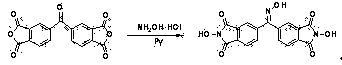Synthesis and application of novel catalyst used in preparation of arone by carrying out catalytic oxidation on ethylbenzene and derivative of ethylbenzene
A catalytic oxidation and catalyst technology, applied in the direction of organic compound/hydride/coordination complex catalyst, carbon-based compound preparation, organic compound preparation, etc., can solve environmental pollution and other problems, achieve reduced production costs and high conversion rate , cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation and characterization of the above catalysts
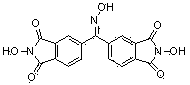
[0037] The preparation method of the above-mentioned catalyst I is as follows: 3,3',4,4'-benzophenone tetracarboxylic dianhydride is used as raw material, and it is mixed with hydroxylamine hydrochloride at 115°C, DMF is used as a solvent, and pyridine is used as an acid-binding agent. The reaction was carried out for three hours, and then the pyridine hydrochloride formed by the reaction was filtered, recrystallized from acetonitrile, and then the target product was separated by plate chromatography. Pale yellow solid with a purity of 97% and a yield of 75%. The NMR characterization of the prepared catalyst is as follows: 1 HNMR (d-DMSO, ppm): 12.255 (1H, C=N-OH), 10.901~10.920 (2H, N-OH), 7.450~8.042 (6H, Ph).
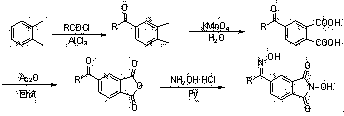
[0038] The preparation method of the above-mentioned catalyst II is similar to that of I, that is, using ortho-xylene as the starting material, dimethylphenone is obtained through the Friedel-Crafts a...
Embodiment 1
[0041]Put 2 mL (16 mmol) of ethylbenzene, 10 mL of acetonitrile, and 10 mol% of catalyst I into a 70 mL stainless steel polytetrafluoroethylene sealed autoclave, replace the air in the autoclave with oxygen three times, and seal it. Turn on the stirring, and the oil bath heating starts to heat up. After reaching the set temperature of 80°C, inject oxygen to 0.3 MPa, and start timing. During the reaction process, if the pressure drops, supplement oxygen at any time to keep the oxygen pressure at 0.3MPa. After reacting for 10 h, it was left to cool to room temperature, and the reactor was depressurized, and the oxidation product was analyzed by GC. In this example, the separation of the liquid phase mixture and the purification of acetophenone adopt conventional vacuum distillation method, and acetophenone with a purity of 99.0% can be obtained. The conversion rate of ethylbenzene was 80.5%, the selectivity of acetophenone was 92.3%, and the yield was 74.3%.
Embodiment 2
[0043] The operation method is the same as in Example 1, except that acetonitrile is changed to acetone, the conversion rate of ethylbenzene is 42.3%, the selectivity of product acetophenone is 90.1%, and the yield is 38.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com