Method for preparing oxo-isophorone by catalytic oxidation using metal free catalytic system
A technology for oxoisophorone and isophorone, which is applied in the field of catalyzed synthesis of oxoisophorone by a metal-free catalytic system, can solve the problems of harsh reaction conditions, corrosion, and unsatisfactory effects, and achieves high selectivity. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 3.26g of N-hydroxyphthalimide catalyst and 100ml of acetonitrile into a 250ml round-bottomed three-neck flask, put it in a water bath at 40°C, and then add 2.76g of α-isophorone after 20 minutes. 0.02g of benzoyl peroxide, then feed oxygen to react, the reaction process is monitored by gas chromatography, and ethyl benzoate is used as internal standard to calibrate, after 36h, the conversion rate of α-isophorone is measured to be 50% , the selectivity of oxyisophorone was 91%.
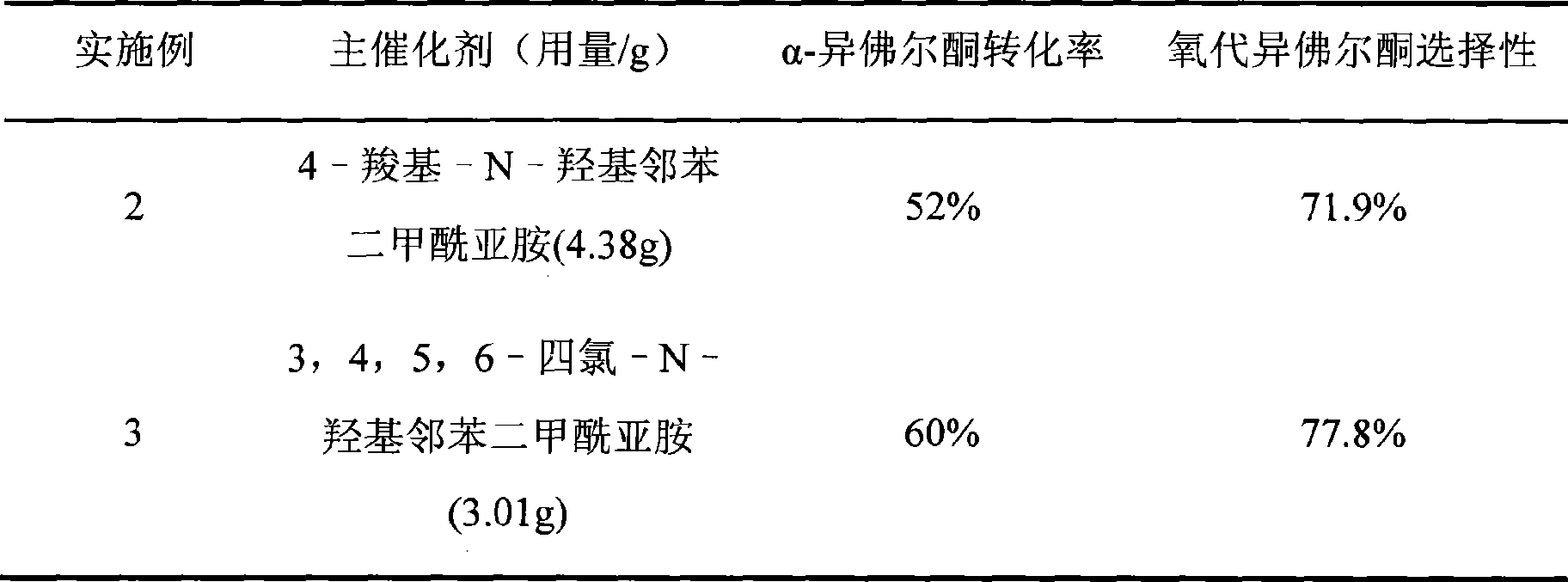
Embodiment 2-3
[0018] Similar to Example 1, the main catalyst was changed, the reaction temperature was carried out under the condition of 55°C, the reaction time was 24h, and the following results were obtained after the reaction (Table 1):
[0019] Table 1 The influence of the change of the main catalyst on the catalytic effect of a-isophorone
[0020]
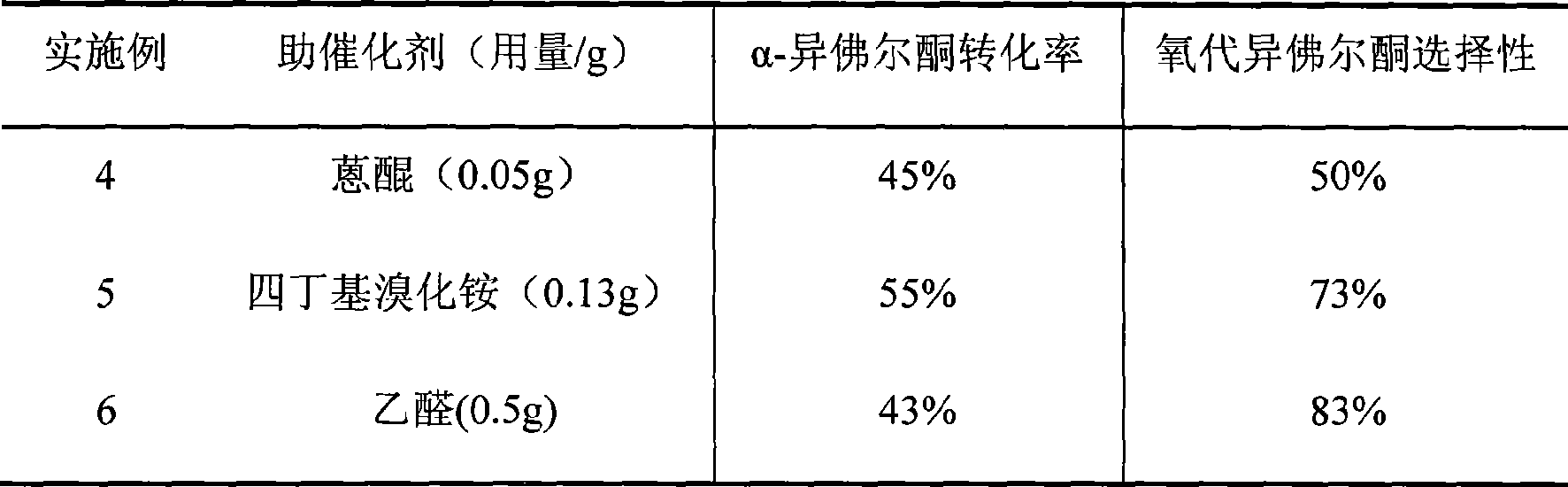
[0021] Example 4-6
[0022] Similar to Example 1, the co-catalyst was changed, the reaction temperature was carried out under the condition of 60°C, the reaction time was 24h, and the following results were obtained after the reaction (Table 2):
[0023] Table 2 The influence of the change of co-catalyst on the catalytic effect of a-isophorone
[0024]
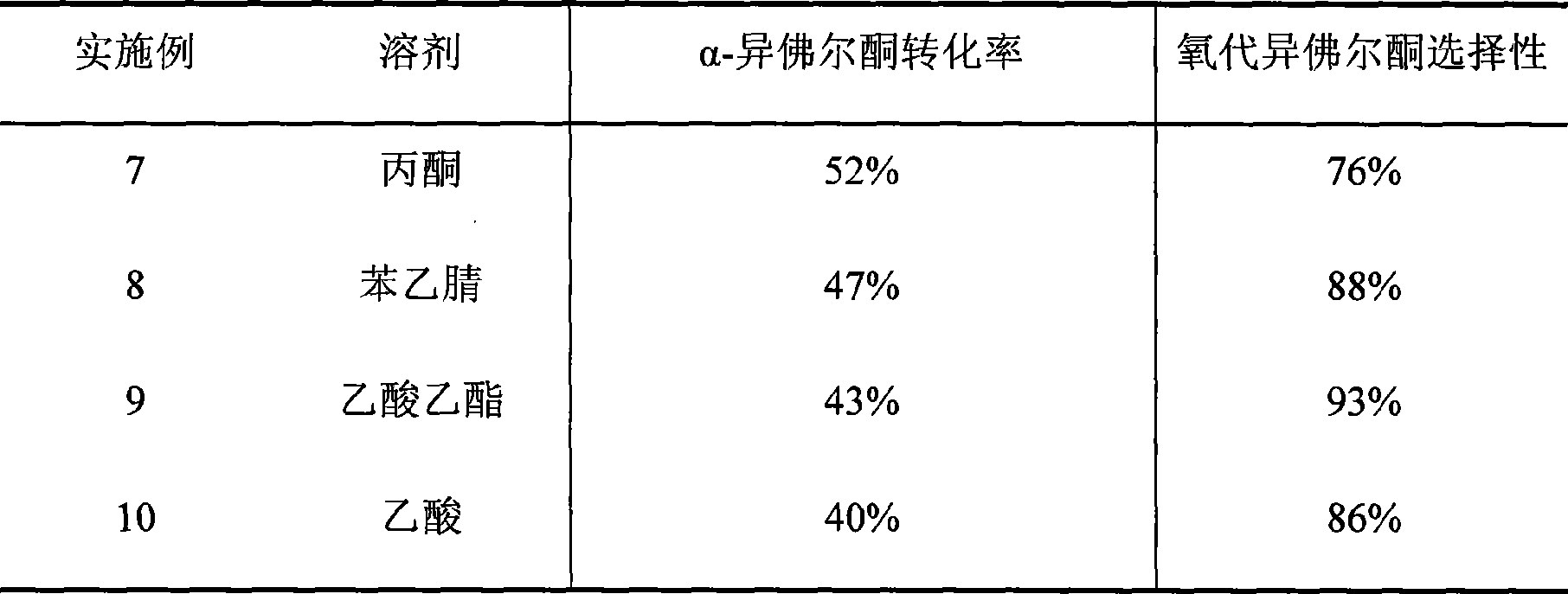
Embodiment 7-10
[0026] Similar to Example 1, the solvent was changed, the reaction temperature was 50°C, the reaction time was 24h, and the following results were obtained after the reaction (Table 2):
[0027] The influence of the change of table two solvents on the catalytic effect of a-isophorone
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com