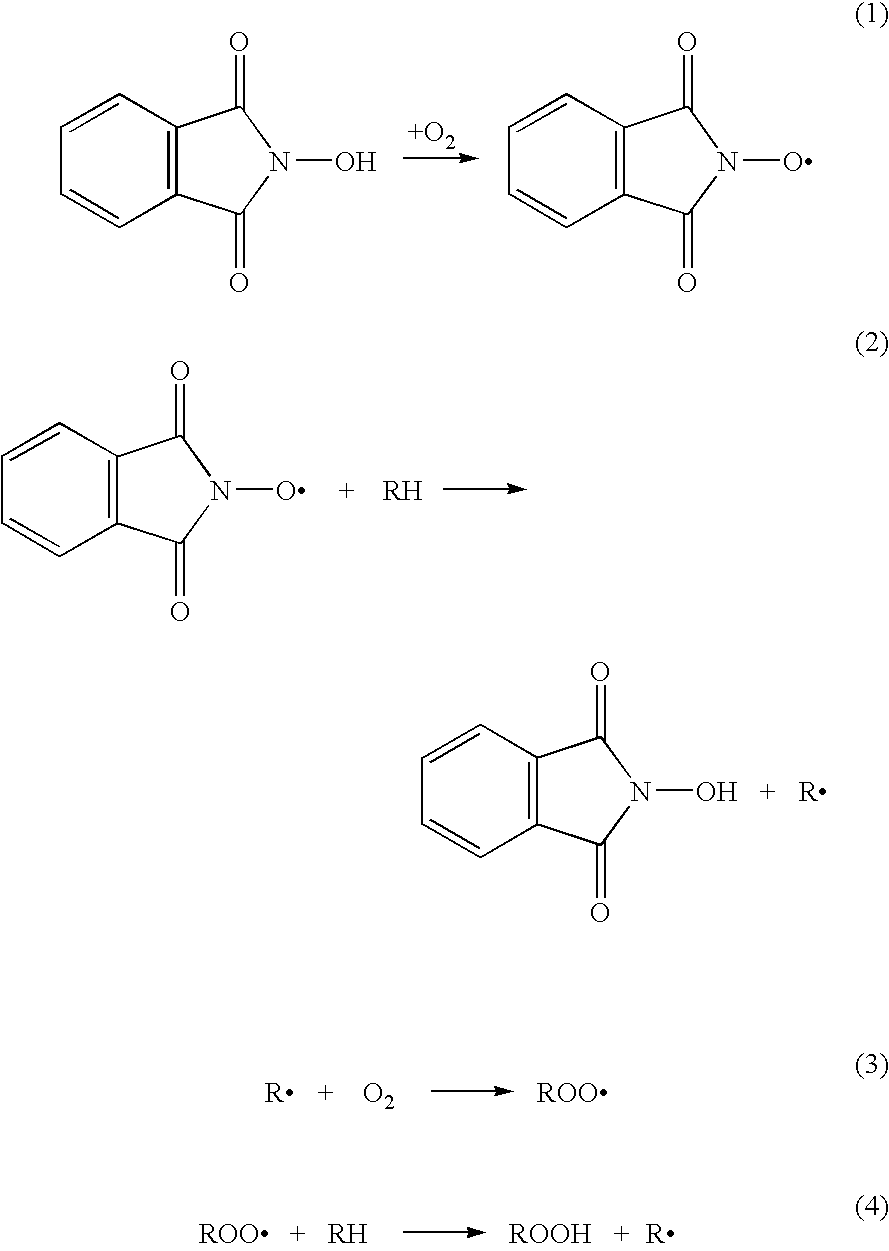
N-Hydroxyphthalimide is the N-hydroxy derivative of phthalimide.The compound is used, inter alia, as catalyst for oxidation reactions, in particular for the selective oxidation (e. g. alkanes to alcohols) with molecular oxygen under mild conditions.
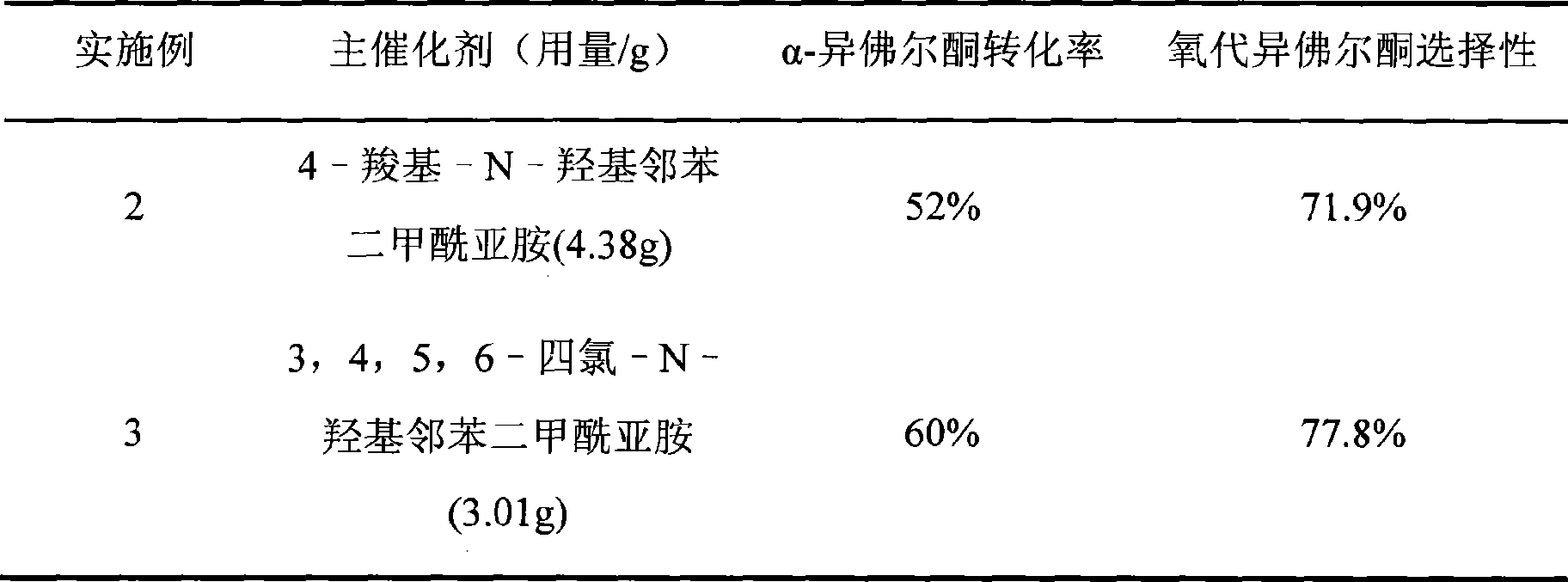
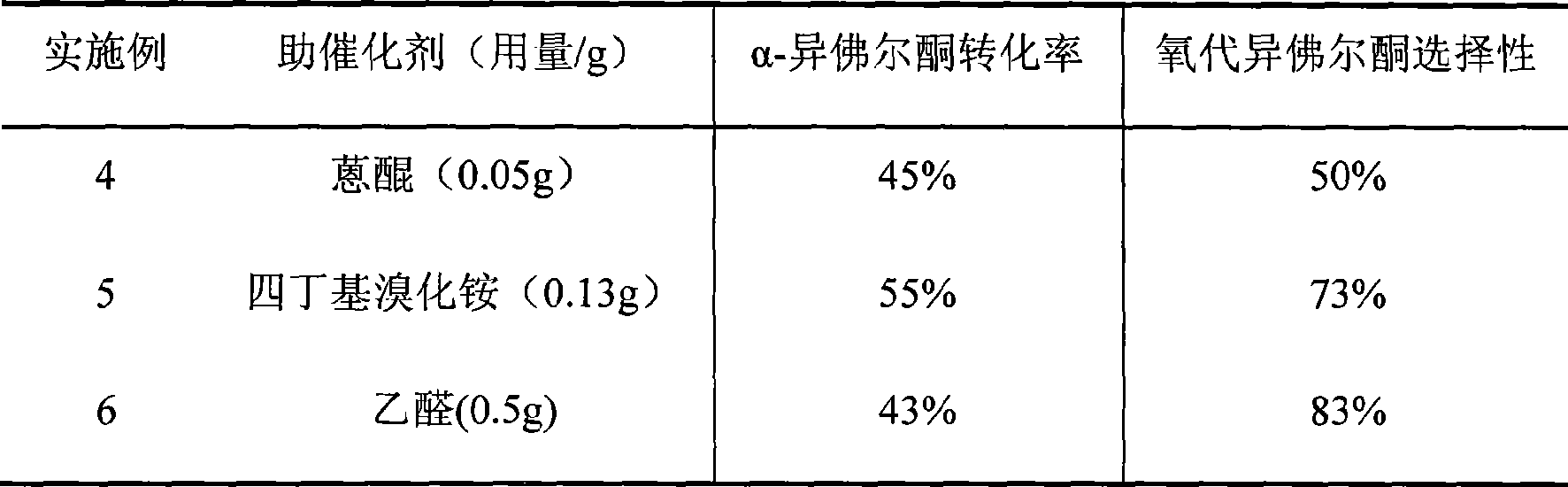
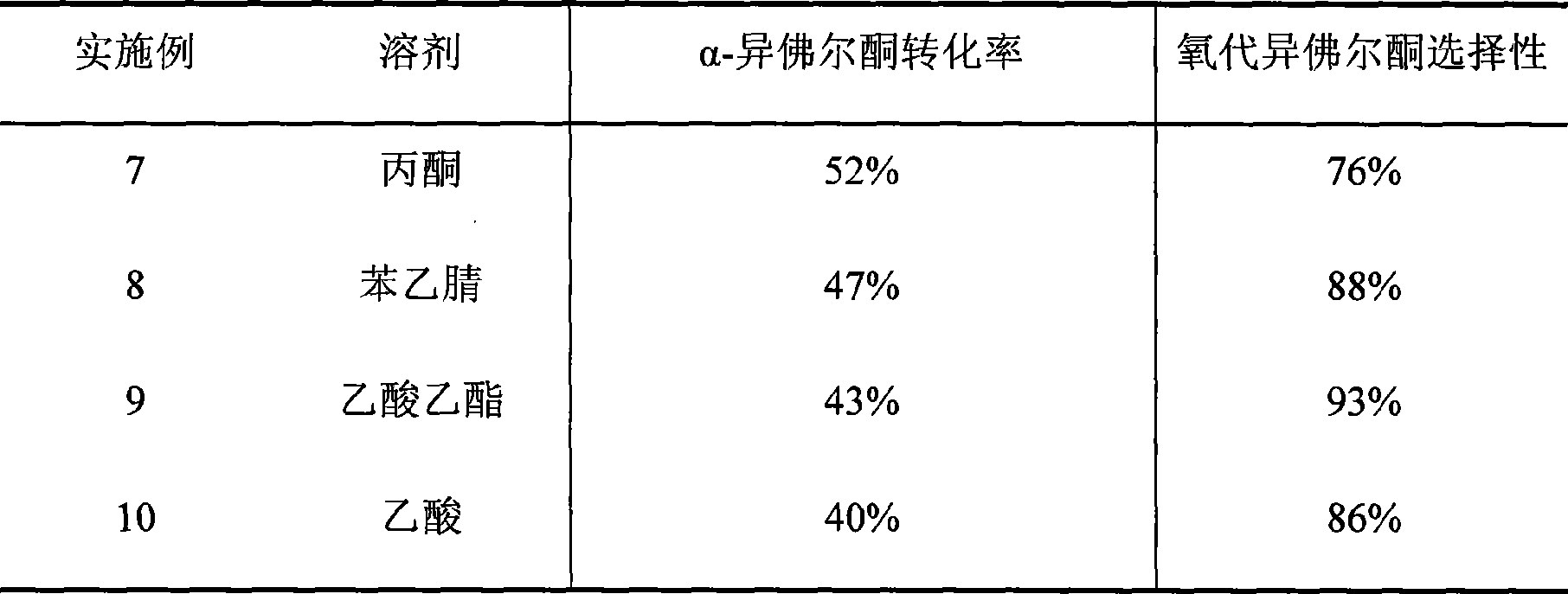
Method for preparing oxo-isophorone by catalytic oxidation using metal free catalytic system
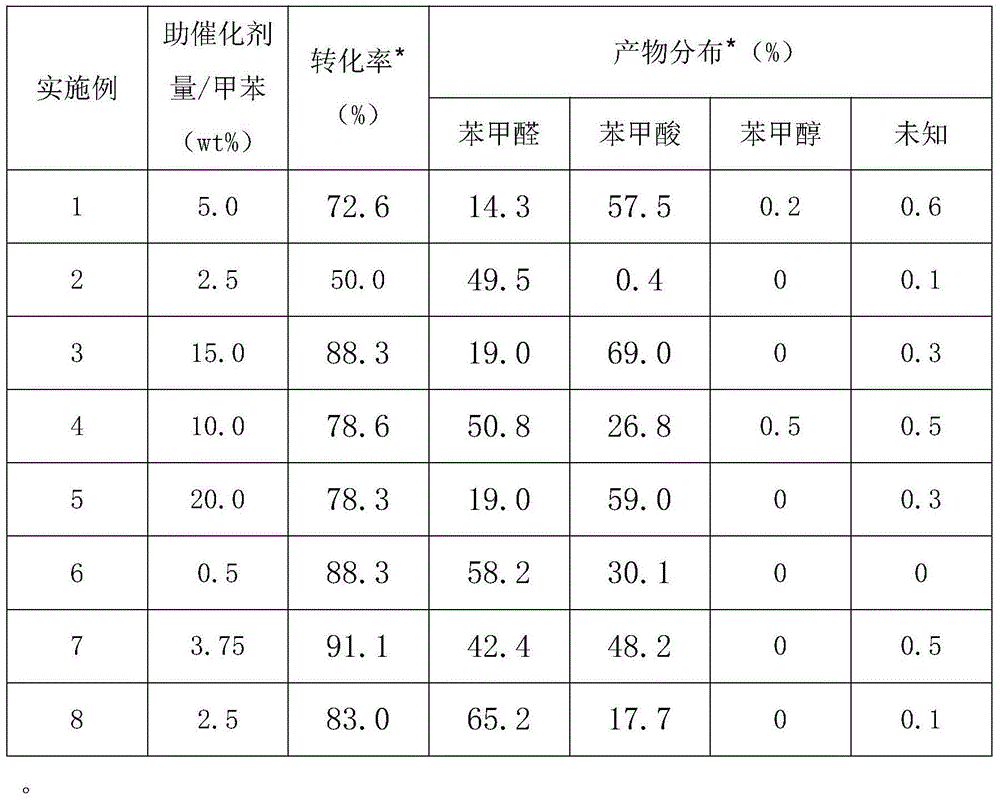
InactiveCN101417936AHigh selectivityMild conditionsOrganic compound preparationCarbonyl compound preparationCatalytic oxidationReaction temperature
The invention relates to a method for preparing keto-isophorone by the catalytic oxidation of a metal free co-catalyst system, which is characterized in that, Alpha-isophorone is used as the raw material; in the presence of an organic solvent, oxygen or an oxygen rich gas is used as an oxidant; under the effect of the co-catalyst system composed by the main catalyst N-hydroxyphthalimide and the analogues of N-hydroxyphthalimide, and organic cocatalyst, keto-isophorone is prepared by catalytic oxidation; reaction temperature ranges from 10 to 100 DEG C; reaction time ranges from 1 to 70 hours; keto-isophorone with high selectivity can be generated. The method has the advantages that, the catalyst is metal free, cheap and easy to be obtained; reaction conditions are mild; operation is simple; the product has high selectively and other advantages.
Owner:ZHEJIANG UNIV +1
Method for preparing tert-butyl hydrogen peroxide and tert-butyl alcohol by oxidating isobutane
InactiveCN102391167AImprove conversion rateHigh selectivityPreparation by oxidation reactionsOrganic compound preparationCatalytic oxidationSolvent
The invention discloses a method for preparing tert-butyl hydrogen peroxide and tert-butyl alcohol by oxidating isobutene. Molecular oxygen is taken as an oxygen source; N-hydroxy phthalimide (NHPI) or a derivative thereof is taken as a catalyst; in the presence of a polar solvent, carrying out catalytic oxidation on the liquid phase of the isobutane to prepare the tert-butyl hydrogen peroxide and the tert-butyl alcohol; after finishing the reaction and rapidly cooling, the catalyst and the products are subjected to solid-liquid separation; and the catalyst is recycled through centrifugal sedimentation and washing. Compared with the existing process without catalytic optimization, the method disclosed by the invention has mild reaction conditions, the temperature and the pressure needed in the reaction can be remarkably lowered; and in addition, under the condition of having higher rate of conversion of the isobutane, the high selectivity of the tert-butyl hydrogen peroxide and the tert-butyl alcohol can be maintained.
Owner:XIANGTAN UNIV
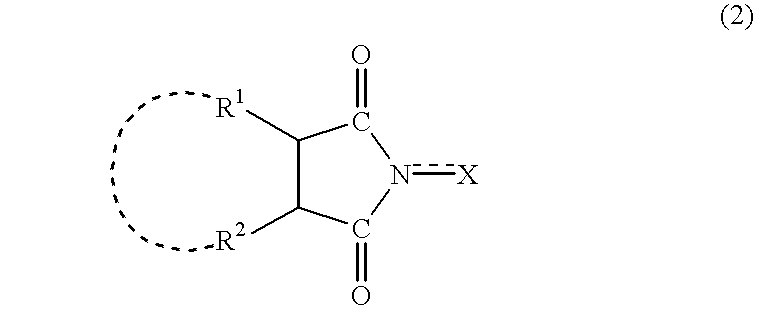
Method for separation of reaction products from catalysts
InactiveUS20040014985A1Efficiently and simply separatingEfficient separationOrganic compounds purification/separation/stabilisationOrganic compound preparationImideOrganic solvent
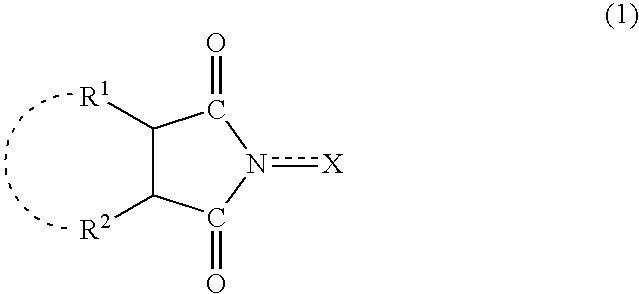
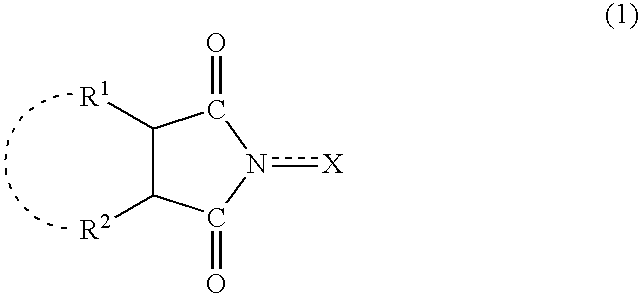
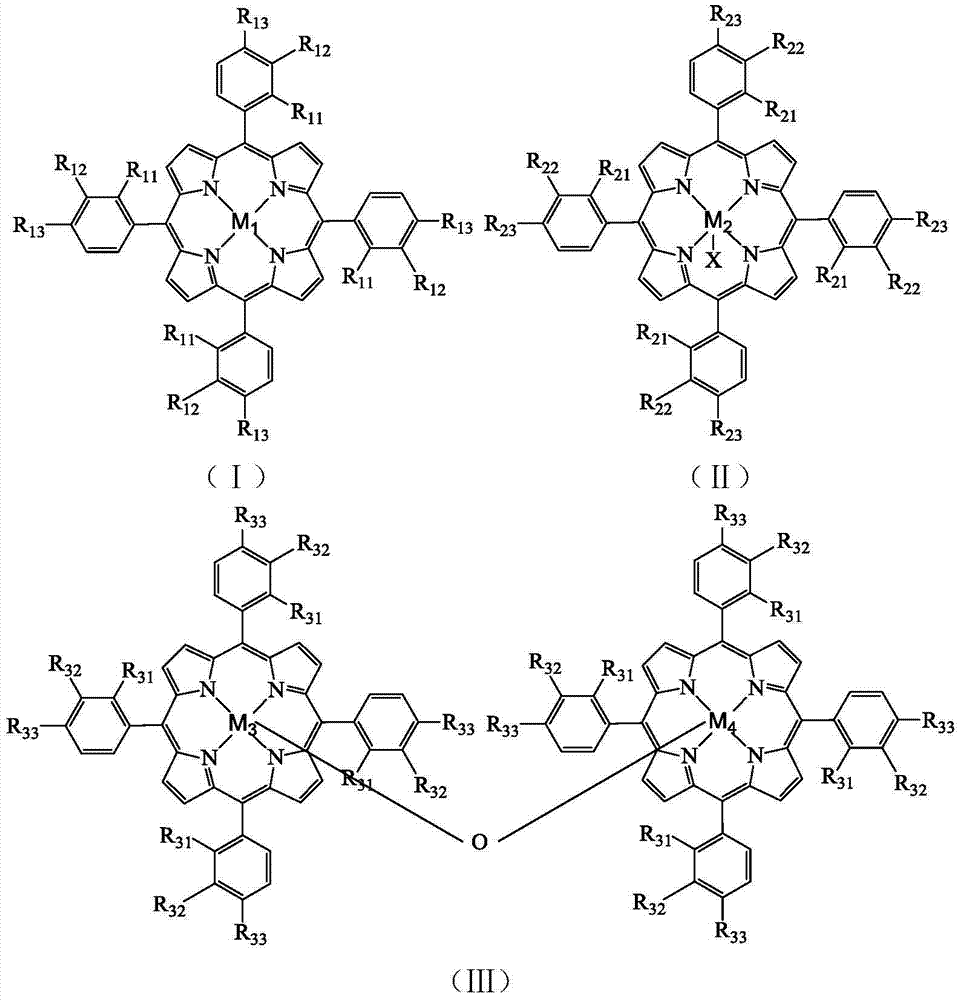
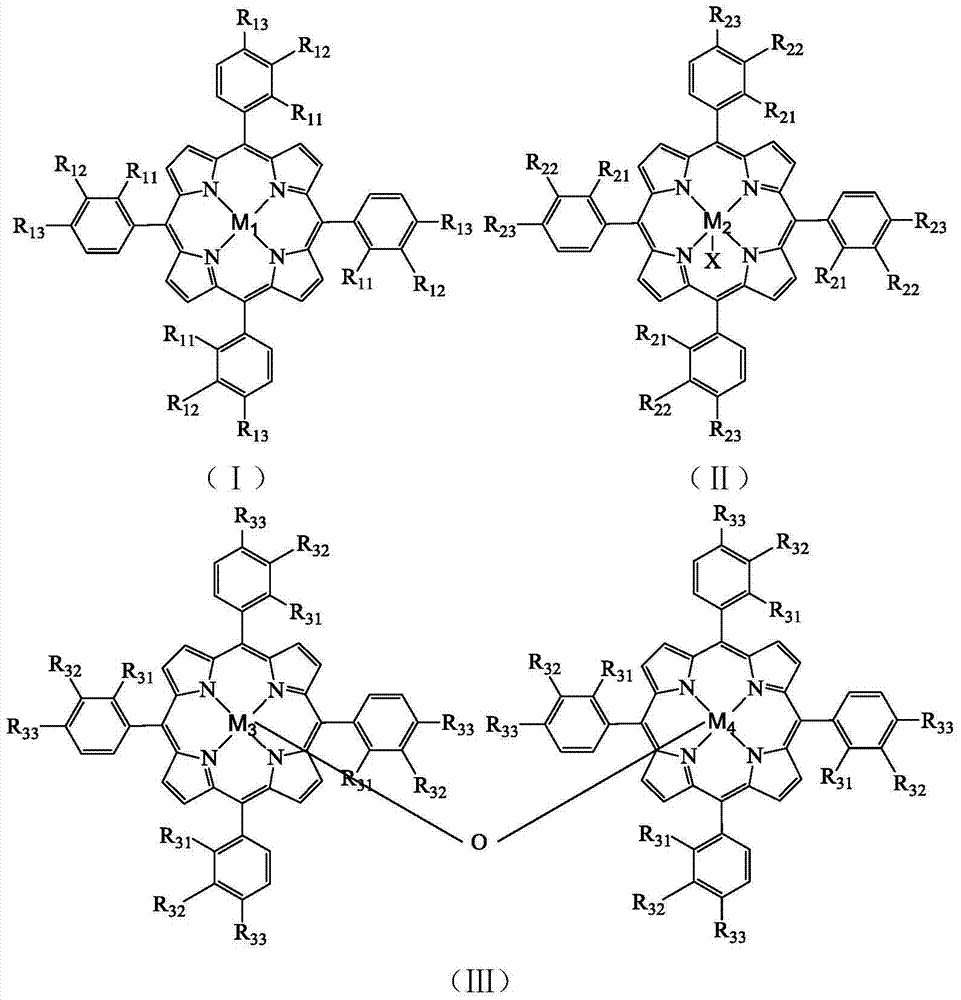
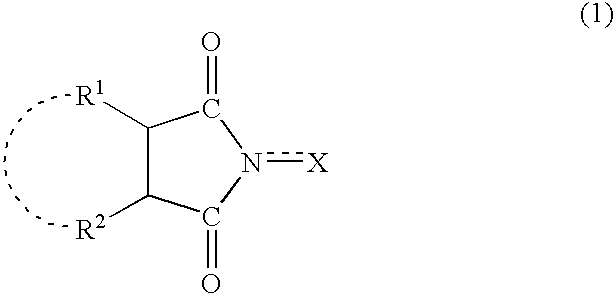
A method separates a reaction product from an imide compound catalyst represented by Formula (1) or an altered derivative thereof in a reaction mixture obtained as a result of a reaction in the presence of the imide compound catalyst by performing an extraction process using two organic solvents separable from each other to thereby separate the reaction product into one organic solvent layer and the imide compound catalyst or an altered derivative thereof into the other organic solvent layer, respectively: wherein R<1 >and R<2 >are each, for example, a hydrogen atom or an alkyl group, where R<1 >and R<2 >may be combined to form a double bond, an aromatic ring, or a non-aromatic ring; and X is an oxygen atom or a hydroxyl group. The method of the invention can efficiently and simply separate a reaction product from a catalyst and / or an altered derivative of the catalyst in a reaction mixture obtained as a result of a reaction of a substrate such as a hydrocarbon using N-hydroxyphthalimide or another imide compound as the catalyst.
Owner:DAICEL CHEM IND LTD
Preparation of fluorinated acetophenones
InactiveUS20050043559A1Carboxylic acid nitrile preparationOrganic compound preparationArylAcetophenone
The invention relates to a process for preparing fluorinated acetophenones from fluorinated aryl compounds in the presence of N-hydroxyphthalimide and the use thereof.
Owner:LANXESS DEUTDCHLAND GMBH
Method of preparing adipic acid by directly oxidizing cyclohexane
ActiveCN104109083AImprove conversion rateHigh yieldOrganic compound preparationCarboxylic compound preparationManganesePolyethylene glycol
The invention relates to a method for preparing adipic acid by directly oxidizing cyclohexane. The problems of low cyclohexane conversion rate and low adipic acid yield of preparation of adipic acid by directly oxidizing cyclohexane in the prior art are mainly solved. The method for preparing adipic acid by directly oxidizing cyclohexane comprises is characterized in that adipic acid is obtained by oxidizing cyclohexane with an oxygen-containing gas as an oxidant in the presence of a solvent, a free radical catalyst, a metal catalyst and a cocatalyst; the solvent is at least one selected from acetic acid, acetonitrile and ethyl acetate; the free radical catalyst is at least one selected from NHPI (N-hydroxyphthalimide) and NAPI (N-acetoxylphthalimide); the metal catalyst is at least one selected from cobalt and manganese; and the cocatalyst is at least one selected from polyethylene glycol and crown ether. The method well solves the problems, and can be used in the industrial production of adipic acid.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method and application of immobilized catalyst for hydrocarbon oxidation
ActiveCN104148110AEasy to prepareAvoid condensationPreparation by oxidation reactionsOrganic compound preparationMesoporous materialOxygen
Owner:XIANGTAN UNIV
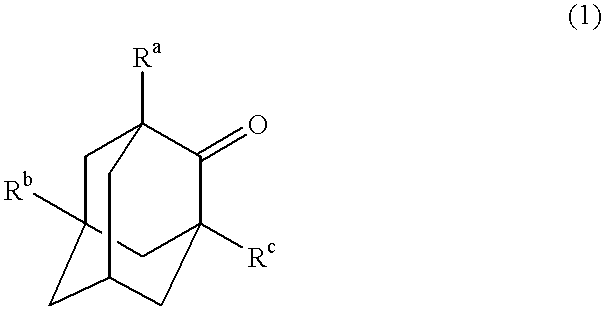
Process for the preparation of hydroxyadamantanone derivatives
InactiveUS6229050B1High yieldEasy to purifyOrganic compound preparationOrganic chemistry methodsManganeseOxygen
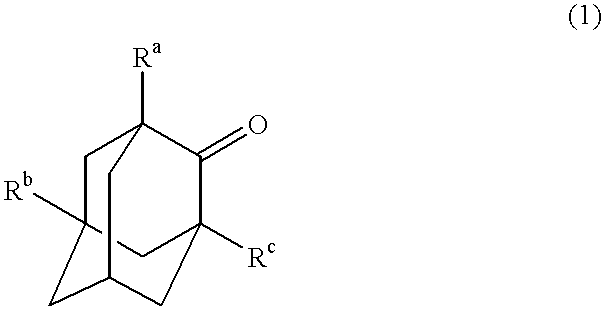
The invented process produces a corresponding 5-hydroxy-2-adamantanone derivative by allowing a 2-adamantanone derivative of the following formula (1):wherein each of Ra, Rb, and Rc is, identical to or different from one another, a hydrogen atom, a halogen atom, an alkyl group, a hydroxyl group which may be protected by a protective group, a hydroxymethyl group which may be protected by a protective group, an amino group which may be protected by a protective group, a carboxyl group which may be protected by a protective group, or a nitro group, and of carbon atoms constituting an adamantane skeleton, the other carbon atoms than carbon atoms at bridgehead positions and at a bonding position of an oxo group may have a substituent, to react with oxygen in the presence of N-hydroxyphthalimide or another imide compound, a vanadium compound, and a manganese compound.
Owner:DAICEL CHEM IND LTD
Process for synthesizing p-tertiary butyl benzaldehyde
ActiveCN101440028AEasy to separateConvenient and quick escapeMolecular sieve catalystsOrganic compound preparationBenzaldehydeManganese
The invention discloses a method for synthesizing p-tert-butylbenzaldehyde. The method comprises the following steps: a normal atmosphere heterogeneous catalytic oxidation method is adopted; p-tert-butyltoluene as a raw material, sufficient oxygen as an oxygen source and n-hydroxyphthalimide as a free radical evocating agent in an acetonitrile solution react for 4 to 7 hours at a temperature between 50 and 70 DEG C under the condition of a catalyst to directly synthesize the p-tert-butylbenzaldehyde; and the catalyst is a cobalt-loaded mesoporous molecular sieve or a cobalt- and manganese-loaded mesoporous molecular sieve. The method use the cobalt-loaded mesoporous molecular sieve or the cobalt- and manganese-loaded mesoporous molecular sieve as the solid-phase catalyst; compared with the prior liquid-phase catalyst, the target product is easier to separate after reaction; a reactant can rapidly contact catalytic activity center; product molecules rapidly escape from a pore canal, thereby avoiding deep oxidation and improving the selectivity of aldehyde; in addition, micro n-hydroxyphthalimide as the free radical evocating agent is added to the system to trigger the chain transmission reaction of free radicals.
Owner:格林生物科技股份有限公司
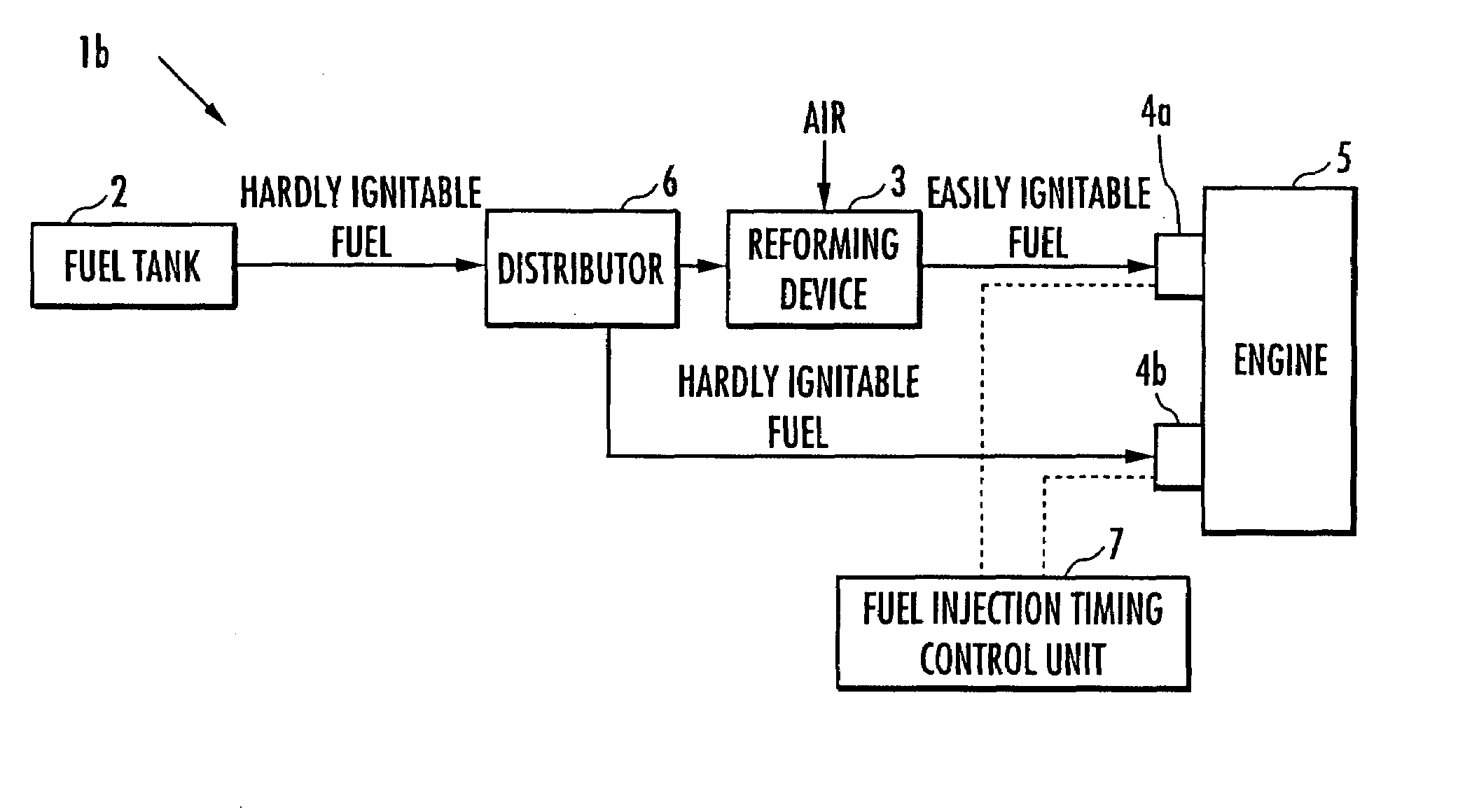
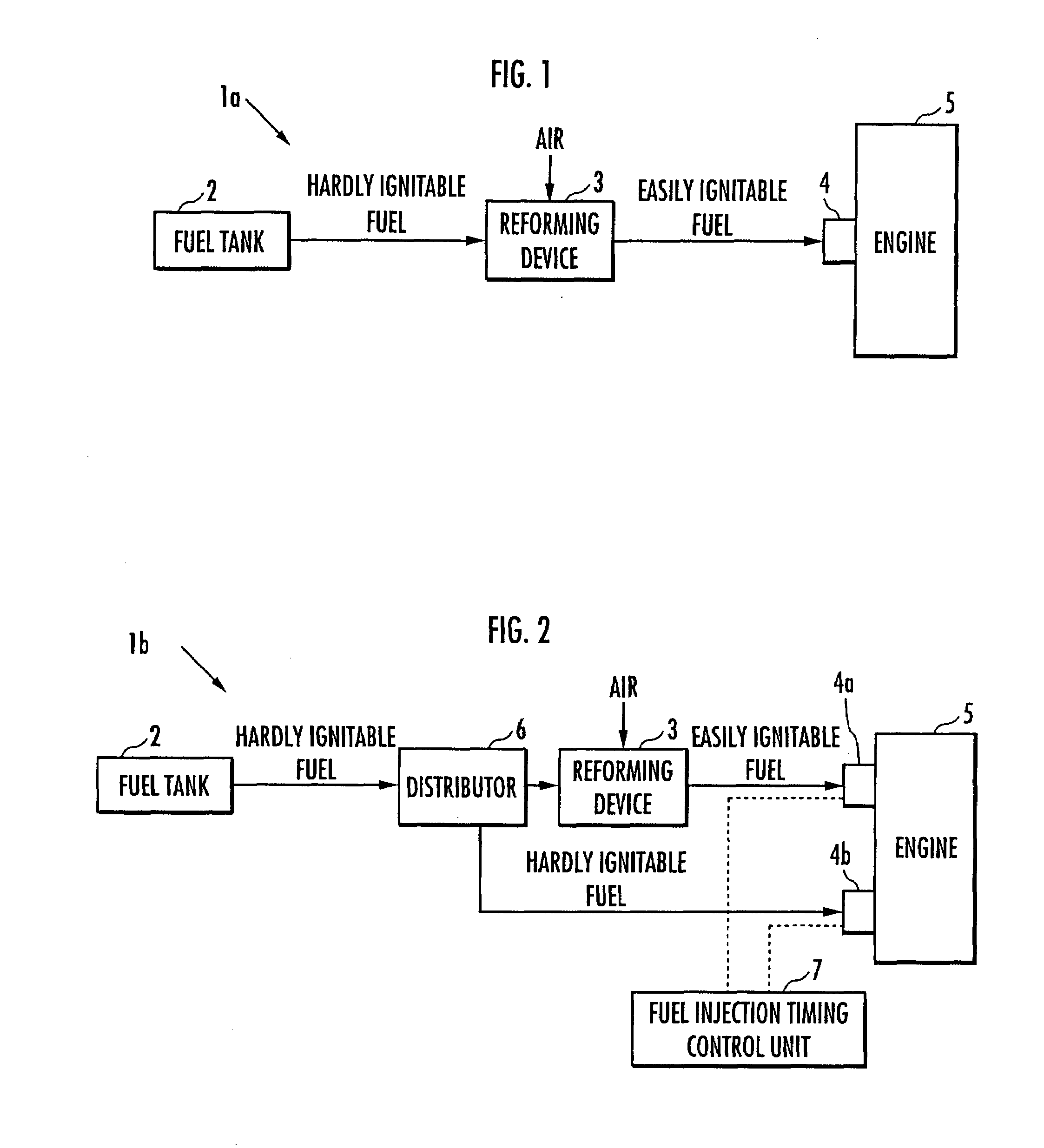
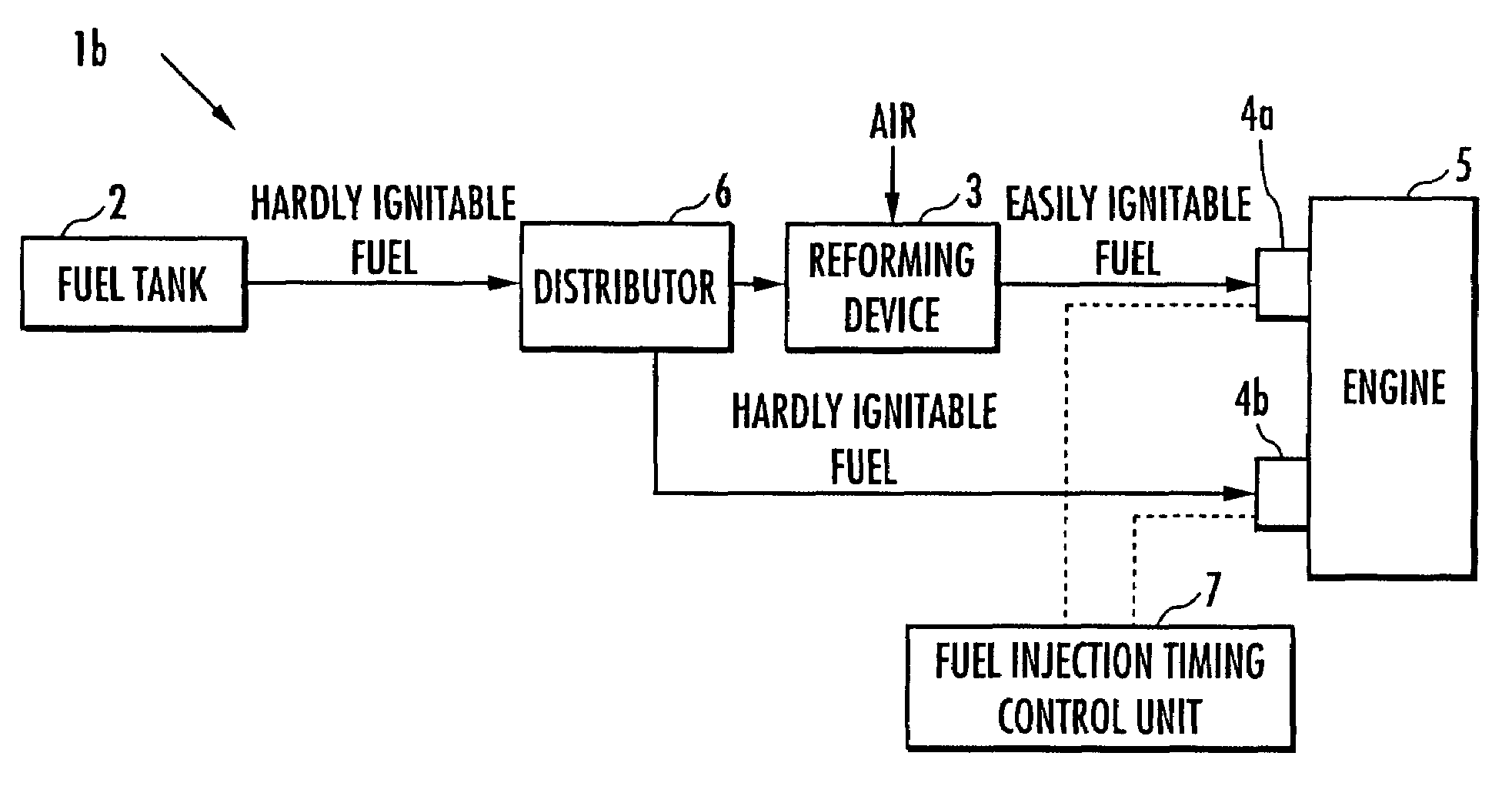
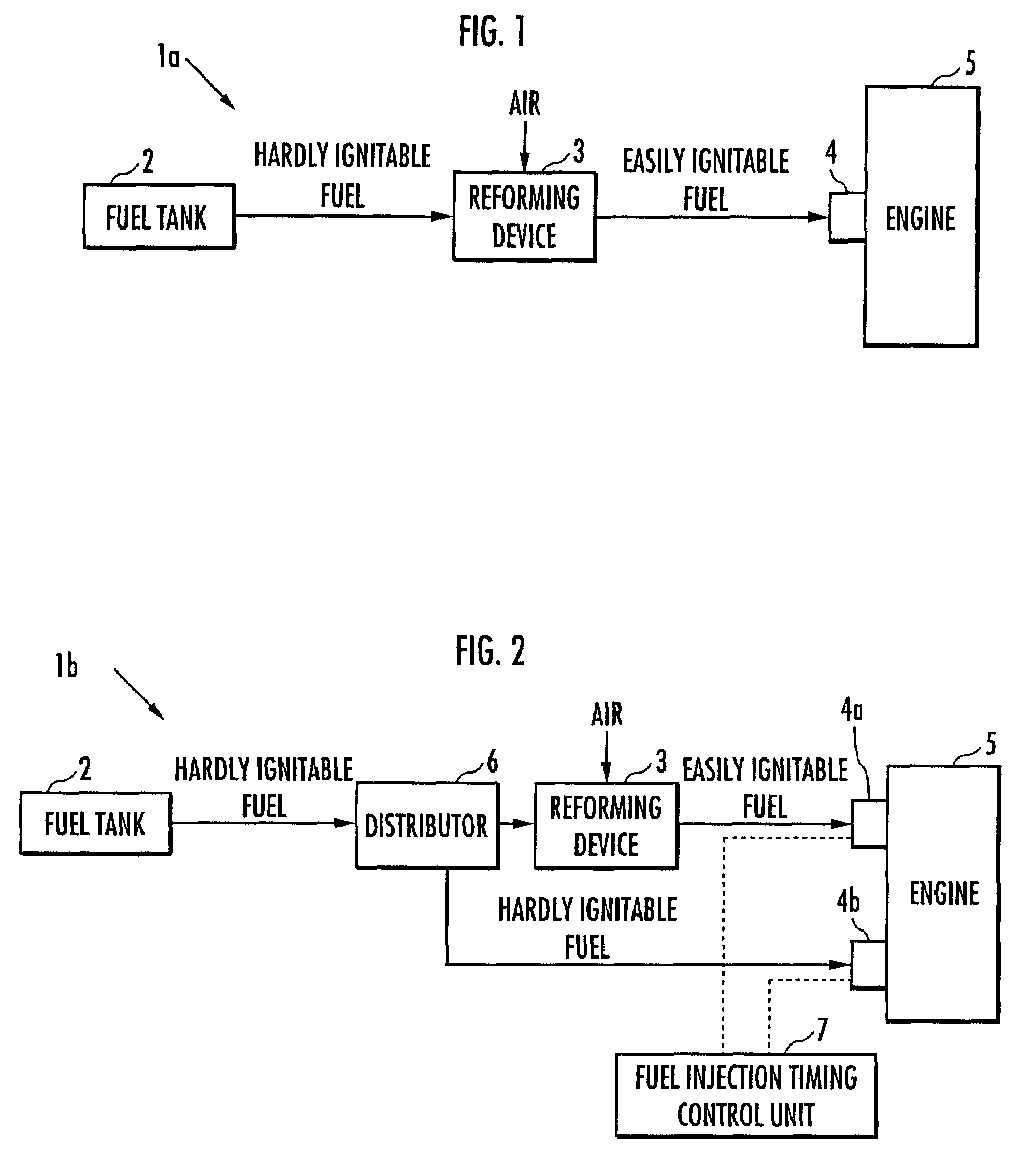
Internal combustion engine
ActiveUS20070215098A1Improve flammabilityGuaranteed uptimeOrganic compound preparationCombustion enginesHomogeneous charge compression ignitionKerosene
The present invention provides an internal combustion engine which can produce several types of fuels from a single fuel as needed. The internal combustion engine comprises reforming means 3 for reforming a first fuel to be used in homogeneous charge compression ignition combustion into a second fuel having high ignitability, by making the first fuel contact with a catalyst formed from N-hydroxyphthalimide. The engine uses the second fuel when conducting diesel combustion. The engine can switch between the homogeneous charge compression ignition combustion with the use of the first fuel and the diesel combustion with the use of the second fuel. The engine conducts the homogeneous charge compression ignition combustion when a load is low, and conducts the diesel combustion when the load is high. The internal combustion engine further comprises a first fuel injector 4b which injects the first fuel to an air intake port when the homogeneous charge compression ignition combustion is conducted, and a second fuel injector 4a which directly injects the second fuel to a combustion chamber when the diesel combustion is conducted. The engine further comprises fuel injection timing control means 7 for switching between the homogeneous charge compression ignition combustion and the diesel combustion, by changing the injection timing of the fuel. The first fuel is at least one of gasoline, kerosene, light oil and alcohol.
Owner:HONDA MOTOR CO LTD
Method for synthesizing N-hydroxy phthalimide with solid-phase process
InactiveCN101845012AEfficient green reaction processHigh yieldOrganic chemistryHydroxylamine HydrochlorideNitrogen gas
The invention discloses a method for synthesizing N-hydroxy phthalimide with the solid-phase process by grinding the raw materials of phthalic anhydride and hydroxylamine hydrochloride and the mineralizer of sodium chloride and ferric oxide under the protection of nitrogen. The method for synthesizing N-hydroxy phthalimide with the solid-phase process ensures the thorough reaction, high yield, low energy consumption and less pollution and is suitable for industrial production, thereby being a promising preparation method.
Owner:JIANGSU CHENPAI PHARM GRP CO LTD
Preparation method of crosslinked polystyrene microsphere surface synthesized and immobilized N-hydroxyphthalimide catalyst
ActiveCN105080604AHigh degree of immobilizationEasy to separateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMicrosphereCatalytic oxidation
The invention belongs to the technical field of a polymeric microsphere surface immobilized N-hydroxyphthalimide catalyst and specifically relates to a preparation method of a crosslinked polystyrene microsphere surface synthesized and immobilized N-hydroxyphthalimide catalyst. According to the preparation method, chloromethylated crosslinked polystyrene microspheres CMCPS are immersed in a de-watering solvent to undergo swelling so as to prepare modified microspheres CPS-PA with the surface bonded with phthalic anhydride; and then, the modified microspheres CPS-PA with the surface bonded with phthalic anhydride is used to prepare crosslinked polystyrene microsphere surface synthesized and immobilized N-hydroxyphthalimide. The method provided by the invention has advantages as follows: good stability, simple process, convenience in operation and high immobilization capacity. The product is easy to separate in a catalytic oxidation system and has good reusability, and the system is easy for purification.
Owner:铁居科技(海南)有限公司
Glucose oxidase mediation free radical initiating system and method for preparing hydrogel by using glucose oxidase mediation free radical initiating system
InactiveCN104418971ACrushableImprove mechanical propertiesConcentration ratioNanocomposite hydrogels
The invention relates to a glucose oxidase mediation free radical initiating system and a method for preparing hydrogel by using the glucose oxidase mediation free radical initiating system. The glucose oxidase mediation free radical initiating system consists of a N-hydroxyphthalimide derivative, glucose oxidase and glucose and has the advantages that the initiation condition is gentle, the preparation is simple and convenient, the pH value application range of a reaction medium is relatively wide, and the environment can be protected. According to the method provided by the invention, by adjusting the pH value of the reaction medium or adjusting the concentration ratio of components of a ternary initiating system, monomer polymerization can be initiated within 3-30 minutes at the room temperature under control to prepare hydrogel, and high-strength nano composite hydrogel can be also prepared, so that the method has remarkable application prospect in fields such as controlled-release of medicines, enzyme immobilization, tissue engineering and substance separation.
Owner:TONGJI UNIV
Method for preparing aromatic ketone or aromatic aldehyde by selectively oxidizing alkyl arene
InactiveCN101100418AEasy to handleRaw materials are cheap and easy to getOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationBromineAryl radical
A non-metallic catalytic system with pyramine compound, molecule bromine and N-hydroxy-o-dimethyl-acylimine is prepared by catalyzing aryl radical oxide with molecular oxygen as oxygen source to generate aryl ketone or aryl aldehyde under gentle condition and oxidation reacting selectively. It costs low, is non-toxic and has less environmental pollution.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
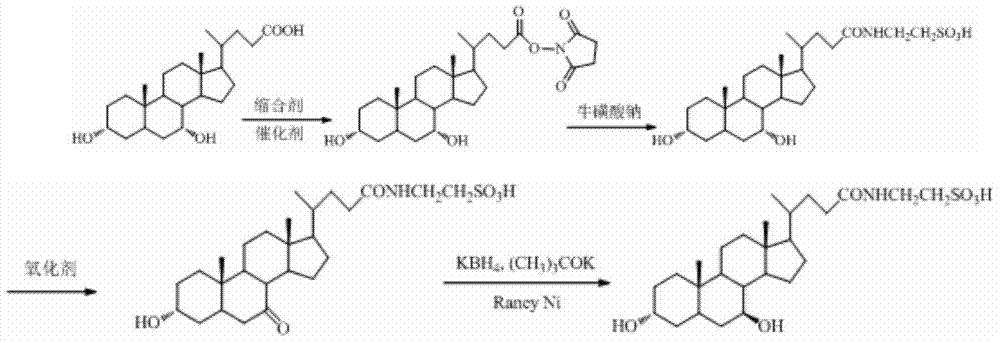
Tauroursodeoxycholic acid synthesis method
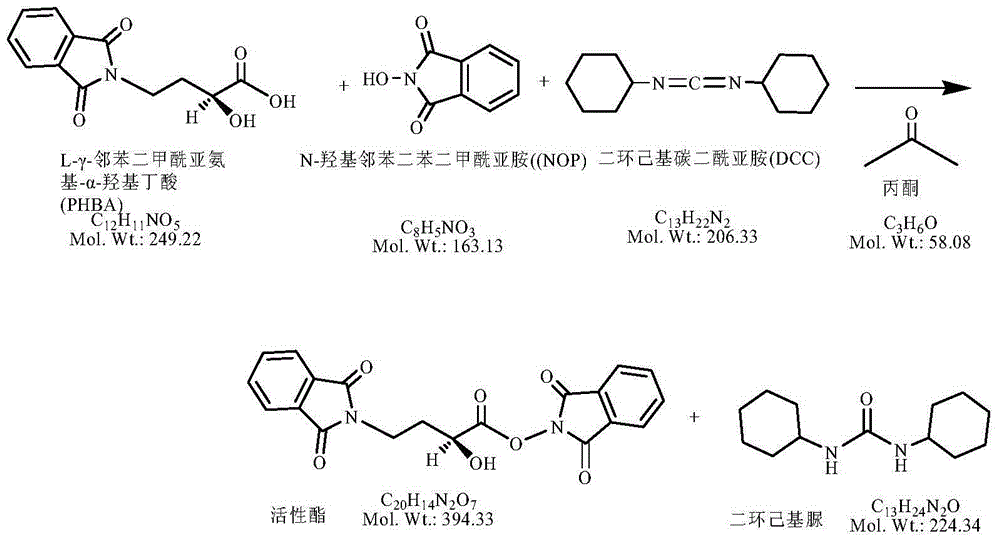
The present invention discloses a tauroursodeoxycholic acid synthesis method, which comprises that chenodeoxycholic acid is adopted as a raw material, the chenodeoxycholic acid and N-hydroxy succinimide or N-hydroxyphthalimide are subjected to condensation under effects of a condensation agent and a catalyst to obtain an active ester, the active ester reacts with sodium taurocholate to obtain taurochenodeoxycholic acid, and further an oxidation reaction and a reduction reaction are performed to synthesize the tauroursodeoxycholic acid. The synthesis method of the present invention adopts the chenodeoxycholic acid as the raw material, and has characteristics of low cost, simple and controllable purification process, high target product purity, and easy industrialization.
Owner:GUANGZHOU YINGYU PHARMA TECH CO LTD
Process for cooxidizing organic compounds, process for producing epoxy compounds and process for producing esters or lactones
InactiveUS6229023B1Easy to operateMild conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTetralinKetone
Owner:DAICEL CHEM IND LTD
A synthetic method of amikacin
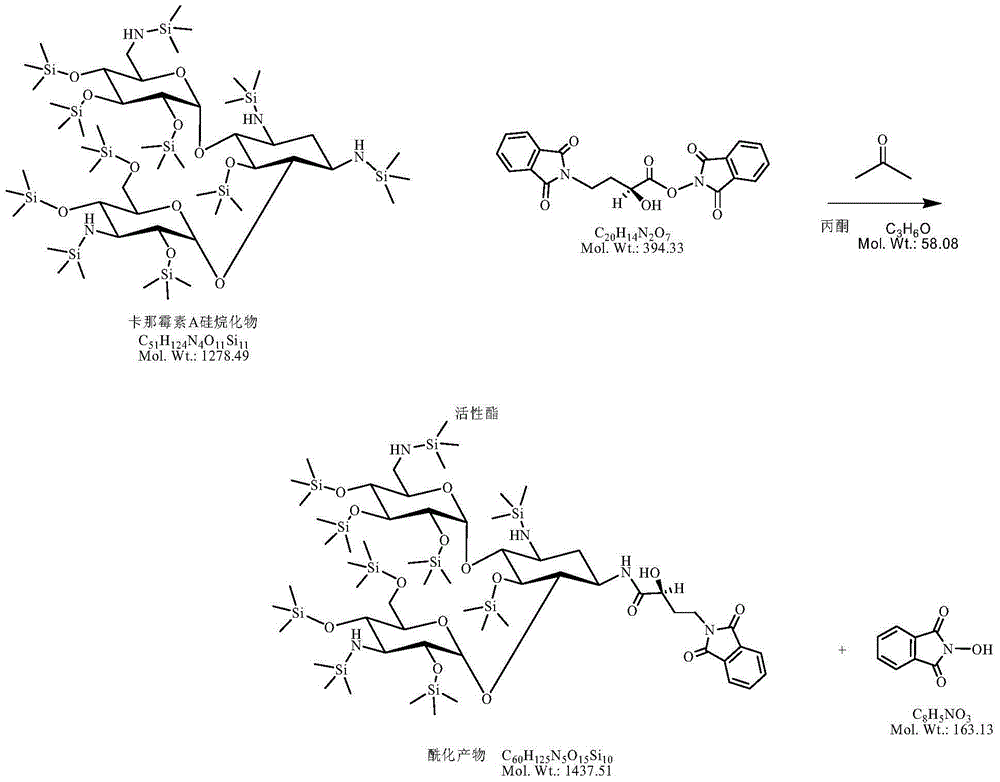
InactiveCN105440090AReduce manufacturing costEasy to operateSugar derivativesSugar derivatives preparationAmikacinSilylene
A synthetic method of amikacin is disclosed. Gamma-4-phthalimido-2-hydroxy butyric acid is adopted as a raw material for direct acylation. 4-dimethylaminopyridine (DMAP) or 1-hydroxybenzotriazole (HOBT) is adopted as a catalyst. N,N'-dicyclohexylcarbodiimide is adopted as a condensing agent. Silyl kanamycin A is directly acylated to obtain an acylation product and the acylation product is subjected to acidolysis and hydrazinolysis to obtain the amikacin. A production operation for specially preparing active ester for acylation is not needed in the method, thus simplifying operation steps and production equipment. N-hydroxyphthalimide for preparing the active ester is not used in the direct acylation, thus reducing the production cost. By optimization of acylation conditions, selectivity of the acylation is improved, the synthesis yield is ensured, contents of impurities is lowered, and beneficial conditions for subsequent purification of the amikacin are provided.
Owner:CHONGQING DAXIN PHARMA +2
Method for preparing ketone or carboxylic acid through catalytic oxidation of secondary alcohol or primary alcohol
InactiveCN110483273AHigh yieldGood catalyticOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationReaction temperature
The invention relates to a method for preparing ketone or carboxylic acid through catalytic oxidation of alcohol. The method specifically comprises the following steps: adding secondary alcohol or primary alcohol into a certain amount of organic solvent to serve as a raw material, forming an N-hydroxyphthalimide (NHPI)-phthalocyanine catalytic system and taking oxygen as an oxidant, and reacting for 9-36 hours at the reaction temperature of 60-120 DEG C under the condition of normal pressure to obtain the ketone or carboxylic acid with higher yield. Compared with the prior art, the method hasthe advantages of green and environment-friendly oxidant, cheap and easily-prepared catalyst, easiness in separation from the product, mild reaction conditions and the like, and is a green alcohol oxidation method.
Owner:SHANGHAI INST OF TECH
Novel synthesis method for phosphate compounds
InactiveCN107522741AMild reaction conditionsEasy to operateGroup 5/15 element organic compoundsPhosphateSynthesis methods
The invention discloses a novel synthesis method for phosphate compounds. Specifically, the method comprises steps as follows: alcohol with the structure (I), H-phosphite with the structure (II), bivalent cobalt salt and n-hydroxyphthalimide are dispersed in a solvent sequentially, oxygen is introduced into a mixture, heating and stirring are performed, and the phosphate compounds with a structure (III) shown in the description can be obtained. The novel method for efficiently synthesizing the phosphate target compounds from cheap and available alcohol and H-phosphite compounds as starting materials for the reaction, bivalent cobalt salt and n-hydroxyphthalimide as co-catalysts as well as oxygen as an oxidizing agent through aerobic oxidative coupling is provided for the first time. The method has the advantages that reaction conditions are mild, operation is simple, the raw materials are cheap and available, product structures are diversified, the yield is high and the like, and great application value and broad industrial prospect are shown.
Owner:XINYANG NORMAL UNIVERSITY
Method for preparing p-methoxybenzaldehyde perfume in presence of metalloporphyrin through catalytic oxidation of p-methoxytoluene
InactiveCN103694093AReduce pollutionReduce dosageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationPorphyrin
The invention relates to a method for preparing p-methoxybenzaldehyde perfume in presence of metalloporphyrin through catalytic oxidation of p-methoxytoluene. The method comprises the steps of: by taking metalloporphyrin with the concentration of 10-100ppm as a catalyst, N-hydroxyphthalimide as a free radical initiator and, introducing oxygen under 0.1-1.0Mpa and reacting for 1-12h at 60-100 DEG C in an acetonitrile solvent to obtain p-methoxybenzaldehyde, wherein the molar ratio of N-hydroxyphthalimide to p-methoxytoluene is (0.01-0.1):1. The method has the advantages that the consumption of the catalyst is extremely low, separation and recovery are not needed, the catalyst is higher in activity and selectivity, and a reaction condition is mild. According to the method provided by the invention, not only can resources be utilized and saved effectively, but also environmental pollution is reduced and the purpose of reducing energy consumption is achieved to further realize energy saving and emission reduction in an all-round way, and thus, the method has a wide application prospect in the industry.
Owner:BEIJING UNIV OF TECH
Preparation method and application of a solid-supported catalyst for hydrocarbon oxidation
ActiveCN104148110BEasy to prepareAvoid condensationPreparation by oxidation reactionsOrganic compound preparationMesoporous materialOxygen
Owner:XIANGTAN UNIV
Method for increasing yield of amikacin
ActiveCN104356182AReduce lossesReduce degradationSugar derivativesSugar derivatives preparationHydroxybutyric acidKanamycin
The invention discloses a method for increasing yield of amikacin. The method for increasing yield of amikacin comprises the following steps: heating, refluxing and carrying out reaction on gamma-amino-alpha-hydroxybutyric acid and fluorenylmethyl chloroformate, so as to generate a reaction liquid containing gamma-amino-alpha-hydroxybutyric acid; directly adding N-hydroxyl phthalimide and N,N-dicyclohexylcarbodiimide into the reaction liquid, and carrying out reaction to generate a reaction liquid containing active ester; directly adding a kanamycin A silanization compound into the reaction liquid containing the active ester for carrying out acylation reaction, after complete reaction is finished, adding an HBr aqueous solution until the pH is adjusted to be 2-3, and carrying out suction filtration to remove solid impurities; and then carrying out reduced pressure distillation for removing the solvent, adjusting the pH to be 7-8 with concentrated liquor, purifying by virtue of a CD180 macroporous resin column, concentrating, and carrying out freeze drying, so that amikacin solid is obtained. The active ester causes selectivity of the acylation reaction to be greatly improved due to enlargement of a protective group, and a follow-up processing step is also greatly simplified, so that the amikacin synthesis yield is increased.
Owner:山东安信制药有限公司
A kind of preparation method of polymer microsphere immobilized n-hydroxyphthalimide catalyst
InactiveCN104069891BHigh degree of immobilizationEasy to separateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMicrosphereCatalytic oxidation
The invention belongs to the technical field of immobilized N-hydroxyphthalimide catalysts, and specifically relates to a preparation method of polymeric-microsphere-carrier immobilized N-hydroxyphthalimide catalyst. The preparation method is characterized in that intermediate product aldehyde group modified microsphere GMA / MMA-AL, modified microsphere GMA / MMA-PA bonded with a phthalic acid group and microsphere GMA / MMA-PPA loaded with phthalic anhydride PPA are prepared, and finally a functional microsphere loaded with N-hydroxyphthalimide functional microsphere group is obtained. With the preparation method provided by the invention, the efficient organic catalyst N-hydroxyphthalimide (NHPI) is easy to separate and can be reused, a system is easy to purity, and therefore, environment-friendly catalytic oxidation is realized.
Owner:ZHONGBEI UNIV
Method for photocatalytic oxidation of alkane by iron oxide
The invention relates to a method for photocatalytic oxidation of alkane by iron oxide. The method employs iron oxide and N-hydroxyphthalimide or a derivative thereof to compose a photocatalytic system, and uses air or oxygen as the oxidant to realize selective oxidation of different hydrocarbons under the conditions of 400-650nm visible light irradiation at room temperature, thus generating an oxygen-containing chemical product. The specific steps include: dissolving a substrate hydrocarbon and N-hydroxyphthalimide or a derivative thereof in a solvent, adding an iron oxide catalyst, then replacing the atmosphere in a photoreactor with air or oxygen, and carrying out reaction at 20-35DEG C under 400-650nm visible light irradiation for 10-600min so as to generate corresponding aldehyde, ketone or organic acid. The synthesis method provided by the invention has important application in mild oxidation of hydrocarbons.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of 2-amino-5-chloro-N,3-dimethylbenzamide
InactiveCN105859574APromote oxidationReduce usageOrganic compound preparationCarboxylic acid amides preparationChemical synthesisBenzoic acid
The invention discloses a synthesis method of 2-amino-5-chloro-N,3-dimethylbenzamide, belonging to the technical field of organic chemical synthesis. The method comprises the following steps: carrying out oxidation under the catalytic actions of N-hydroxyphthalimide and cobalt acetylacetonate to generate benzoic acid, carrying out substitution reaction with chlorine gas to generate 3,5-dichlorobenzoic acid, shielding off 5- chlorine by using a shielding reagent, carrying out methyl substitution on the 5- chlorine by using a Grignard reagent to generate 3-methyl-5-chlorobenzoic acid, carrying out nitro-substitution on the 3-methyl-5-chlorobenzoic acid and nitric acid under the catalytic action of concentrated sulfuric acid to generate 2-nitro-3-methyl-5-chlorobenzoic acid, carrying out catalytic hydrogenation to reduce the nitro group into amino group, carrying out reaction under the actions of N,N'-diisopropylcarbodiimide and 1-hydroxybenztriazole to obtain an intermediate, and carrying out reaction on the intermediate and methylamine to obtain the 2-amino-5-chloro-N,3-dimethylbenzamide. The method is simple to operate, and obviously enhances the synthesis yield to 92% or above.
Owner:CHANGZHOU AMANTE CHEM CO LTD
Synthesis and application of novel catalyst used in preparation of arone by carrying out catalytic oxidation on ethylbenzene and derivative of ethylbenzene
InactiveCN104193670ALow costReduce pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperatureCatalytic oxidation
The invention relates to synthesis and application of a novel catalyst used in preparation of arone by carrying out catalytic oxidation on ethylbenzene and a derivative of ethylbenzene. According to the synthesis, on the basis of a substrate being 16mmol of ethylbenzene or a derivative of ethylbenzene, 1-15mol% of novel catalyst with a double structure of N-hydroxyphthalimide (NHPI) and ketoxime is used as a catalyst; reaction is carried out in the presence of the novel catalyst without any catalyst promoter to obtain a main product acetophenone or other arones, wherein the oxygen pressure is 0.1-0.5 MPa, the reaction temperature is 20-100 DEG C, the reaction time is 1-12h, the ethylbenzene conversion rate is up to 80.5%,the selectivity of the product acetophenone is up to 92.3% and the yield can be up to 74.3%. The catalytic system used in the invention uses molecular oxygen as an oxidant, no catalyst promoter is added and the reaction conditions are mild, so that the cost is reduced, the yield is improved, the application range is wide and a good method is provided to the preparation of acetophenone or other arones.
Owner:UNIV OF JINAN
Internal combustion engine
ActiveUS7597090B2Improve flammabilityGuaranteed uptimeOrganic compound preparationCombustion enginesHomogeneous charge compression ignitionKerosene
The present invention provides an internal combustion engine which can produce several types of fuels from a single fuel as needed. The internal combustion engine comprises reforming means 3 for reforming a first fuel to be used in homogeneous charge compression ignition combustion into a second fuel having high ignitability, by making the first fuel contact with a catalyst formed from N-hydroxyphthalimide. The engine uses the second fuel when conducting diesel combustion. The engine can switch between the homogeneous charge compression ignition combustion with the use of the first fuel and the diesel combustion with the use of the second fuel. The engine conducts the homogeneous charge compression ignition combustion when a load is low, and conducts the diesel combustion when the load is high. The internal combustion engine further comprises a first fuel injector 4b which injects the first fuel to an air intake port when the homogeneous charge compression ignition combustion is conducted, and a second fuel injector 4a which directly injects the second fuel to a combustion chamber when the diesel combustion is conducted. The engine further comprises fuel injection timing control means 7 for switching between the homogeneous charge compression ignition combustion and the diesel combustion, by changing the injection timing of the fuel. The first fuel is at least one of gasoline, kerosene, light oil and alcohol.
Owner:HONDA MOTOR CO LTD
Method for preparing benzaldehyde by selective oxidation of methylbenzene
InactiveCN104151133AGood choiceHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzaldehydePhenanthroline
The invention belongs to a method for preparing benzaldehyde by selective oxidation of methylbenzene. The method comprises the following steps: adding methylbenzene, N-hydroxyphthalimide, a catalyst promoter and a solvent into a reaction kettle, introducing oxygen, and reacting under the pressure of 0.1-1.5MPa at the temperature of 30-150 DEG C for 60-600 minutes, wherein the catalyst promoter is 1,10-phenanthroline or a metal complex of 1,10-phenanthroline derivative, and the total amount of the catalyst promoter is 0.05%-20% based on the mass percentage of methylbenzene. The method has the beneficial effects that the reaction condition is mild, and benzaldehyde is good in selectivity and high in yield in comparison with benzaldehyde produced by using other catalyst promoters formed by transition metal ions.
Owner:ZHENGZHOU UNIV
Method for preparing oxo-isophorone by catalytic oxidation using metal free catalytic system
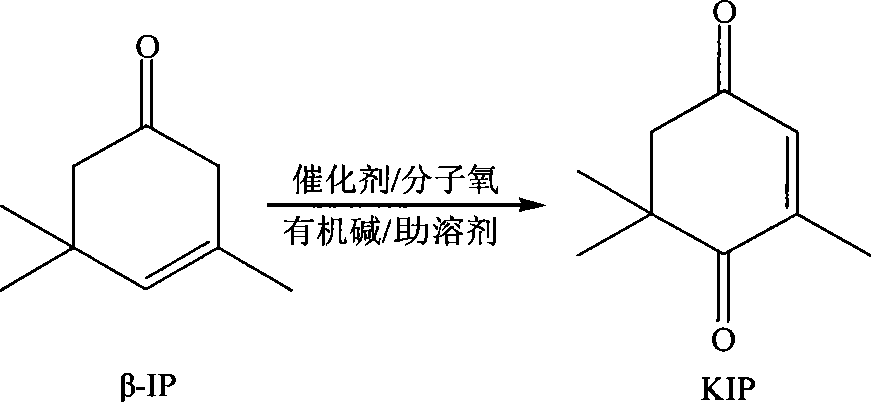
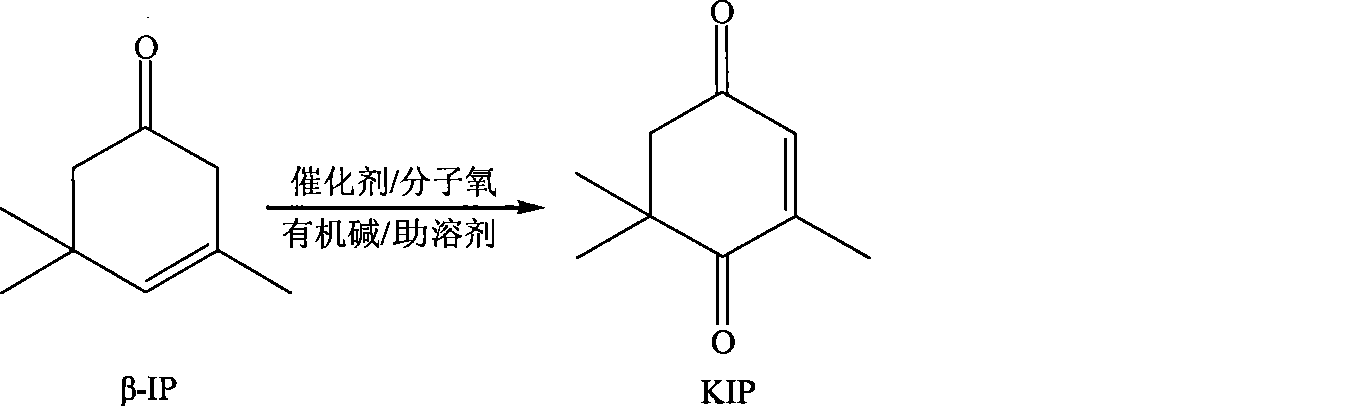
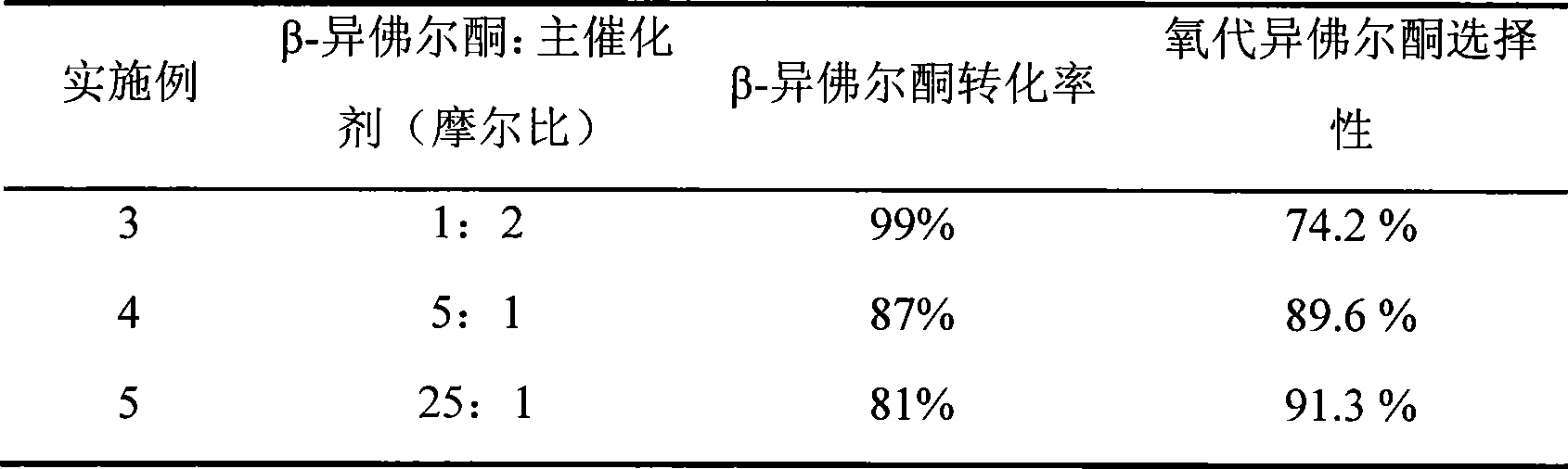
ActiveCN101417935AEasy to reuseEasy to separateOrganic compound preparationCarbonyl compound preparationCatalytic oxidationReaction temperature
The invention relates to a new method for preparing keto-isophorone, which utilizes metal free co-catalyst system to catalyze and oxidize Beta-isophorone. Beta-isophorone is used as the raw material; in the presence of an organic solvent, molecular oxygen or an oxygen rich gas is used as an oxidant; under the effect of the co-catalyst system composed by the main catalyst N-hydroxyphthalimide and the analogues of N-hydroxyphthalimide, and organic cocatalyst, keto-isophorone is prepared by catalytic oxidation; reaction temperature ranges from 0 to 120 DEG C; reaction time ranges from 5 to 50 hours; keto-isophorone with high selectivity can be generated. The method has the advantages that, the catalyst is metal free, cheap and easy to be obtained; reaction conditions are mild; operation is simple; the product has high selectively; recycling is easy, and multiple usage can be realized.
Owner:ZHEJIANG NHU CO LTD +1
Method of preparing aromatic isopropyl hydrogen peroxide compounds by microchannel oxidation process
InactiveCN109574898AAvoid explosiveLarge specific surface areaOrganic compound preparationPeroxy compound preparationReaction temperatureCatalytic oxidation
The invention discloses a method of preparing aromatic isopropyl hydrogen peroxide compounds by a microchannel oxidation process. The method includes: with a N-hydroxyphthalimide hydroxylamine compound as a catalyst and an azodiisobutyronitrile azo compound as an initiator, using a JD-SSIC silicon carbide plate micro-channel reactor to continuously introduce oxygen at certain flow rate maintained,with reaction pressure reaching a range of 0.5-4.5 MPa; mixing an aromatic isopropyl compound, the catalyst and the initiator, adding suitable solvent, preheating, allowing a flow into a microchannelthrough a metering pump, allowing oxidative reaction at 60-95 DEG C for 30-180 min, and carrying out gas-liquid separation after reaction to obtain finished aromatic isopropyl hydrogen peroxide compound solution, with the product yield reaching 49%. An organic-phase homogenous catalytic oxidation system and the microchannel reactor are utilized; mass transfer and heat transfer are effective; oxidative reaction time is short; explosion proneness of reaction is effectively avoided; therefore, the yield is high, selectivity is good, and safety is high.
Owner:ZHEJIANG UNIV OF TECH
Process for producing dicarboxylic acids
InactiveUS20040024248A1Increased space-time yieldLow conversion rateOrganic compound preparationCarboxylic preparation by oxidationAlkaneCatalytic oxidation
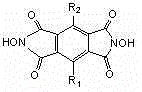
A process of the present invention produces a corresponding dicarboxylic acid by oxidative cleavage of a cycloalkane with oxygen and performs a reaction in the presence of a catalyst including an imide compound and a metallic compound, the imide compound having a cyclic imide skeleton represented by following Formula (I): wherein X is an oxygen atom or an -OR group, and wherein R is a hydrogen atom or a hydroxyl-protecting group, under conditions of a reaction temperature of 80° C. or higher and a concentration of the cycloalkane in a system of 21% by weight or more. The imide compound includes, for example, N-hydroxyphthalimide. The amount of the imide compound is, for example, from about 0.000001 to about 0.01 mole per mole of the cycloalkane. In the production of a corresponding dicarboxylic acid by catalytic oxidation of a cycloalkane with oxygen, the present invention can yield the dicarboxylic acid in a high space time yield even using a small amount of the catalyst.
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com