Tauroursodeoxycholic acid synthesis method
A technology of tauroursodeoxycholic acid and tauroursodeoxycholic acid, which is applied in the field of synthesis of tauroursodeoxycholic acid, can solve the problems of high cost of raw materials and troublesome separation methods, and achieve low cost and easy Simple and controllable effect of industrialization and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
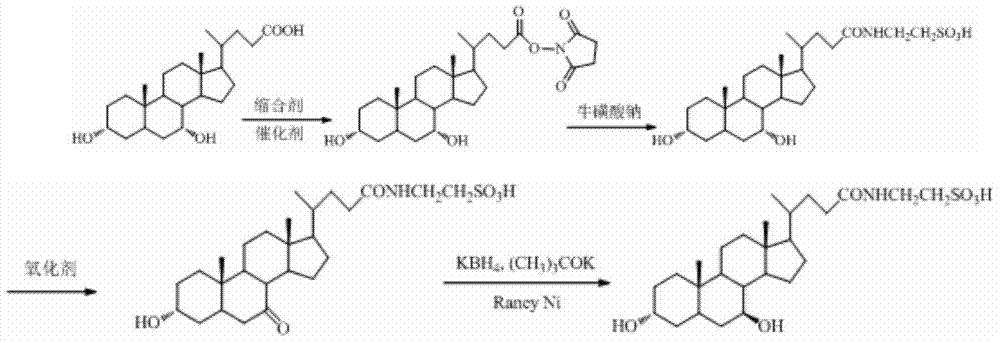
[0022] A kind of synthetic method of tauroursodeoxycholic acid, the concrete processing step that it comprises is as follows:
[0023] (1) Dissolve chenodeoxycholic acid in an organic solvent, control the reaction temperature between 2-6°C, add N-hydroxybutanediimide or N-hydroxyphthalimide, stir, and then add Condensing agent and catalyst 4-dimethylaminopyridine, stirring for 0.5-2h, adding 3% sodium taurate aqueous solution and triethylamine to the reaction solution, continuing to stir the solution for 3-4h, adding HCl to adjust the pH of the reaction solution For 1-2, stir the reaction solution for 1-2h, concentrate the solvent, filter the precipitate with suction, wash with water until neutral, and dry at 70°C to obtain the crude taurochenodeoxycholic acid. Dissolve the crude taurochenodeoxycholic acid in acetone In a mixed solvent with water, heat until completely dissolved, cool to -3-3°C, stand for crystallization for 24-48h, filter, wash the filter cake with ice water,...
Embodiment 1
[0029] A kind of synthetic method of tauroursodeoxycholic acid, its concrete processing step is as follows:
[0030](1) Dissolve 19.6g (0.05mol) of chenodeoxycholic acid in 50ml of dioxane, control the reaction temperature between 0-5°C, add 6.9g (0.06mol) of N-hydroxybutanediimine 12.38 g (0.06 mol) of dicyclohexylcarbodiimide and 0.06 g of catalyst 4-dimethylaminopyridine (1% of the raw material) were added, stirred at 10°C for 2 hours, and then added to the reaction solution. Sodium sulfonate aqueous solution (250ml, 3%) was added dropwise to triethylamine (17ml, 0.06mol), and the solution was stirred for 2h, and HCl was added to adjust the pH of the reaction solution to 1-2, the reaction solution was stirred for 1h, the solvent was concentrated, and the The precipitate was filtered, washed with water until neutral, and dried at 70°C to obtain 19.5 g of crude taurochenodeoxycholic acid, with a yield of 78%. Taurochenodeoxycholic acid crude product 18g was dissolved in 20ml...
Embodiment 2
[0034] A kind of synthetic method of tauroursodeoxycholic acid, its concrete processing step is as follows:
[0035] (1) Dissolve 19.6g (0.05mol) of chenodeoxycholic acid in 50ml of dioxane, control the reaction temperature between 0-5°C, add 10.6g (0.06 mol), stir, then add 7.56g (0.06mol) of diisopropylcarbodiimide and 0.06g (1% of raw material) of catalyst 4-dimethylaminopyridine, maintain 10 ℃ and stir for 2h, in the reaction solution Add sodium taurate aqueous solution (250ml, 3%), then drop triethylamine (17ml, 0.06mol), continue to stir the solution for 2h, add HCl to adjust the pH of the reaction solution to 1-2, stir the reaction solution for 1h, concentrate Solvent, filter the precipitate with suction, wash with water until neutral, and dry at 70°C to obtain 19.0 g of crude taurochenodeoxycholic acid, with a yield of 76%;
[0036] (2) Add 12g (0.024mol) of taurochenodeoxycholic acid into 360ml of dioxane to dissolve, stir to dissolve, add 7.5g (0.048mol) of m-chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com