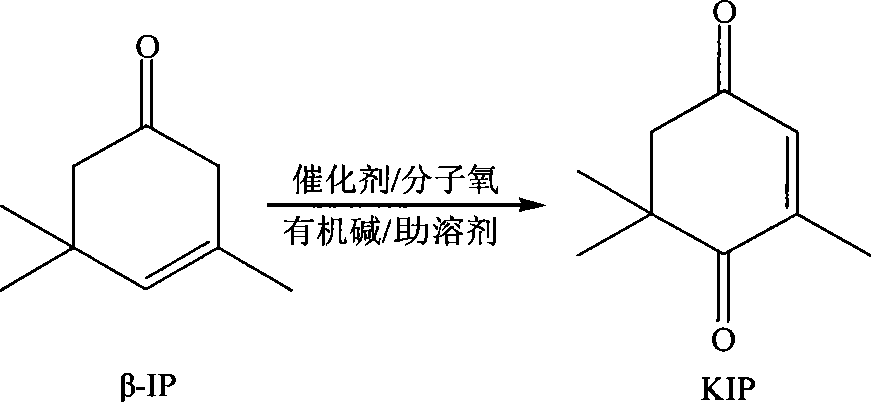
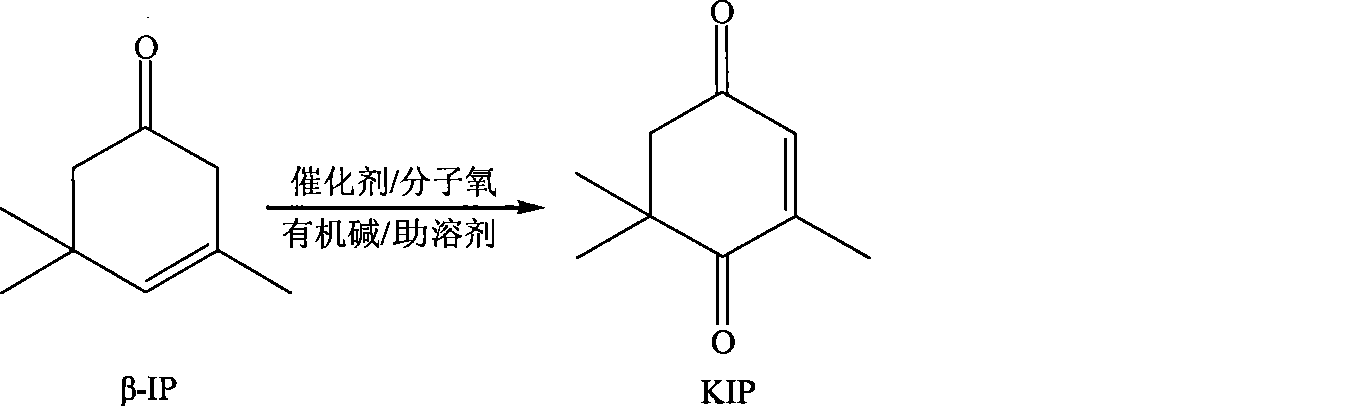
Method for preparing oxo-isophorone by catalytic oxidation using metal free catalytic system
A metal-free catalytic technology for oxyisophorone, which is applied in the field of catalytic oxidation of metal-free catalytic systems to prepare oxoisophorone, can solve the problems of reduced reaction selectivity, catalyst poisoning, and high price, and achieve easy separation and The effect of control, mild reaction conditions, and easy reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.82g of N-hydroxyphthalimide catalyst and 40ml of acetone into a 50ml round bottom four-neck flask, put it in a water bath at 60°C, and then add 6.9g of β-isophorone after 20 minutes , 0.02g of benzoyl peroxide, then pass into oxygen to react, the reaction process is monitored by gas chromatography, and ethyl benzoate is used as the internal standard to calibrate, and the conversion rate of β-isophorone measured after 20h is 93 %, the selectivity of oxyisophorone is 88.5%.
Embodiment 2
[0029] Add 1.46g of 4-alkoxyformyl-N-hydroxyphthalimide catalyst and 40ml of acetone into a 50ml round bottom four-neck flask, put it in a water bath at 60°C, and then add 6.9g after 20 minutes β-isophorone, 0.02g of o-phenanthroline, and then pass into oxygen to react, the reaction process is monitored by gas chromatography, and ethyl benzoate is used as internal standard for calibration, and β-isophorone is measured after 20h The conversion rate of ketone was 97%, and the selectivity of oxyisophorone was 78.5%.
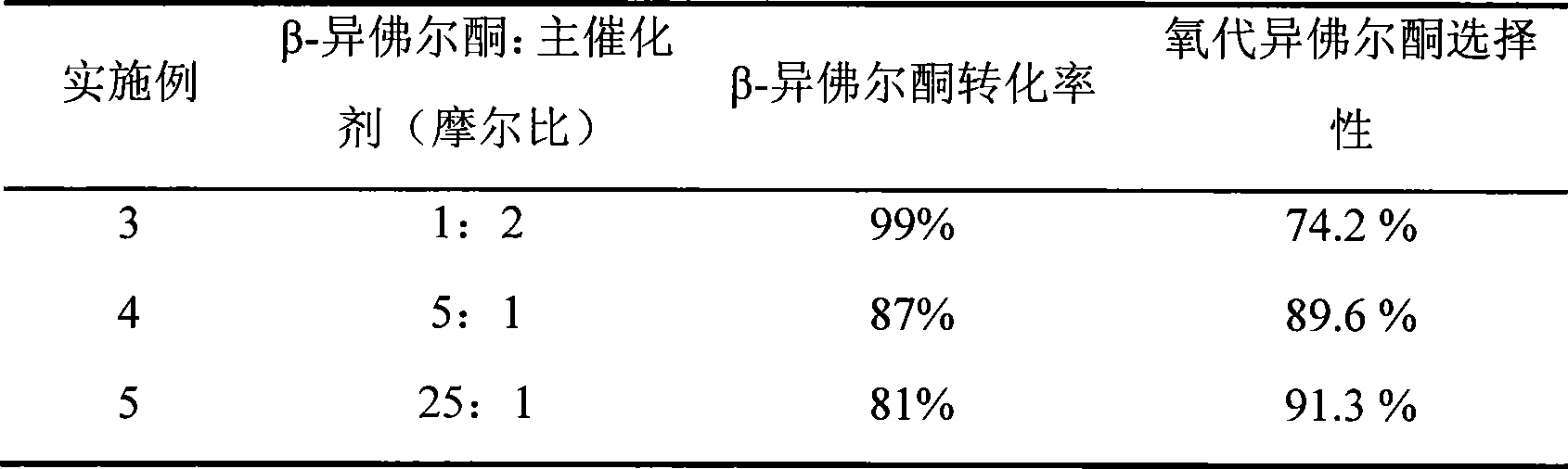
Embodiment 3-5
[0031] Similar to Example 1, the reaction temperature is 60°C, the reaction time is 20h; the molar ratio of β-isophorone and N-hydroxyphthalimide is changed, and the following table is obtained after the reaction:
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com