Method for increasing yield of amikacin
A technology of star yield and amikacin, which is applied in the field of amikacin preparation, can solve the problems of multiple reaction steps, low selectivity of acylation reaction, and complicated post-treatment methods, so as to achieve improved synthesis yield and simplified treatment process , Reduce the effect of material degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
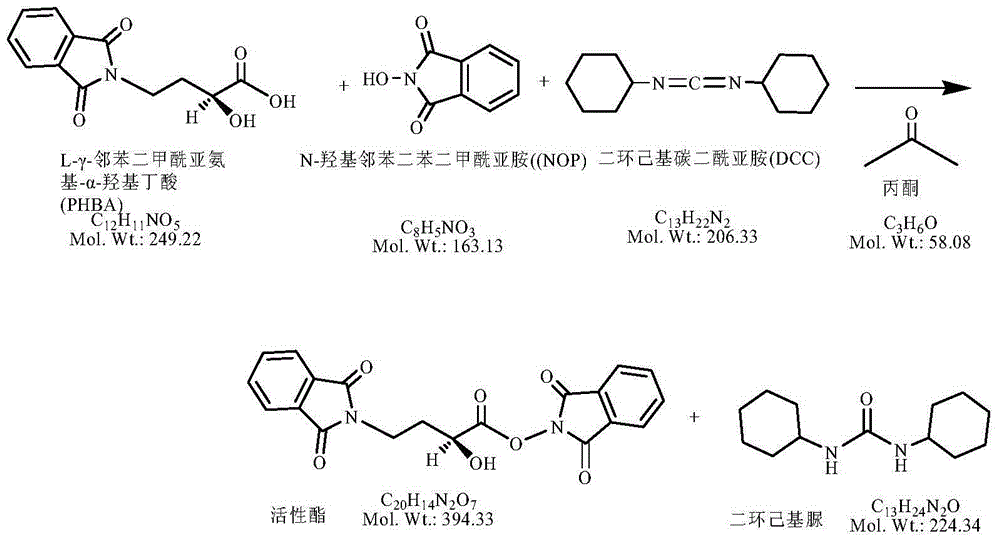
[0041] (1) Weigh 5.96g of AHBA (50.0 millimoles), add 300ml of acetone, add 12.94g of fluorenyl methoxycarbonyl chloride (50.0 millimoles) dropwise, reflux with nitrogen and react for 2 hours. After the plate reaction is complete, place it for later use ;
[0042] (2) Add 8.16g of NOP (50.0 millimoles) and 8.26g of DCC (50.0 millimoles) to the above reaction solution, and react at 20-25°C for 2 hours, and then cool to room temperature after the plate is finished;
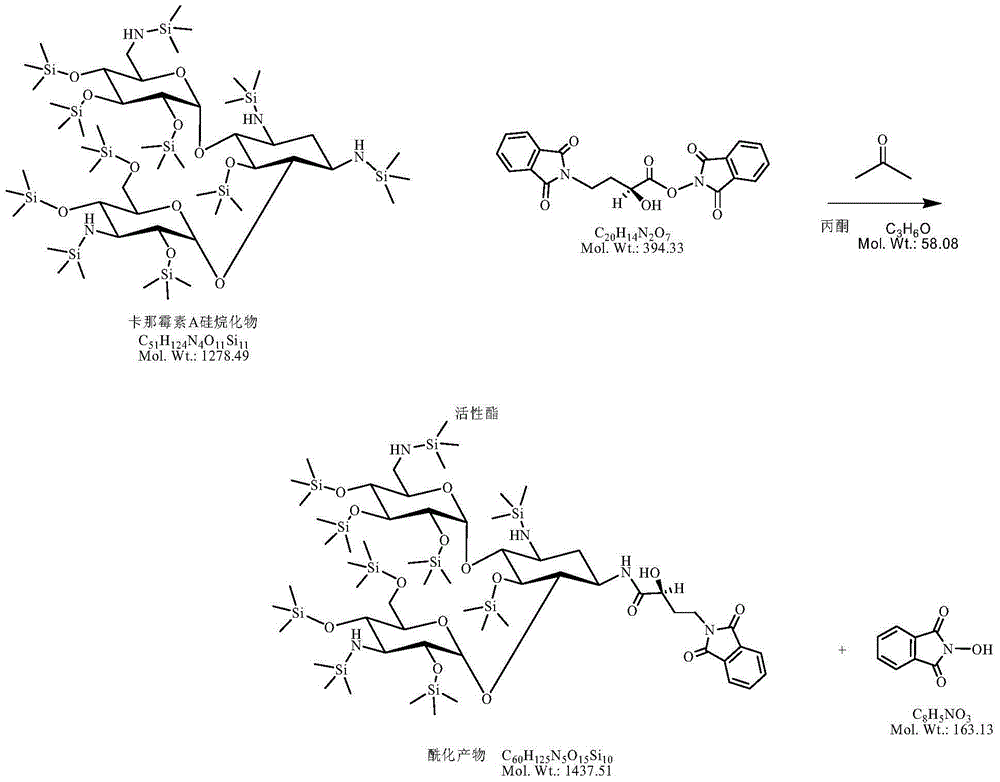
[0043] (3) Add 63.93g of kanamycin A silanide (50.0 millimoles) to the above reaction solution, control the temperature at 10-15°C and react for 2 hours, and the plate reaction is completely ready for use;
[0044] (4) Add 40% hydrobromic acid in the previous step to adjust the pH to 2.0-3.0; remove the solid impurities by suction filtration, and evaporate the solvent under reduced pressure; add concentrated ammonia water to adjust the pH to 7-8, and separate on the CD180 resin column. -3.0mol / L ammonia water was subjecte...
Embodiment 2
[0045] (1) Weigh 5.96g of AHBA, add 300ml of acetone, add 13.00g of fluorenyl methoxycarbonyl chloride dropwise, and react under reflux with nitrogen for 2 hours. After the plate reaction is complete, place it for use;
[0046] (2) Add 8.20g NOP and 8.30gDCC to the above reaction solution, and react at 25-30°C for 1.5 hours. After the platen is finished, the temperature is reduced to room temperature;
[0047] (3) Add 63.93g of kanamycin A silanide to the above reaction solution, control the temperature at 15-20°C and react for 1.5 hours, the spotting reaction is complete and ready for use;
[0048] (4) Add 40% hydrobromic acid in the previous step to adjust the pH to 2.0-3.0; remove the solid impurities by suction filtration, and evaporate the solvent under reduced pressure; add concentrated ammonia water to adjust the pH to 7-8, and separate on the CD180 resin column. -3.0mol / L ammonia water was subjected to gradient release to obtain pure amikacin feed liquid, concentrated to 30° ...
Embodiment 3
[0049] (1) Weigh 5.96g AHBA, add 200ml acetone, add 13.58g fluorenyl methoxycarbonyl chloride dropwise, and reflux with nitrogen for 1.5 hours. After the plate reaction is complete, place it for later use;
[0050] (2) Add 8.56g NOP and 10.83g DCC to the above reaction solution, control the temperature at 20-25℃ and react for 2 hours, and then cool to room temperature after the plate is finished;
[0051] (3) Add 63.93g of kanamycin A silanide to the above reaction solution, control the temperature at 10-15°C and react for 2 hours, the spotting reaction is complete and ready for use;
[0052] (4) Add 35% hydrobromic acid in the previous step to adjust the pH to 2.0-3.0; remove the solid impurities by suction and evaporate the solvent under reduced pressure; add concentrated ammonia water to adjust the pH to 7-8, and separate on the CD180 resin column to 0.1 -3.0mol / L ammonia water was subjected to gradient release to obtain pure amikacin material liquid, concentrated to 30° of optica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com