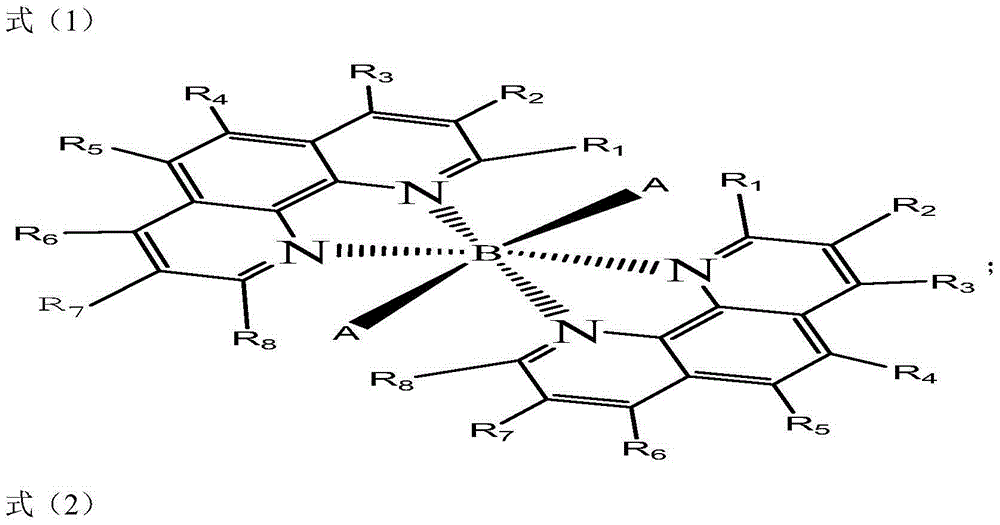
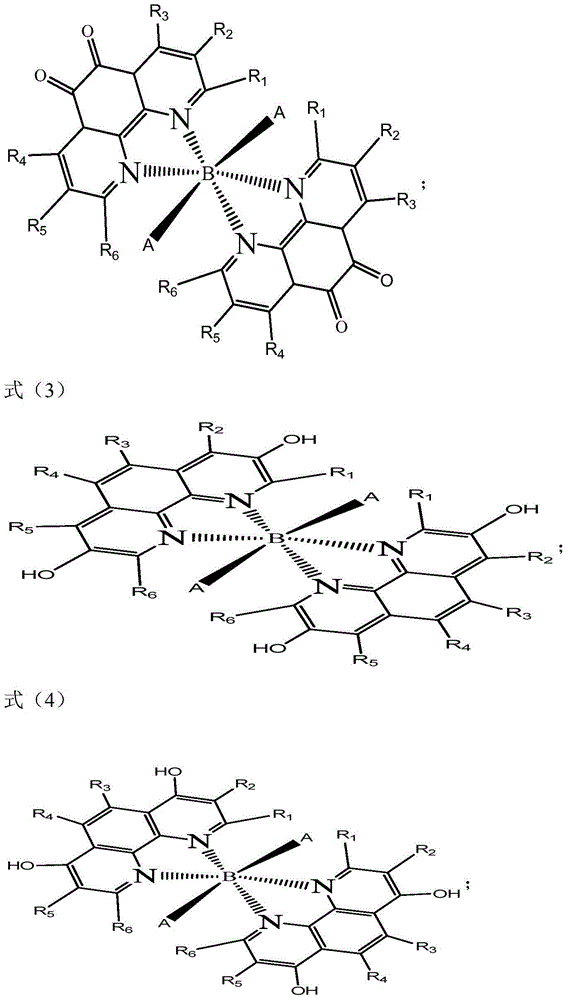
Method for preparing benzaldehyde by selective oxidation of methylbenzene
A selectivity and benzaldehyde technology, which is applied in the direction of hydrocarbon oxidation to prepare oxygenates, carbon-based compounds, chemical instruments and methods, etc., can solve the problem of low selectivity of benzaldehyde, achieve mild reaction conditions, high yield, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of cocatalyst in the present embodiment:
[0023] Add 2.5 grams (0.01mol) of 3,8-dichloro-1,10-phenanthroline, 1.7 grams (0.01mol) of copper chloride dihydrate, 50 mL of absolute ethanol, and 5 mL of water into the reaction flask, and heat to reflux for 5 hours , static, precipitated solids, and dried the solids to obtain 3.0 grams of co-catalysts.
[0024] Catalytic Oxidation of Toluene:
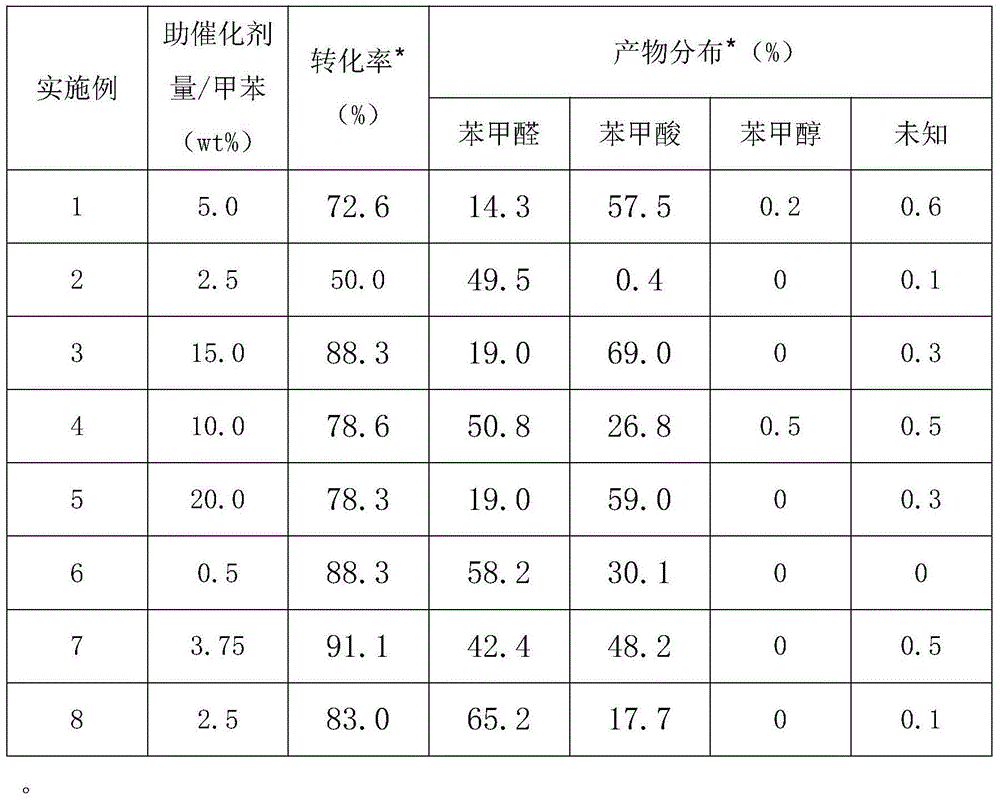
[0025] Add 50mL toluene and 50mL acetonitrile, 2.0 grams of N-hydroxyphthalimide, 2.0 grams of the cocatalyst prepared above, feed oxygen into the reaction kettle, keep the pressure of the reaction kettle at 0.3MPa, and the reaction temperature at 80°C. The time was 360 minutes to obtain the reaction product. After detection by high-pressure liquid chromatography, the benzaldehyde in the product was 14.3%, the benzoic acid was 57.5%, and the benzyl alcohol was 0.2%. The reaction results are shown in Table 1.
Embodiment 2
[0027] The synthesis of cocatalyst in the present embodiment:
[0028] Add 3.3 grams (0.01mol) of 4,7-diphenyl-1,10-phenanthroline, 2.4 grams (0.01mol) of cobalt chloride hexahydrate, 50 mL of acetone, and 10 mL of water into the reaction flask, and heat to reflux for 5 hours. After standing still, a solid precipitated out, and after the solid was dried, 3.5 g of cocatalyst was obtained.
[0029] Catalytic Oxidation of Toluene:
[0030] Add 50mL toluene and 5mL anhydrous acetic acid, 2.0 grams of N-hydroxyphthalimide, 1.0 gram of the cocatalyst prepared above, feed oxygen into the reactor, keep the pressure of the reactor at 0.1MPa, and the reaction temperature at 120 ℃, the reaction time is 120 minutes, promptly obtains the reaction product, detects through high-pressure liquid chromatography, and benzaldehyde is 49.5% in the product, and benzoic acid is 0.4%, and the product obtains 9.7 grams of benzaldehyde through purification separation, and reaction result is shown in T...
Embodiment 3
[0032] The synthesis of cocatalyst in the present embodiment:
[0033] Add 3.7 grams (0.01mol) of 3,8-dibromo-1,10-phenanthroline-5,6-dione, 1.7 grams (0.01mol) of manganese acetate, 40mL of anhydrous acetic acid, 10mL of water into the reaction flask, Heated to reflux for 10 hours, stood still, and a solid precipitated out. After the solid was dried, 2.9 g of a cocatalyst was obtained.
[0034] Catalytic Oxidation of Toluene:
[0035] Add 50mL of toluene and 100mL of dichloroethane, 2.0 grams of N-hydroxyphthalimide, 0.4 grams of the above-mentioned prepared cocatalyst into the reactor, feed oxygen, keep the pressure of the reactor at 1.5MPa, and the reaction temperature is 150°C. The reaction time was 480 minutes, and the reaction product was obtained. The benzaldehyde in the product was 19.0%, and the benzoic acid was 69.0% through high-pressure liquid chromatography detection. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com