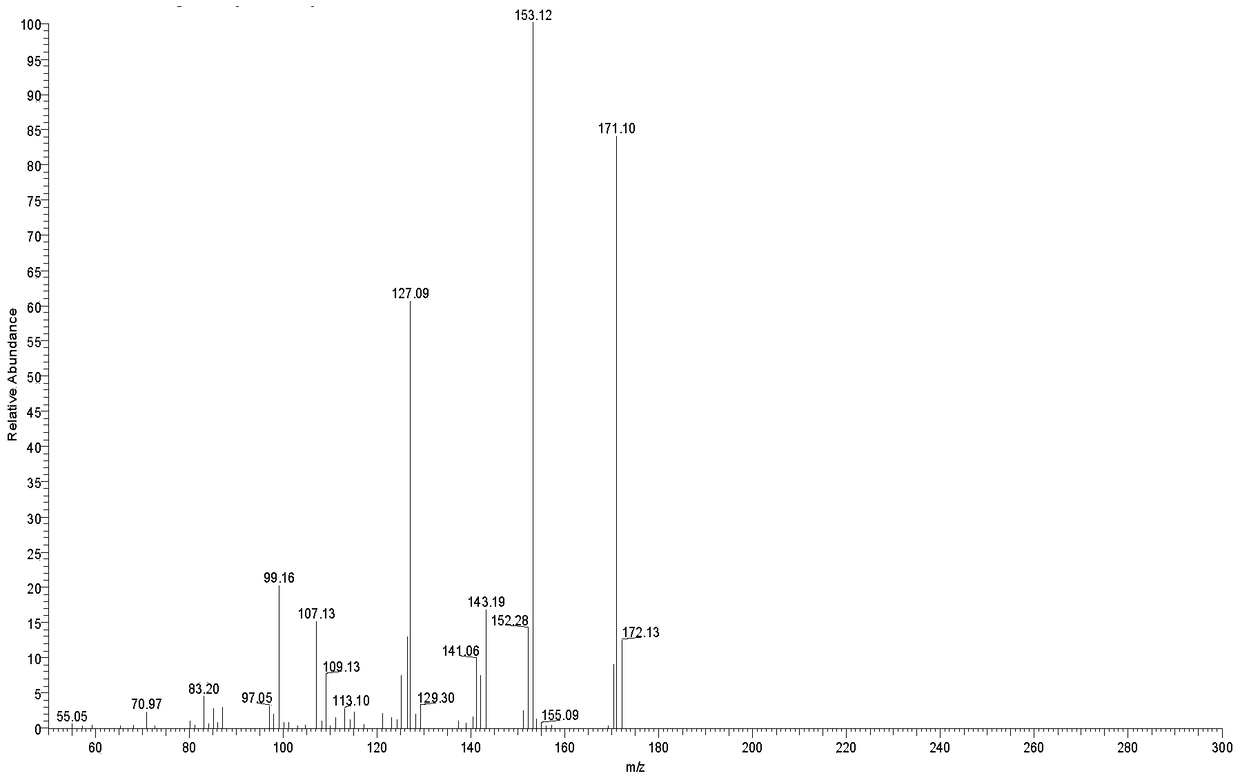
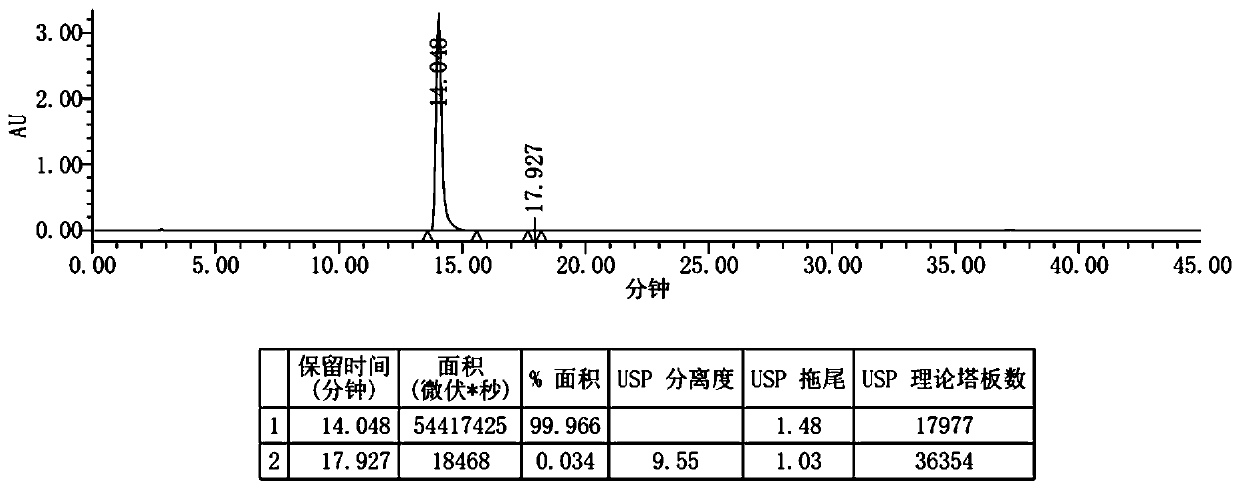
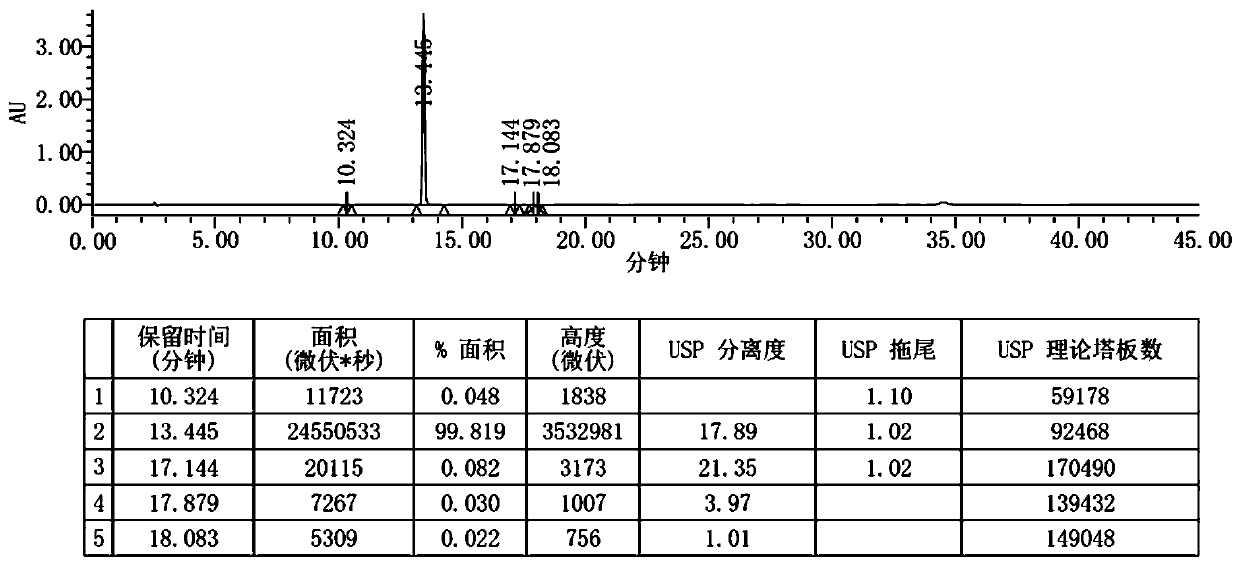
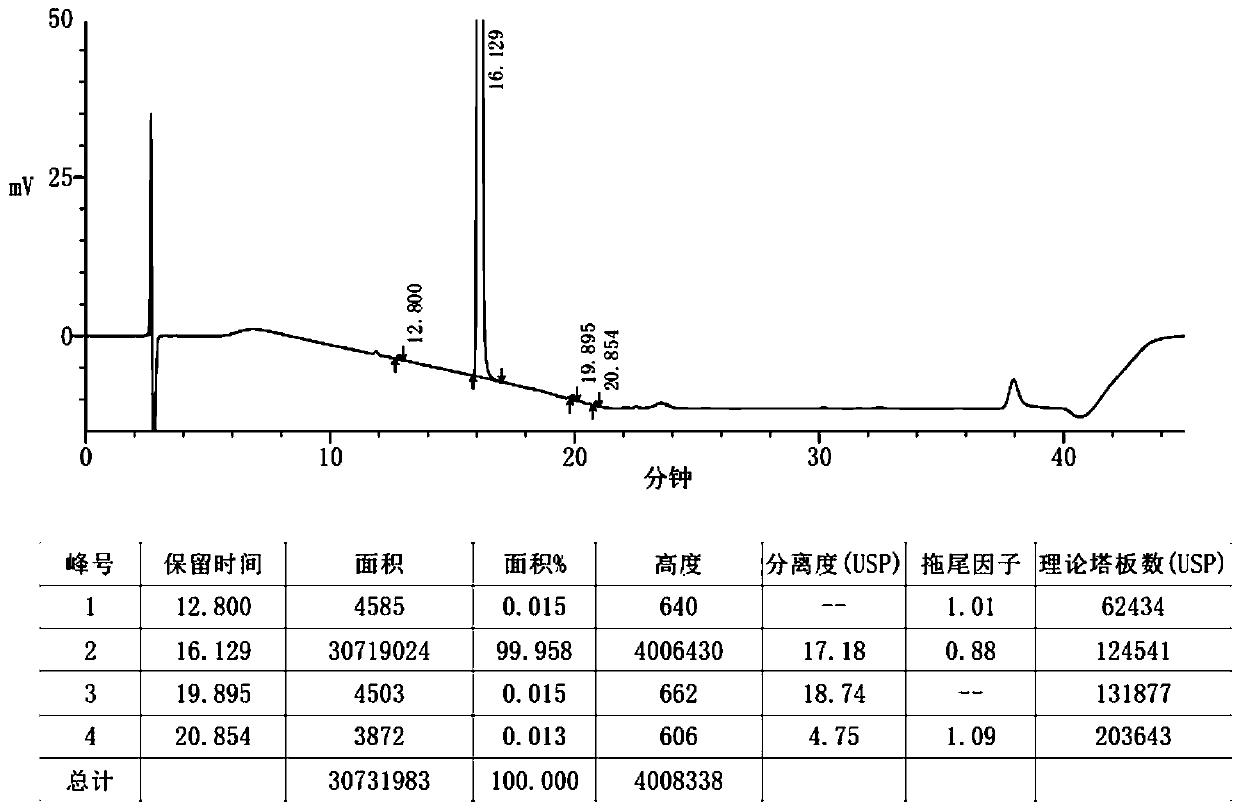
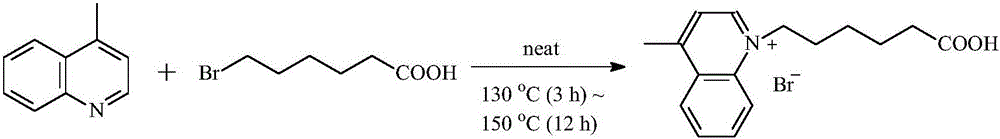Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Methyl tosylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
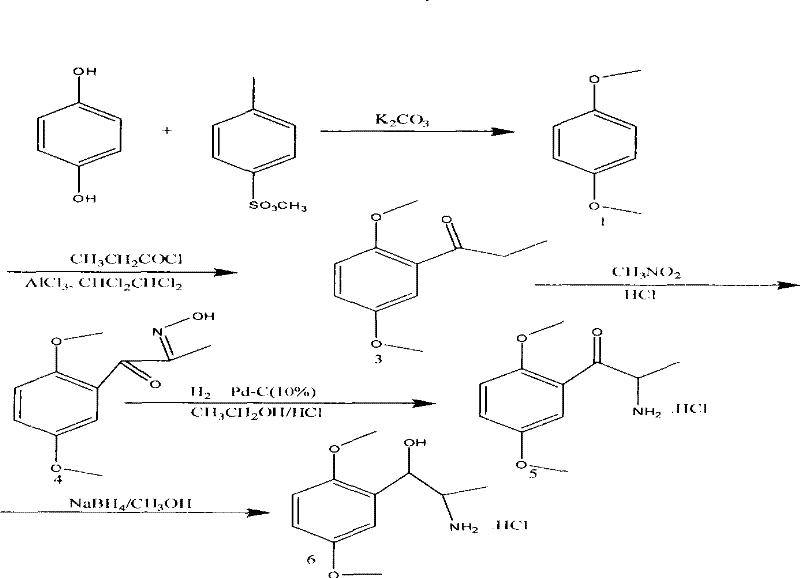
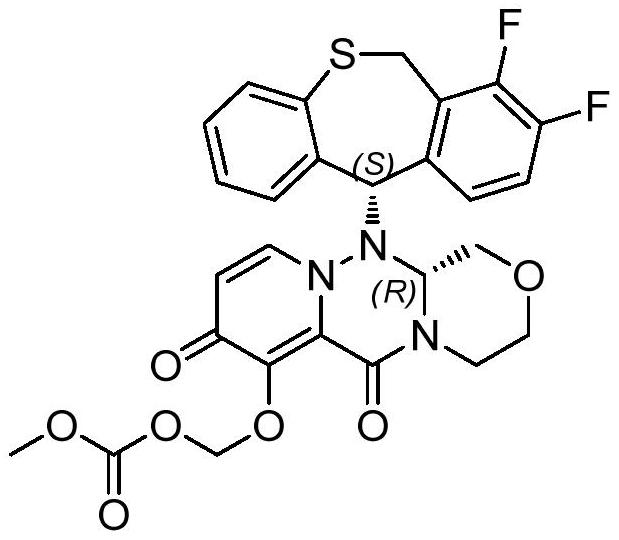
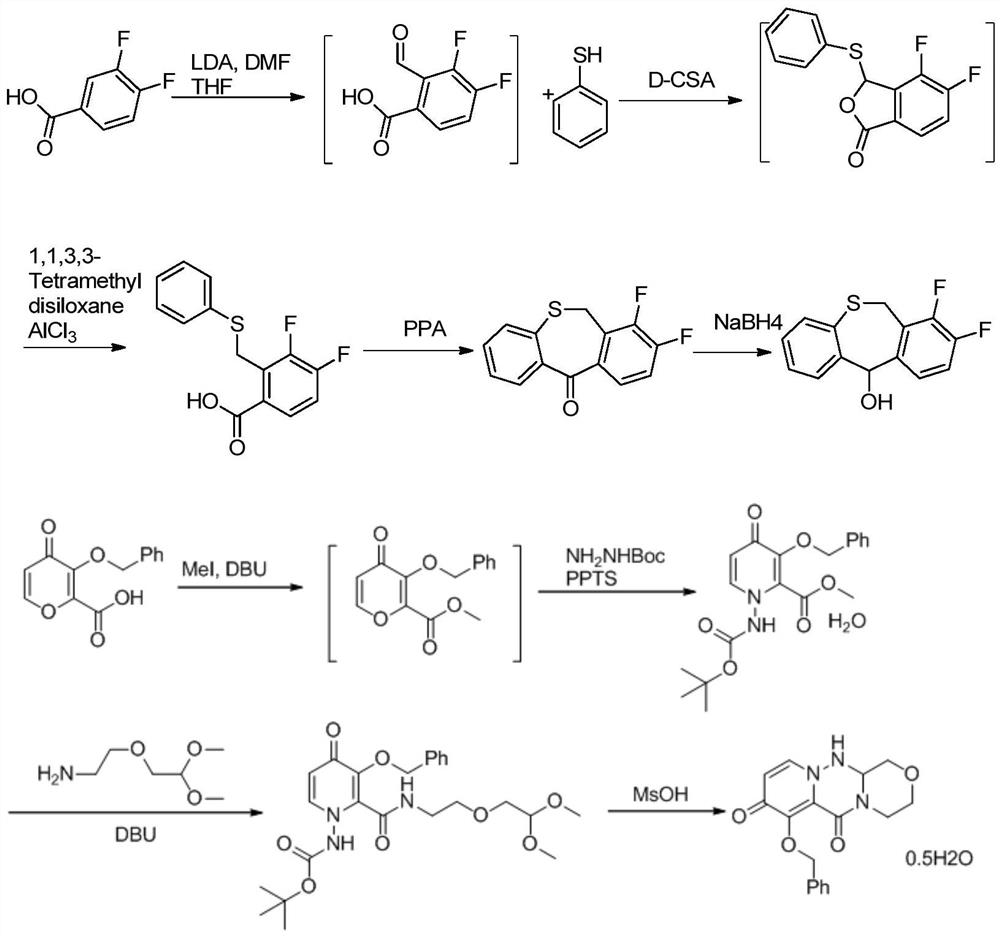
Method for synthesizing novel anti-influenza drug
ActiveCN109504721AReduced operating requirementsReduce negative impactOrganic chemistryFermentationThiosalicylic acidAnti influenza drug
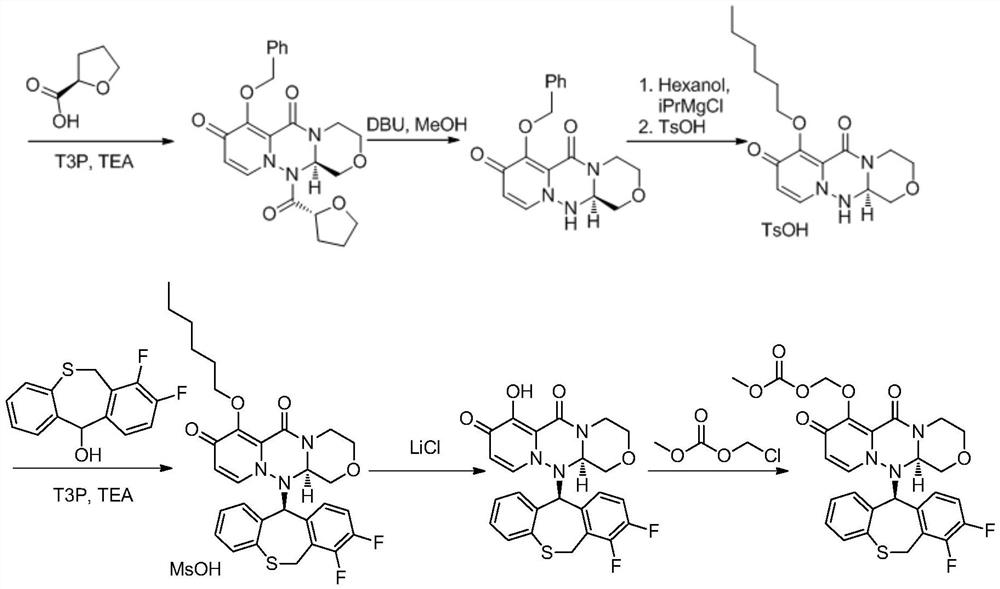
The invention discloses a method for synthesizing a novel anti-influenza drug baloxavir marboxil. The method comprises the following steps: directly docking a thiosalicylic acid compound 1 and a 1-(halogenated methyl)-2,3-difluorobenzene compound 2 serving as initial raw materials so as to obtain a compound 3; performing PPA ring closure to obtain a 7,8-difluorodibenzo[b,e]thiozepine-11(6H)-one compound 4, and obtaining a key chiral thiazem intermediate compound 5 under catalysis of a chiral enzyme; directly condensating the compound 5 and a key chiral fragment compound 6 by virtue of a Mitsubobu reaction so as to obtain a compound 7; finally performing dealkylation protection, and condensating with ((methoxycarbonyl)oxo)4-methyl-toluenesulfonate, so as to obtain the final product compound9, namely the baloxavir marboxil. According to the synthetic route, the process operation magnification difficulty of the route is reduced, the production of by-products is reduced, the product purity is improved, and the cost of the route is reduced.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
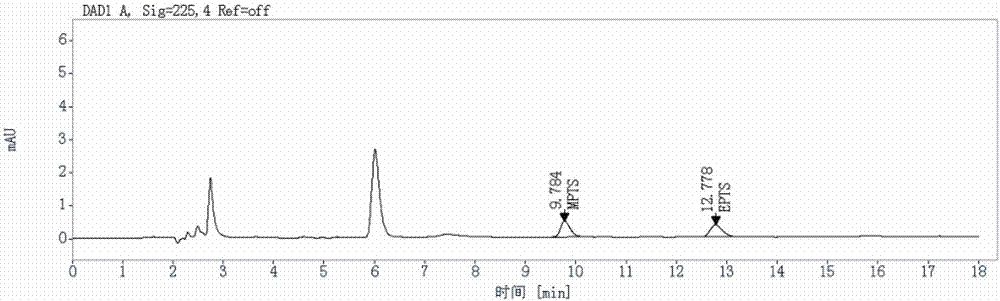
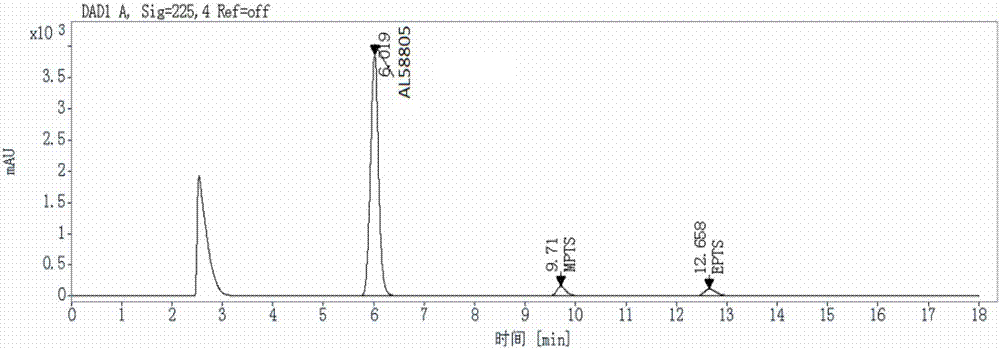
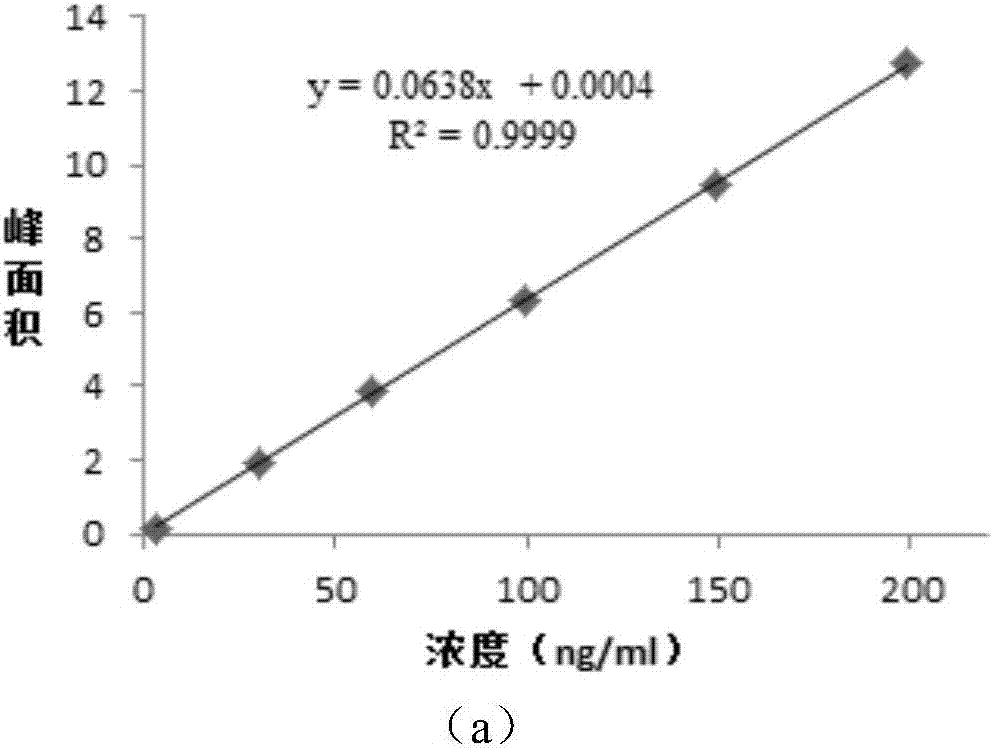
Method for detecting genotoxic impurities in AL58805 bulk drug or medicinal preparation by using high-performance liquid chromatography
ActiveCN107037153AEfficient separationDo not interfere with detectionComponent separationPhosphoric acidColumn temperature
The invention relates to a method for detecting genotoxic impurities in an AL58805 bulk drug or medicinal preparation by using high-performance liquid chromatography. The method is directed at methyl p-toluenesulfonate (MPTS) and ethyl p-toluenesulfonate (EPTS); a sample undergoes pre-treatment at first; and then high-performance liquid chromatography is employed for determination, wherein a C18 chromatographic column is employed; detection wavelength is 225 nm; an aqueous acetonitrile-phosphoric acid solution is used as a mobile phase; flow velocity is 0.8 to 1.2 mL / min; column temperature is 30 to 40 DEG C; a sample size is 20 [mu]L; and elution time is 18 min. Results show that the detection limit of MPTS is 0.3 ppm and the limit of quantitation of MPTS is 1 ppm; and the detection limit of EPTS is 0.5 ppm and the limit of quantitation of EPTS is 1.4 ppm. The method has the advantages of good specificity, simple operation, high sensitivity and accurate results and is applicable to determination of MPTS and EPTS in AL58805.
Owner:常州佳德医药科技有限公司
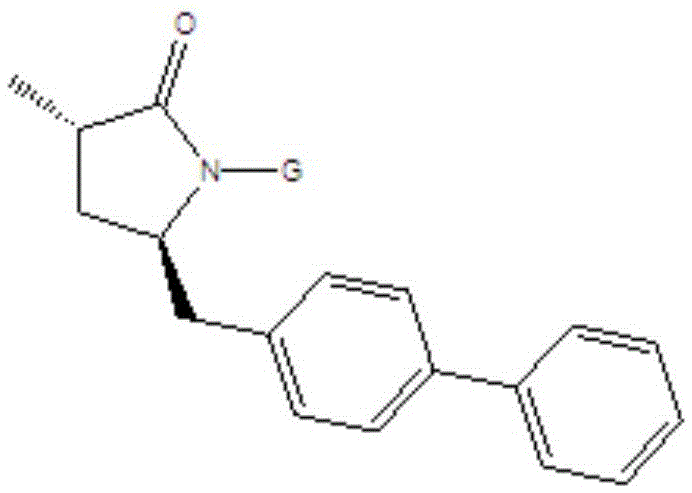
Preparation method for Sacubitril intermediate
InactiveCN105566194AHigh purityHigh yieldOrganic chemistry methodsPhenylmagnesium bromideMethyl methanesulfonate
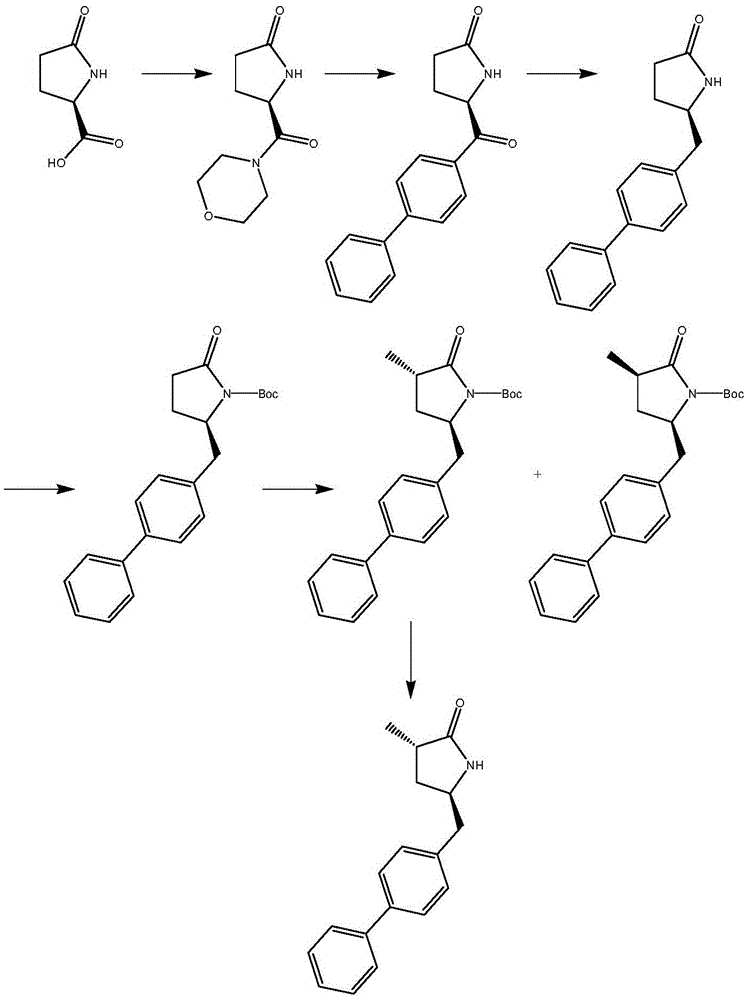
The invention relates to a preparation method for a Sacubitril intermediate. The preparation method comprises the following steps that (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone is esterified with toluene sulfochloride or methanesulfonyl chloride to obtain (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone; (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone is coupled with 4-diphenylmagnesium bromide or 4-diphenylmagnesium chloride to obtain (3R,5S)-3-methyl-5-(1,1'-diphenyl-4-yl-methyl)-2-pyrrolidinone. According to the preparation method, the method is novel, the raw materials are easy to obtain, the technology is simple, and the purity and yield of the product are both very high.
Owner:张伯引
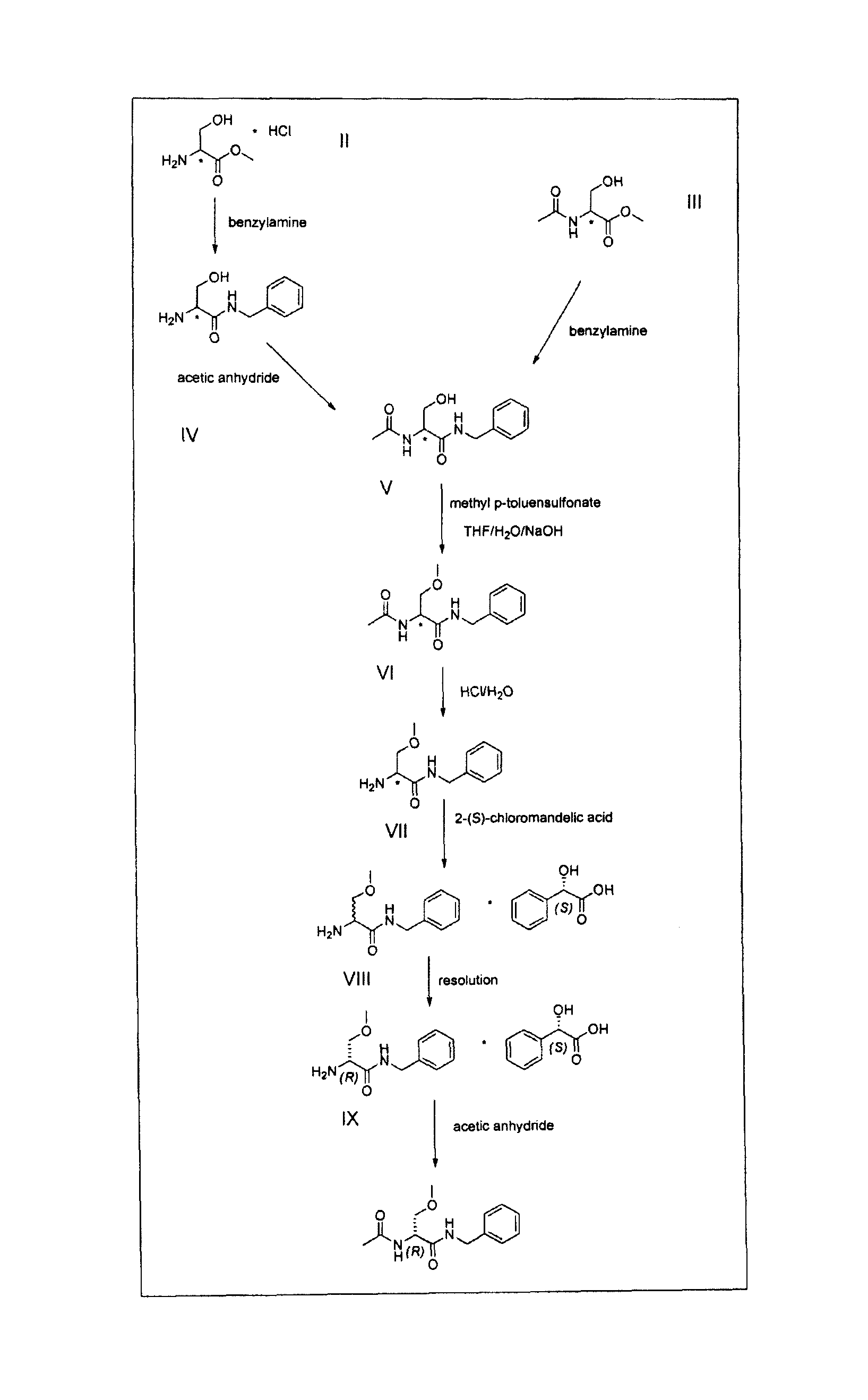
Process for the synthesis of lacosamide
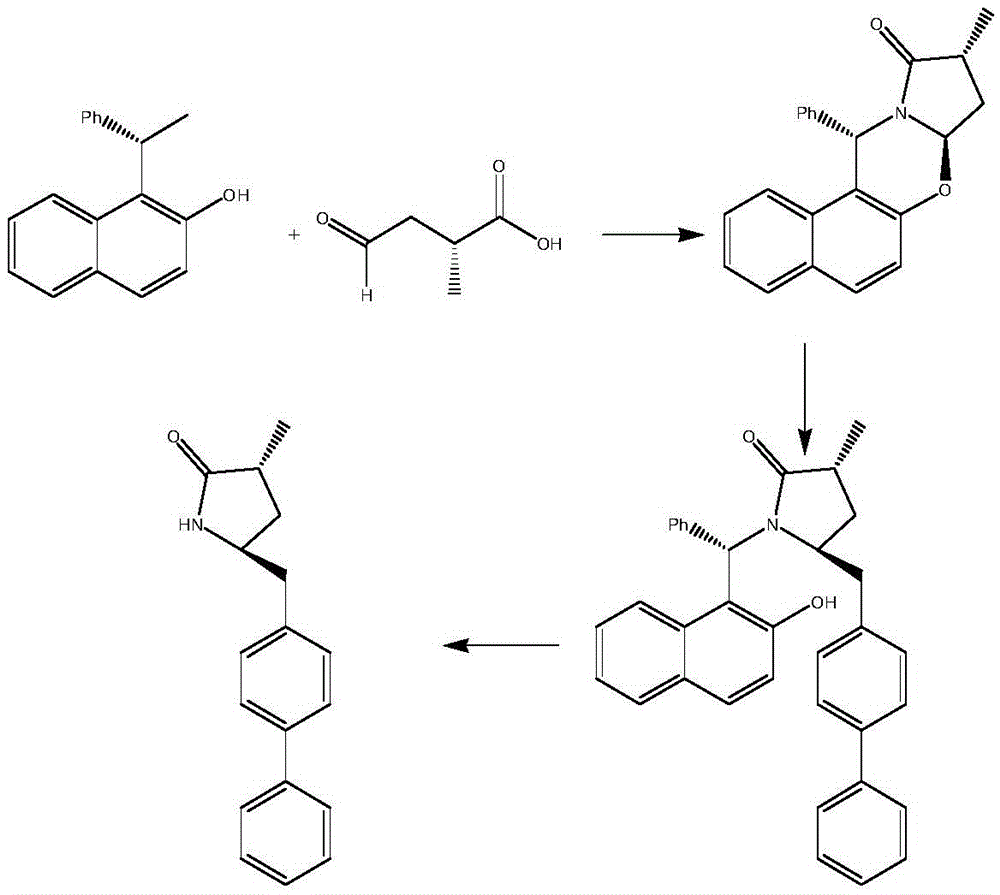
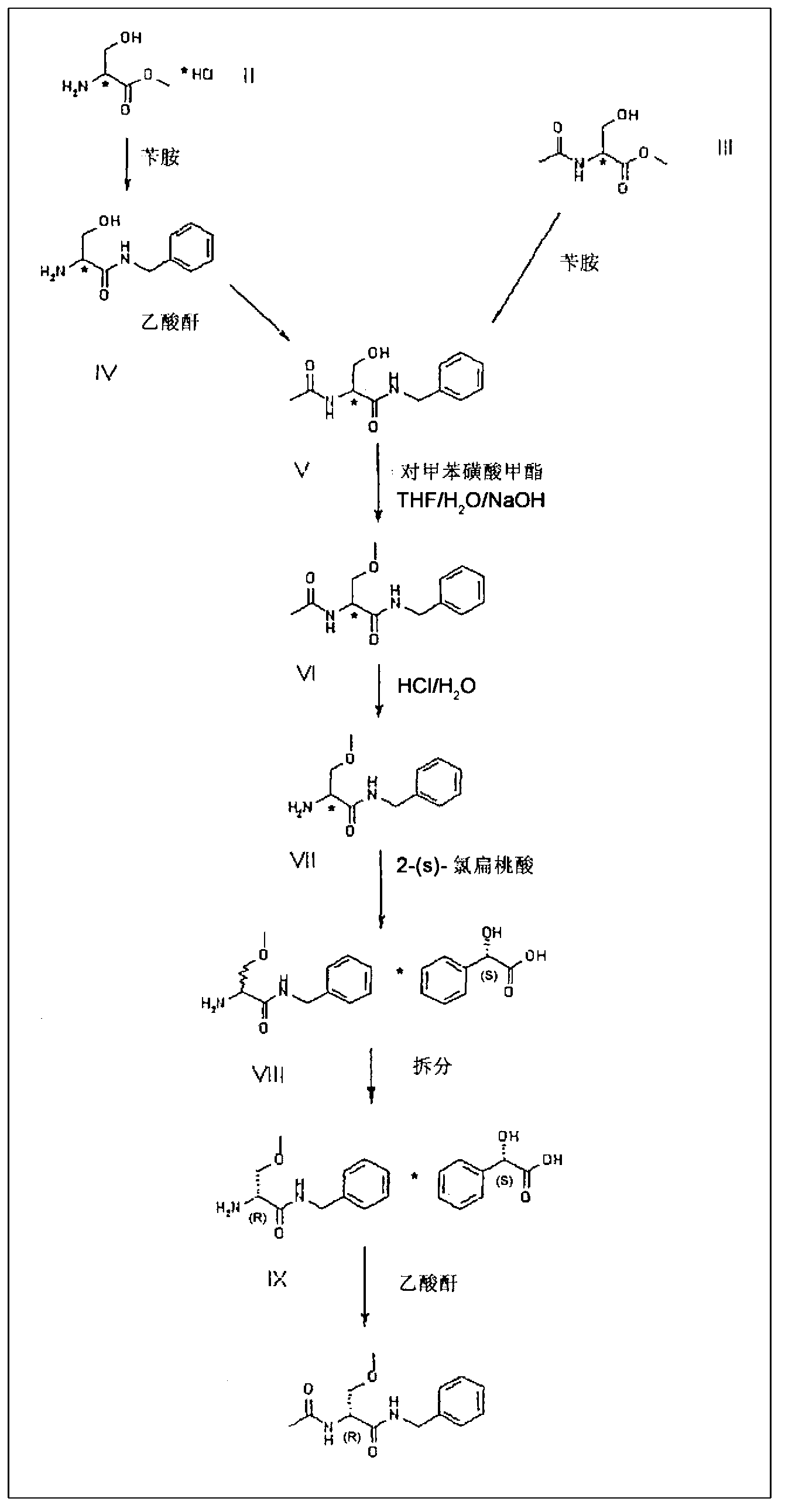
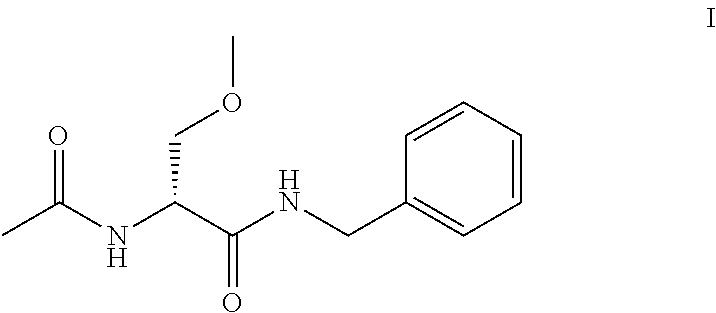
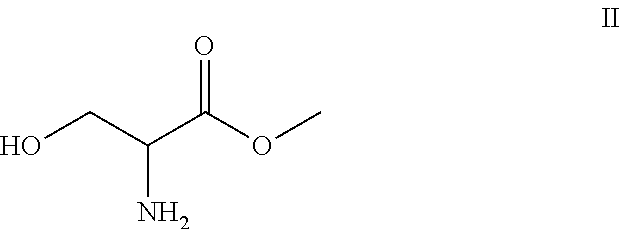
A novel process for the synthesis of Lacosamide using D,L-serine as starting material is described, where the methylation reaction of hydroxyl is carried out using an inexpensive base such as NaOH and an inexpensive alkylating agent, non-toxic and non-carcinogenic, such as methyl p-toluenesulfonate; the R enantiomer is isolated from the racemic mixture of Lacosamide after selective hydrolysis of the acetamide, salification of the racemic mixture with a chiral acid (HX*) in an organic solvent, resolution of the diastereoisomeric mixture, preferably by precipitation of the R enantiomer, and subsequent acetylation of the optically pure intermediate.
Owner:EUTICALS
High-yield high-purity DAST source powder synthetic process
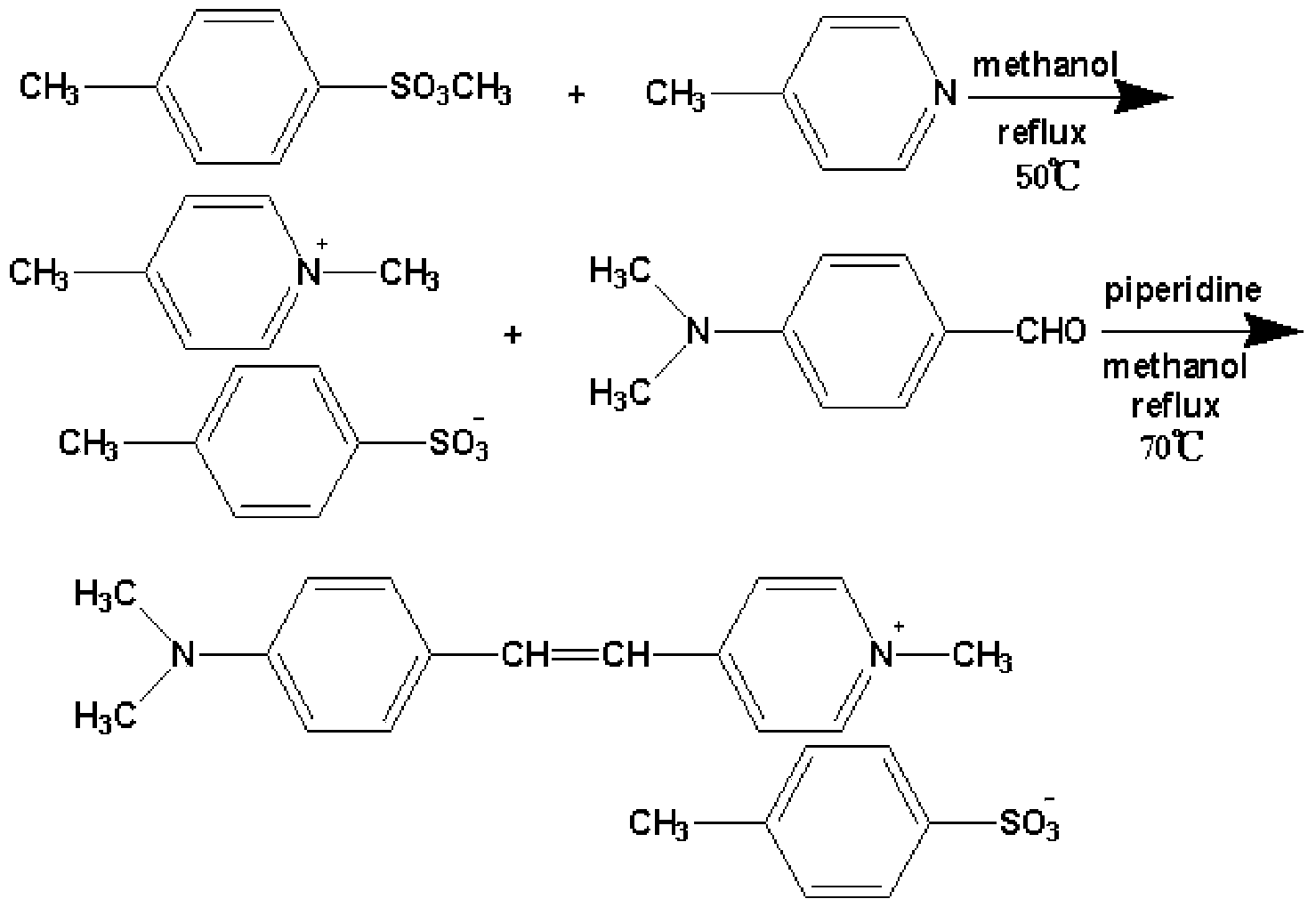
The invention discloses a high-yield high-purity DAST source powder synthetic process. The synthetic process comprises the following two steps: 1, reacting 4-methylpyridine with methyl p-toluenesulfonate in the presence of absolute ethyl alcohol serving as a solvent to obtain an absolute ethyl alcohol solution of 4-methyl-N-methylpyridine tosilate; and 2, reacting 4-methyl-N-methylpyridine tosilate with p-dimethylaminobenzaldehyde in the presence of absolute ethyl alcohol serving as a solvent and under the catalytic action of di-n-butylamine or piperidine, so as to obtain high-yield (85-95%) high-quality (90-95%) DAST source powder. According to the synthetic process, absolute ethyl alcohol is used as a reaction solvent, harm of poisonous and harmful solvents such as methylbenzene and methyl alcohol to the body of an operator can be avoided and the pollution of waste liquid to the environment can be avoided. The research success of the high-yield high-purity DAST source powder synthetic process is beneficial to culture of high-quality large-sized DAST crystal, thereby laying a good material and theoretical basis for research on the DAST crystal and related products.
Owner:CHINA ELECTRONICS TECH GRP NO 46 RES INST
Preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate
InactiveCN103467366AEasy to prepareThe reaction steps are simpleSulfonic acids salts preparationBenzaldehydeIon exchange
The invention belongs to the technical field of functional material synthesis, and relates to a preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate, which comprises the following steps: mixing 4-methylpyridine and methyl-p-toluene sulfonate, putting in absolute methanol, and pouring into a three-hole flask to carry out ion-exchange reaction; dissolving dimethyl amino benzaldehyde in absolute methanol, and putting into the three-hole flask to carry out condensation reflux reaction to obtain a crystallized product; and flushing the crystallized product with trichloromethane, quickly filtering to obtain a solid with green metal luster, heating the solid with green metal luster to completely dissolve the solid in the absolute methanol, cooling, airing, and recrystallizing to obtain the 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate. The preparation method has the advantages of simpleness, simple reaction steps, short reaction time, low cost and high yield.
Owner:QINGDAO UNIV
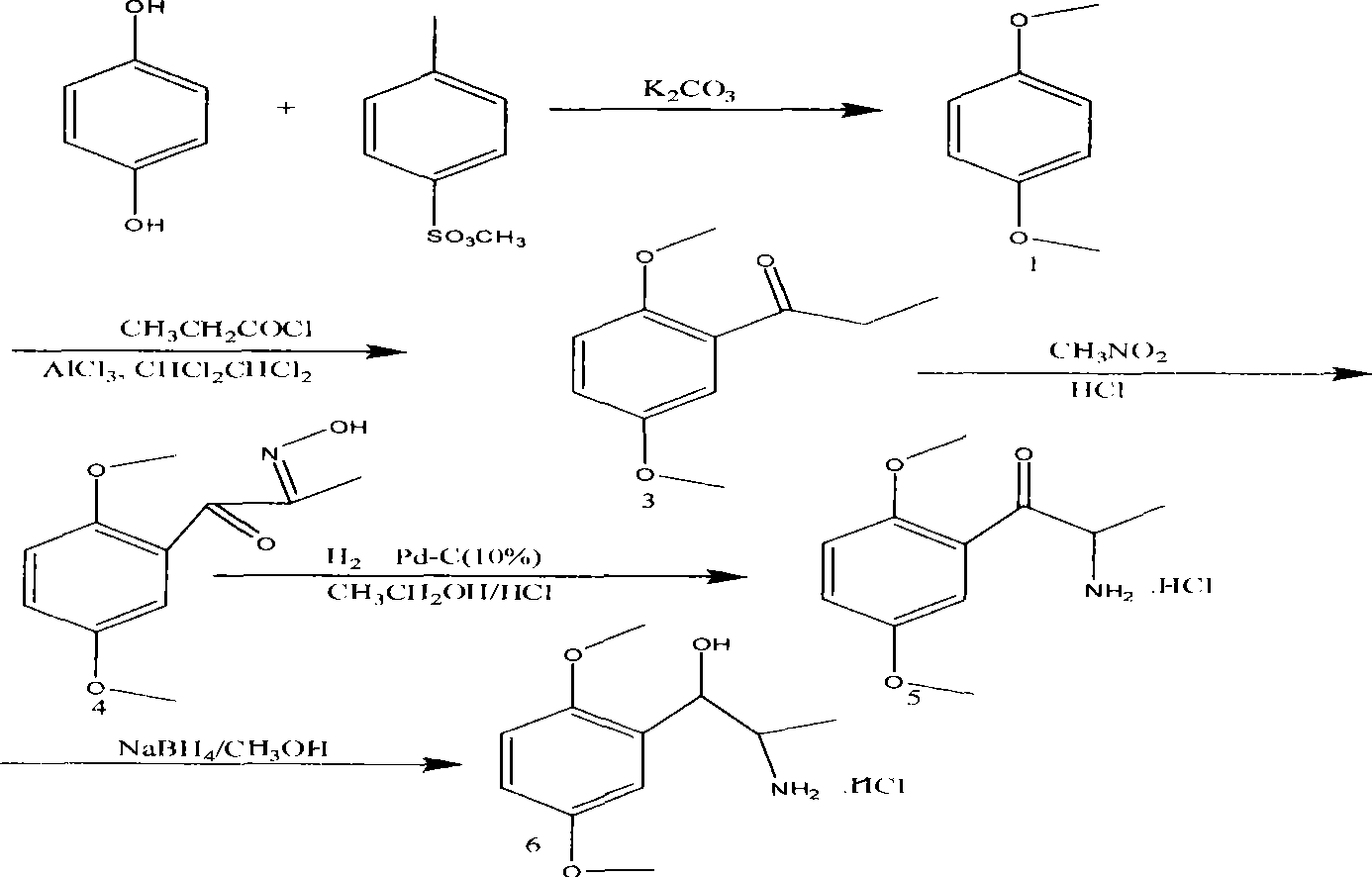
Synthesis method of methoxamine hydrochloride
ActiveCN101417956AMild responseSimple conditionsOrganic compound preparationAmino-hyroxy compound preparationHydrogenation reactionHydroquinone Compound
The invention discloses a novel synthesis method for methoxamine hydrochloride, which comprises the following steps: under the protection of nitrogen, liquid methyl p-toluenesulfonate is added into certain amount of acetonitrile solution of potassium carbonate, the temperature is controlled to between 12 and 18 DEG C, and proper amount of acetonitrile mixed liquid of hydroquinone is slowly dripped. Then the stirring is performed for 5 minutes, the temperature is increased to 83 DEG C for violent reflux reaction, the nitrogen is turned off when the reflux appears, and the complete reaction can be finished 22 hours later. Then a Friedel-Crafts acylation reaction, an oximation reaction, a hydroximino reduction reaction and a ketoamine hydrogenation reaction are orderly performed, and finally a target product is synthesized by an economical and environment-friendly route. The yields of 5-step operations are 94 percent, 72 percent, 80 percent, 79 percent and 88 percent respectively. The method has the advantages that the method has mild reactions and simple conditions, is steady and easy for scale-up production, and improves the yield compared with the prior synthesis of the methoxamine hydrochloride.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation of novel nucleic acid dye for polyacrylamide gel electrophoresis
InactiveCN106008495ASolve the technical problems of poor dyeing effectIncreased sensitivityOrganic chemistryPreparing sample for investigationTetrafluoroborateFluorescence
The invention provides preparation of a novel nucleic acid dye for polyacrylamide gel electrophoresis. Following reactions are sequentially conducted:, wherein is 4-methylquinoline, and is 6-bromohexanoic acid; , wherein is 2-(methylmercapto)benzothiazole, and is methyl p-toluenesulfonate; , wherein DMF is N,N-dimethyl formamide, Et3N is triethylamine, and HCl(conc.) is concentrated hydrochloric acid; , wherein is 2-succinimido-1,1,3,3-tetramethyluronium tetrafluoroborate (TSTU), DIPEA is N, N-diisopropyl ethylamine, is taurine, and is a product compound. Improvement is conducted on a molecular structure, therefore, the nucleic acid dye can easily penetrate into a high-density high polymer, the high sensitivity and high stability of the nucleic acid dye are kept, and the technical problem that a fluorescent dye serving as a nucleic acid gel dye is difficult to penetrate into the high polymer and the problem that the nucleic acid dye generally has the high toxicity are solved.
Owner:苏州宇嘉品恒企业管理咨询有限公司
Process for the preparation of lacosamide
Owner:AMRI ITAL SRL
Polyurethane adhesive and preparation method thereof
ActiveCN104031240AGood yellowing resistanceImprove anti-agingPolyureas/polyurethane adhesivesEpoxyPolyurethane adhesive
The invention provides a polyurethane adhesive and a preparation method thereof. The polyurethane adhesive comprises diphenylmethane diisocyanate, styrene grafted polyether polyol, epoxide resin, methyl p-toluenesulfonate, polypropylene glycol, polyoxypropylene pentaerythritol ester, an amine catalyst, isophorone diamine, zeolite, castor oil, N-ethyl-N-zinc phenyl dithiocarbamate, resorcinol, triphenylphosphine, a silane coupling agent and an emulsifying agent. The preparation method comprises the following steps of: firstly mixing the diphenylmethane diisocyanate, the amine catalyst, the epoxide resin, the methyl p-toluenesulfonate, the polypropylene glycol, the polyoxypropylene pentaerythritol ester and the zeolite, and then adding the resorcinol and the triphenylphosphine; stirring, and then adding a mixture of the styrene grafted polyether polyol, the isophorone diamine, the castor oil, the N-ethyl-N-zinc phenyl dithiocarbamate, the silane coupling agent and the emulsifying agent to obtain the polyurethane adhesive. The polyurethane adhesive provided by the invention has the advantages of excellent yellowing resistance, ageing resistance, heat resistant property, hydrolysis resistant property and bonding strength.
Owner:上海汉司实业有限公司
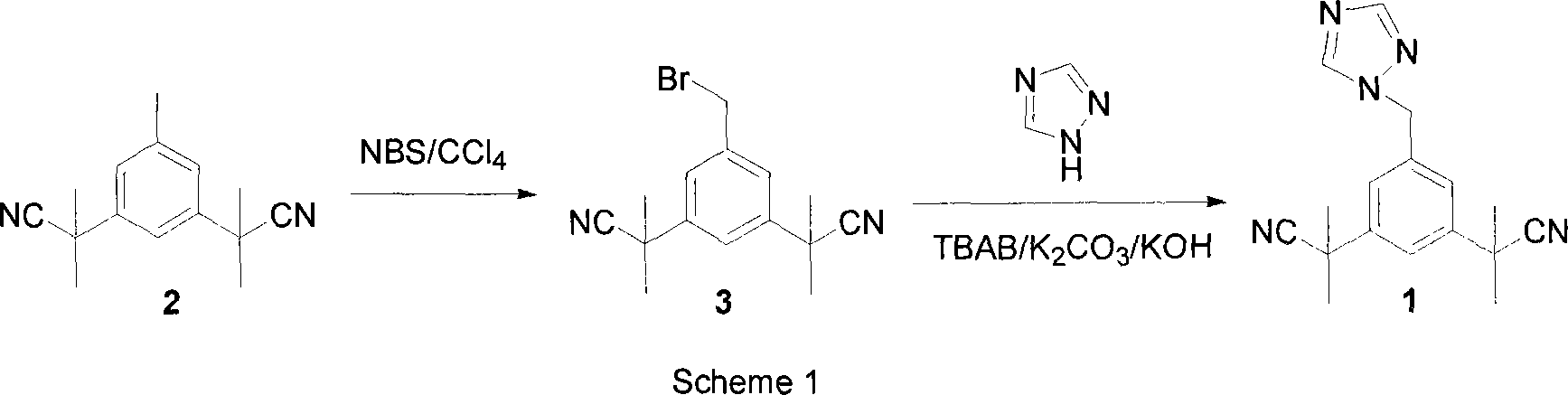
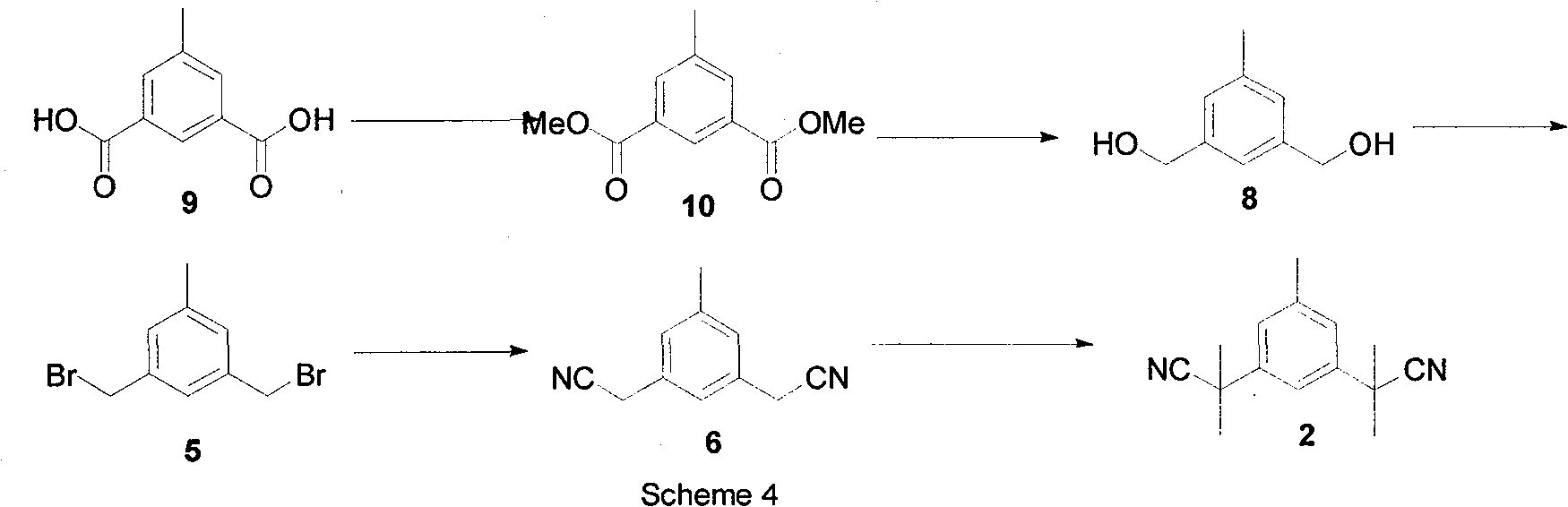
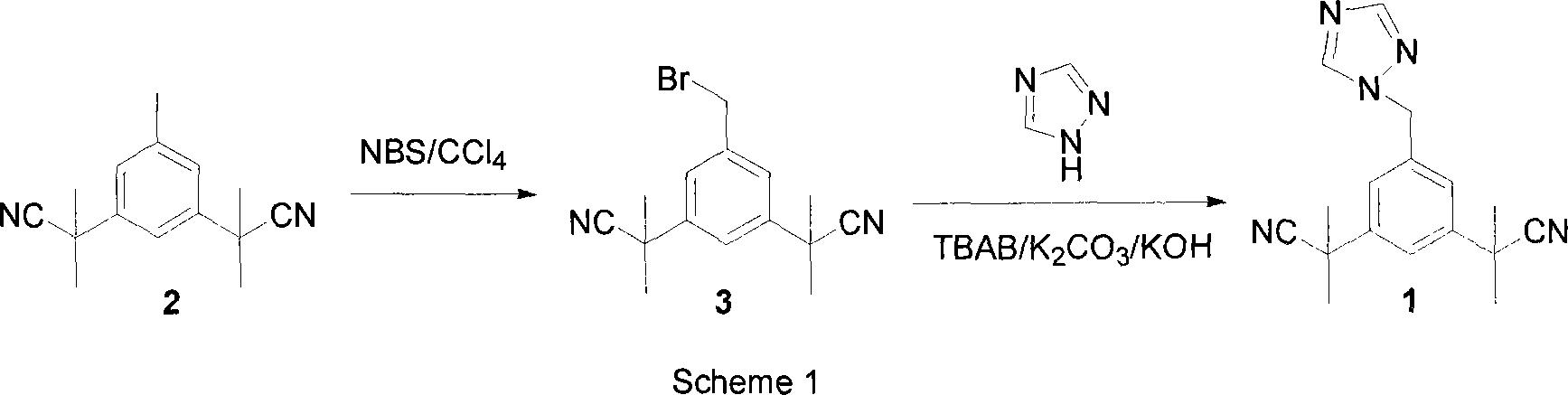
Preparation of 3,5-di(2-cyano-isopropyl)-toluene
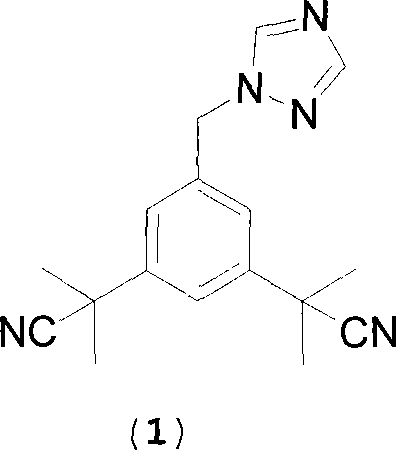
ActiveCN101239932AReduce usageHigh purityPreparation by cyanide reactionChemical synthesisP-Toluenesulfonic acid
The invention relates to a novel synthesis process of pharmaceutical intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene, belonging to the chemical synthesis technology field. The invention uses 5-methyl-1, 3-dimethyl phthalate as the material, synthesizes anastrozole intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene by esterification, reduction, brominating, cyaniding and methylation reaction. The reduction method using borohydrides is performed in aether solvent, the method can completely avoid using high-toxicity solvent such as CCl4 or the like; in the methylation reaction, expensive iodomethane is replaced by p-toluenesulfonic acid methyl; the purity of product 3, 5-bi(2-cyanoindole-isopropyl)-toluene (2) is high.
Owner:苏州第壹制药有限公司
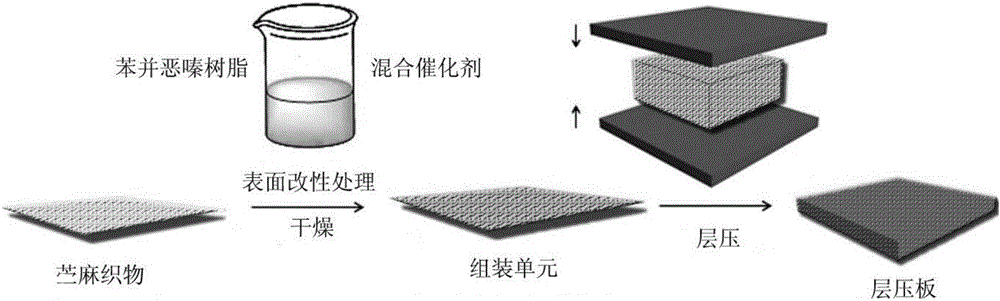
Flame-retardant modified ramie fabric/benzoxazine resin laminated board and preparation method thereof
ActiveCN106079723AImprove flame retardant performanceImprove performanceLamination ancillary operationsLayered product treatmentEngineeringFire retardant
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Thick oil viscosity reducer, preparing method thereof and thick oil viscosity reducing method
ActiveCN105018062AImprove viscosity reduction efficiencySimple implementation of viscosity reductionDrilling compositionMethyl carbonatePotassium hydroxide
The invention discloses a thick oil viscosity reducer, a preparing method thereof and a thick oil viscosity reducing method. The total weight of the thick oil viscosity reducer serves as a benchmark, a component A accounts for 3-30% weight of the thick oil viscosity reducer, a component B accounts for 5-40% weight of the thick oil viscosity reducer, and a solvent accounts for 30-92% weight of the thick oil viscosity reducer. The component A is at least one of halogenate methane, p-toluenesulfonic acid methyl ester, dimethyl sulfate, methyl trifluoromethansulfonate and dimethyl carbonate. The component B is at least one of sodium hydroxide, potassium hydroxide, ammonia water, R1NOH and R2NX, wherein R1 and R2 are independently C1-C4 alkyl groups, and X is F or Cl or Br or I. The higher viscosity reducing efficiency can be achieved when the thick oil viscosity reducer is used for reducing the viscosity of the crude oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
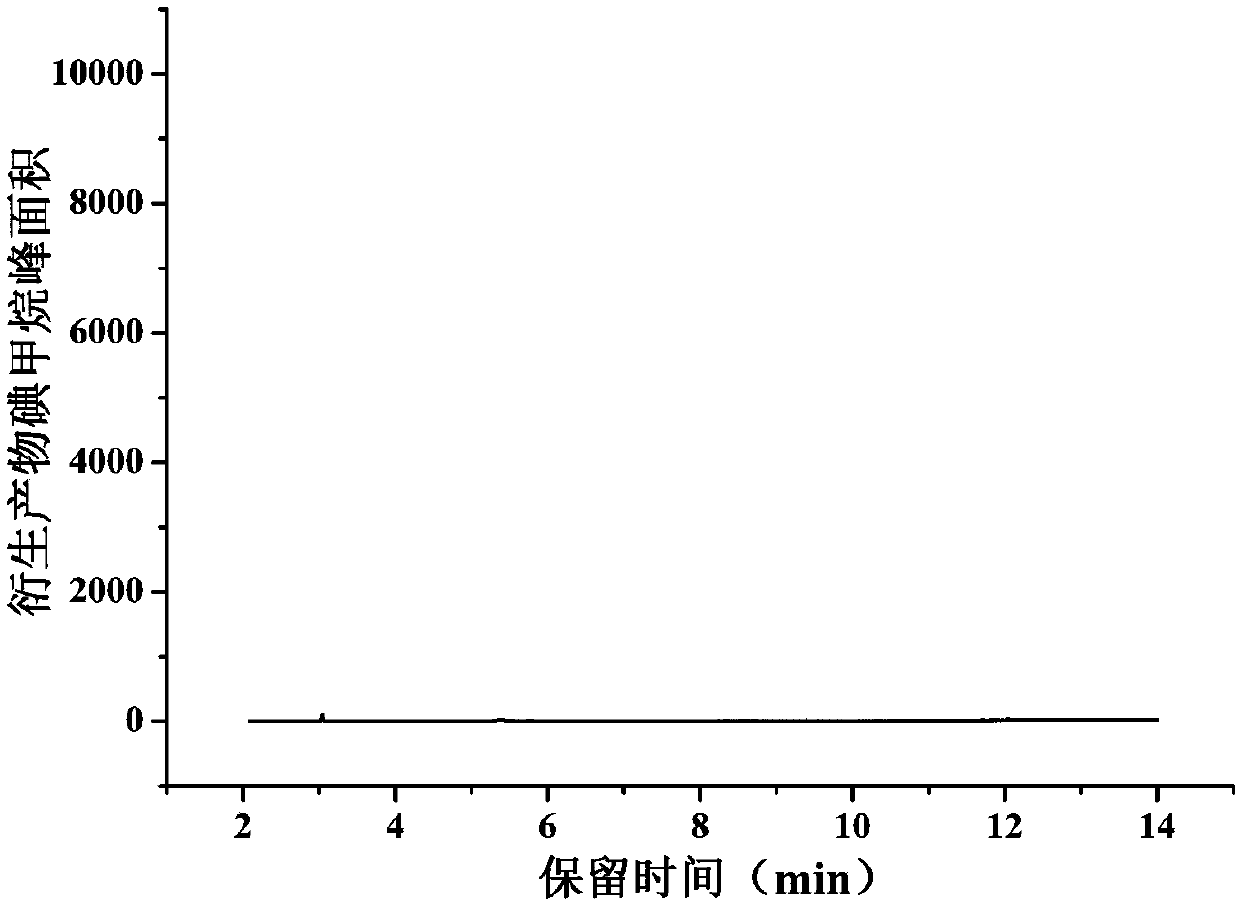
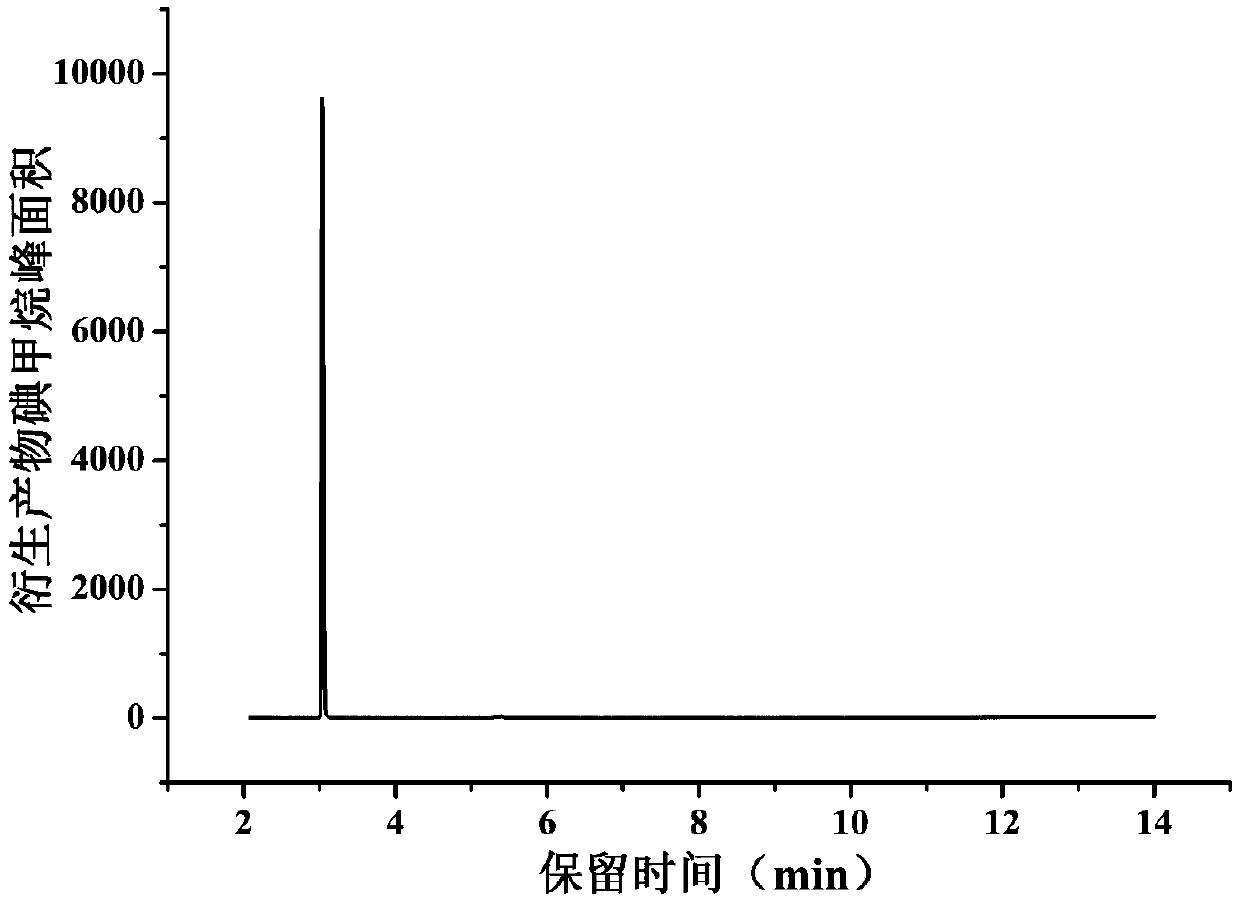
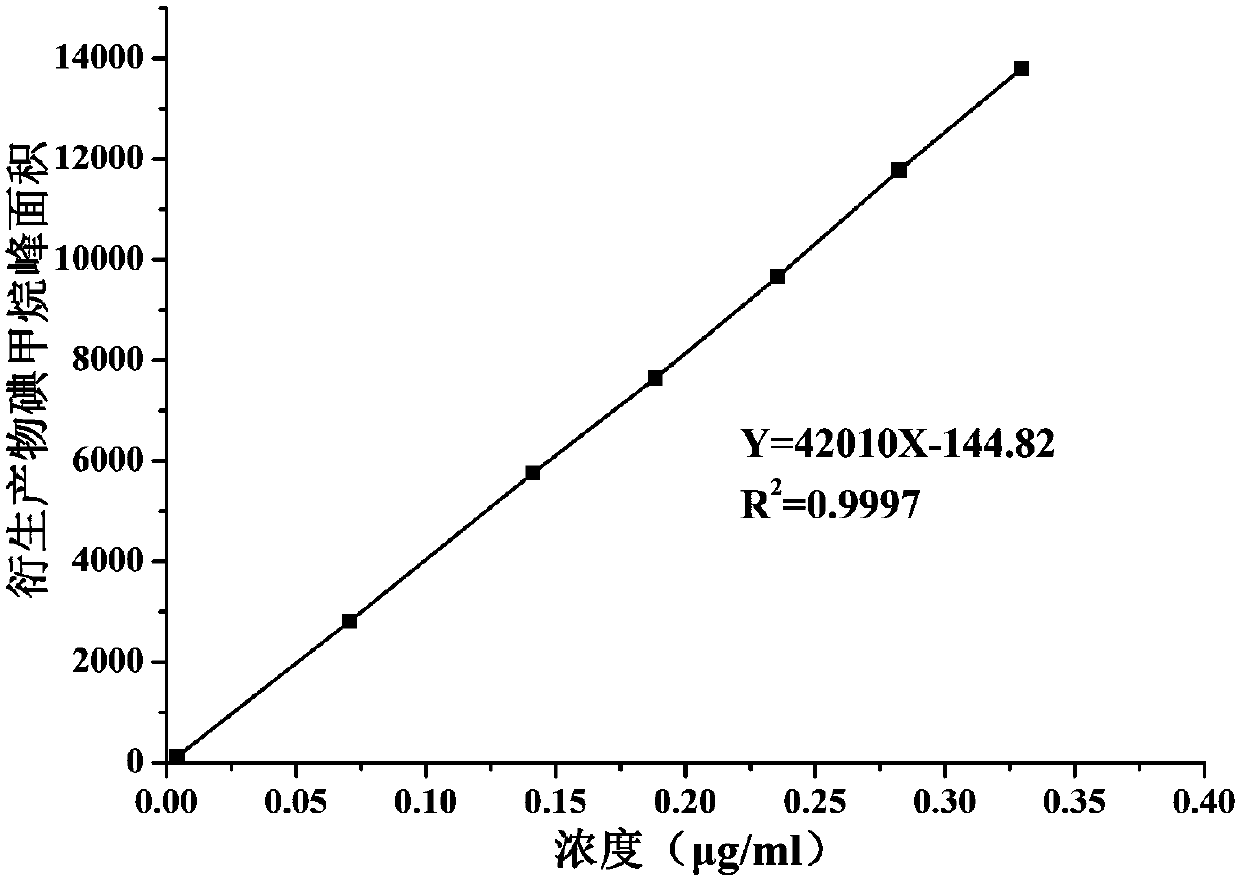
Method for determining methyl p-toluenesulfonate in medicine
The invention relates to a method for determining the content of methyl p-toluenesulfonate in a medicine, and concretely relates to a method for detecting the content of methyl p-toluenesulfonate in amedicine through headspace derivatization gas chromatography-mass spectrometry. The above derivatization method, using a mixed aqueous solution containing vitamin C and sodium iodide as a derivatization solution, has the advantages of wide suitable equilibrium temperature and equilibrium time range, high sensitivity, high precision, high accuracy, good durability, and easiness in operation, and can realize the accurate determination of the methyl p-toluenesulfonate in the medicine in order to ensure the safety of the medicine.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
A mass spectrometry method for detecting sulfonate genotoxic impurities based on dielectric barrier discharge ion source
ActiveCN109192652AFast and accurate detection and analysisImprove throughputSamples introduction/extractionMaterial analysis by electric/magnetic meansP-toluenesulfonateGenotoxicity
The invention provides a mass spectrometry detection method of sulfonate genotoxic impurities based on a dielectric barrier discharge ion source, and belongs to the technical field of pharmaceutical analysis. The method comprises the following steps: a sample solution is dripped as a sample point at a certain interval in the length direction of the sample table, the sample point is dried naturally, the dielectric barrier discharge ion source is aligned with the mass spectrometer at the front end of the sample point, the dielectric barrier discharge ion source is turned on, the heating platformand the mass spectrometer are in a working state, and mass spectrometry analysis and detection are carried out. A method for directly detect genotoxic impurities in medicine by dielectric barrier discharge ionization mass spectrometry is disclosed, From the sample preparation to the result, the rapid detection and analysis of the genotoxic impurity methyl p-toluenesulfonate in the drug can be completed in a few minutes. The established method can detect and analyze the methyl p-toluenesulfonate in the drug sensitively and quickly, thus realizing the high-throughput drug screening, and has good practical application value.
Owner:SHANDONG ANALYSIS & TEST CENT
Synthetic method of betamethasone or prednisolone intermediate
The invention discloses a synthetic method of 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone-21-acetate. The invention employs 11 alpha-hydroxy-1,4-diene-16,17-epoxy progesterone (ii) as a starting material, which together with methyl p-toluenesulfonate is subjected to a Mitunobu reaction for inversion under the catalysis of diethyl azodiformate and triphenyl phosphine to obtain 11 beta-p-toluenesulfonyl-16,17 epoxy-1,4-diene progesterone (iii), namely a 11 beta-hydroxy protector; the (iii) is subjected to protective group removal in the presence of HBr, and 16,17-epoxy addition to obtain 11 beta, 17 alpha-dihydroxy-16 beta-bromo-1,4-allyl progesterone (iv); the (iv) is subjected to 3 steps to synthesize 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone (v), which is added with iodine to obtain 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20- diketone-21-iodine (vi); and the (vi) is subjected to replacement with potassium acetate to obtain the 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone-21-acetate (i). The method provided by the invention can avoid complex strain breeding, has the advantages of simple operation, high yield, little environmental pollution, and has good industrial application prospect.
Owner:SHANGHAI NEW HUALIAN PHARMA
Synthesis method of methyl p-toluenesulfonate
ActiveCN109225312AImprove conversion rateHigh yieldMolecular sieve catalystsSulfonic acid esters preparationSynthesis methodsP-toluenesulfonate
The invention discloses a synthesis method of methyl p-toluenesulfonate. The synthesis method takes concentrated sulfuric acid, methanol, p-toluene sulfonic acid, FeCl3*H3O, zinc oxide and SBA-15 zeolite as main raw materials; and the methyl p-toluenesulfonate is obtained through a synthesis technology by adopting sulfonic acid esterification of the p-toluene sulfonic acid and methanol under the action of a catalyst Fe / Zn / SBA-15 through sulfonic acid esterification reaction. Compared with a manner of taking pure acid as a catalyst to obtain an unsatisfactory esterification effect and relatively low conversion rate and yield, the synthesis method takes the solid-phase loading catalyst Fe / Zn / SBA-15 to greatly improve the esterification conversion rate and yield of the p-toluene sulfonic acid.
Owner:南通沃兰化工有限公司
Preparation method and application of bicyclol
PendingCN111205263ANot suitable for commercial purchaseOvercome the disadvantages of harsh post-processing and low yieldOrganic chemistryComponent separationPharmaceutical SubstancesChemistry
The invention belongs to the field of medicines, and particularly relates to a preparation method and application of bicyclol. The preparation method comprises the following steps: adding bicyclol acid and alkali into a reaction solvent; adding a methyl p-toluene sulfonate solution; and carrying out reactions to obtain bicyclol. According to the provided preparation method, a new methylation reagent namely methyl p-toluene sulfonate is adopted; and a methylation reagent, which is toxic and explosive, is difficult to buy, and is dangerous during the application process, is not used. The defectssuch as difficult post-treatment, low yield and the like caused by adopting a methylation reagent which is toxic and explosive are overcome. Prodrugs meeting the quality requirements can be preparedthrough simple process operation and mild experimental conditions, and the yield is high. The method is stable in process yield, has reproducibility and reliability and is suitable for industrial production.
Owner:北京鑫开元医药科技有限公司
A kind of synthetic method of methoxamine hydrochloride
ActiveCN101417956BMild responseSimple conditionsOrganic compound preparationAmino-hyroxy compound preparationNitrogen gasPotassium carbonate
Owner:NANCHANG HANGKONG UNIVERSITY
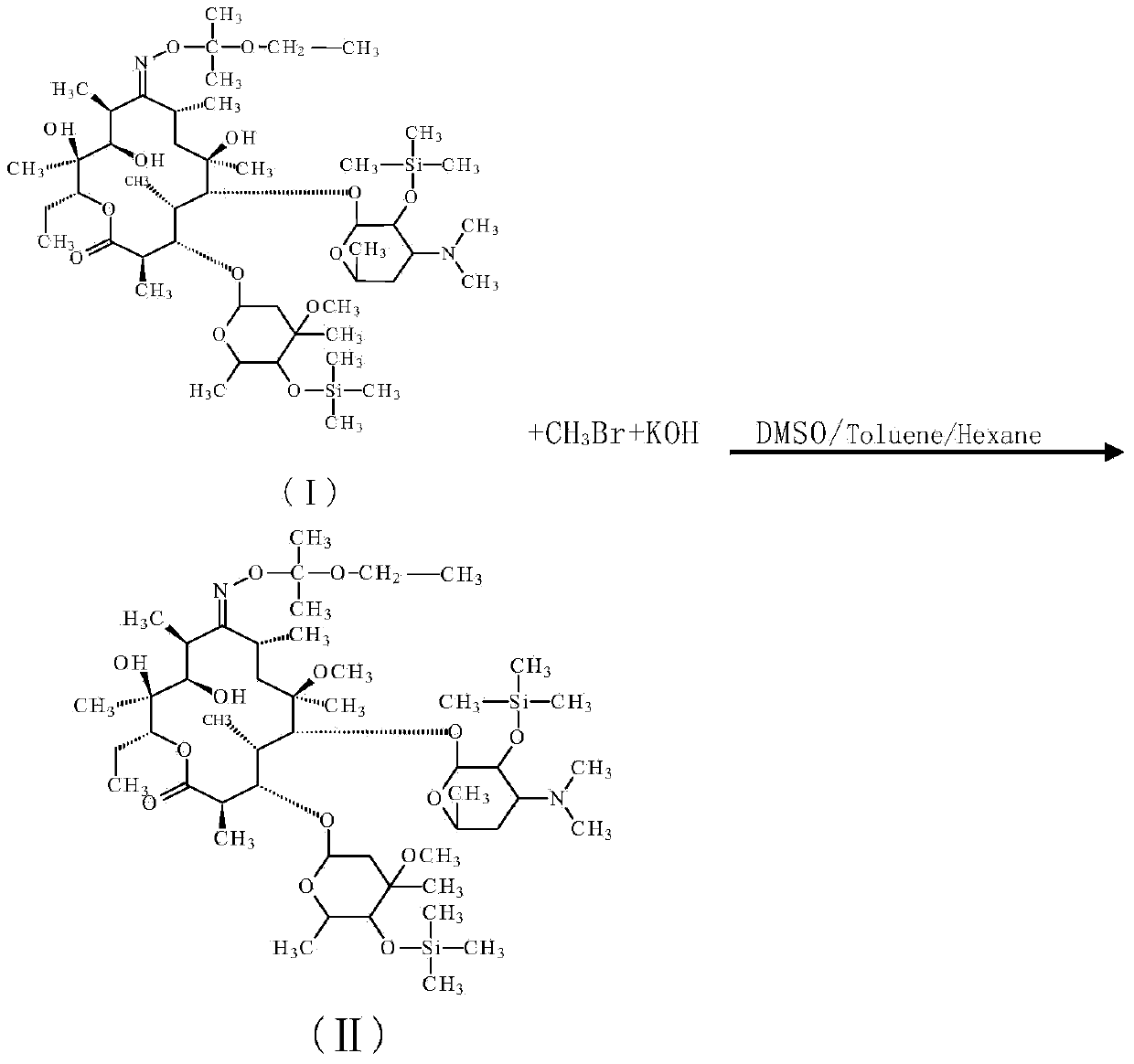
Preparation method for Clarithromycin intermediate
ActiveCN103992346AReduce tolueneDMSOGroup 4/14 element organic compoundsPotassium hydroxideClarithromycin
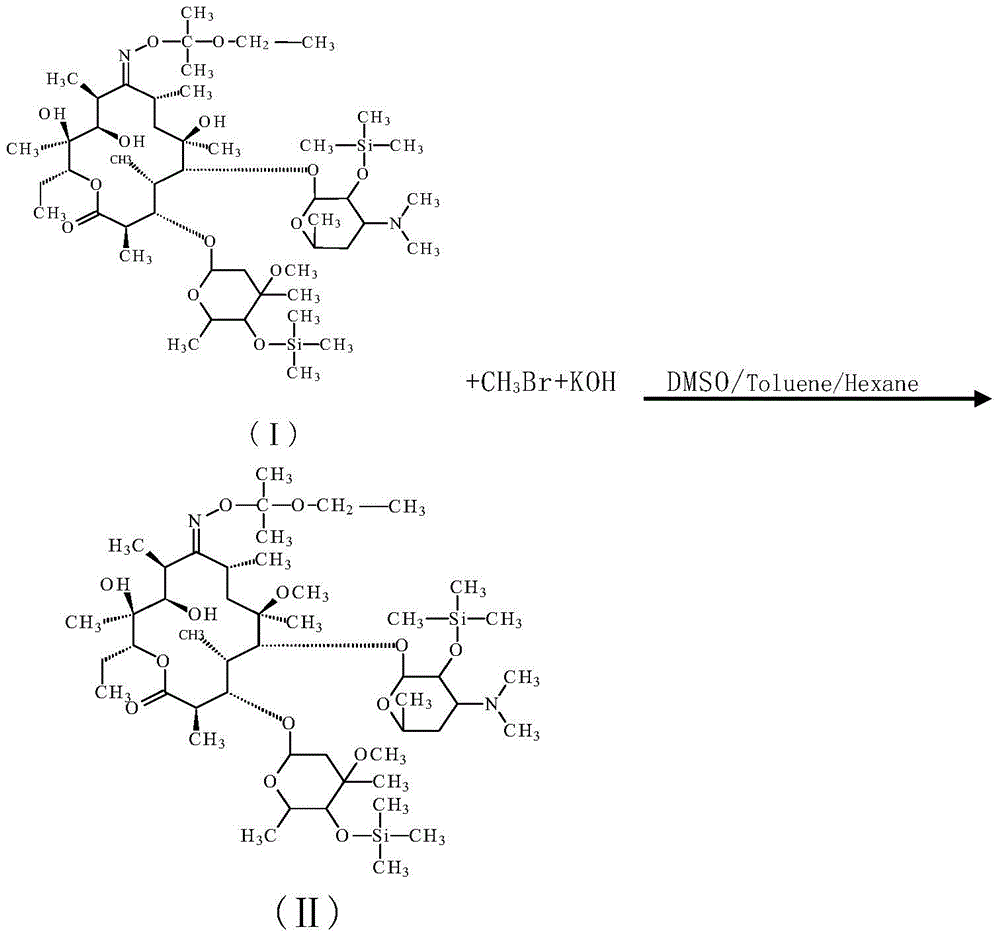
The invention discloses a preparation method for a Clarithromycin intermediate. The method is characterized in that (2',4"-O-bistrimethylsilyl)-erythromycin A-9[O-(1-ethoxy-1-methylethyl)]oxime is used as a raw material, toluene, hexane and dimethyl sulfoxide are used as solvents, bromomethane, iodomethane and methyl p-toluenesulfonate are used as methylation reagents, potassium hydroxide is used as a catalyst, and methylation is carried out so as to prepare the Clarithromycin intermediate. According to the invention, through selection of a solvent system, cooperative control of the amounts of the reaction reagent and the catalyst and synergism of temperature and time, the preparation method for the Clarithromycin intermediate has the advantages of simple process, stable operation, a high conversion rate, low cost, a small amount of waste gas, waste water and industrial residue and suitability for industrial production.
Owner:ZHEJIANG BETTER PHARMA
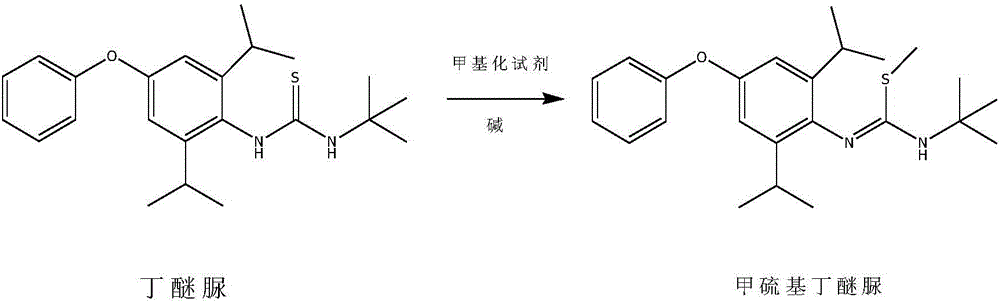
Method for preparing methylmercaptodiafenthiuron
The invention discloses a method for preparing methylmercaptodiafenthiuron. The method comprises the following reaction formula described in the specification, wherein in the formula, a methylation reagent is selected from methyl chloride, methyl bromide, methyl benzenesulfonate, methyl trifluoromethansulfonate, methyl p-toluenesulfonate, dimethyl carbonate, trimethyl phosphate, dimethyl sulfate, diazomethane, methyl trichloroethanimidate, and formaldehyde+formic acid; an alkali is selected from M2CO3, NCO3, MHCO3, MXR1, N(XR1)2, MH, NH2 or NO; M is selected from Li, Na or K; N is selected from Mg or Ca; X is selected from O or S; R1 is selected from H or C1-C6 alkyl. The compound shown in the reaction formula undergoes a reaction with the methylation reagent and the alkali in a proper solvent to prepare the methylmercaptodiafenthiuron. According to the method, the methylation reagent with low price is used, so the product cost is reduced; and the method is mild in reaction condition, simple, convenient and feasible in post-treatment method, economic and effective, and prone to industrialized production.
Owner:HAILIR PESTICIDES & CHEM GRP
Preparation method of composite flame retardant pretreatment liquid
InactiveCN109232963AImprove flame retardant performanceProduce environmental protectionCoatingsPhosphateEvaporation
The invention relates to the field of foam plastic modification, in particular to a preparation method of composite flame retardant pretreatment liquid. The preparation method comprises the followingsteps: a) preparing mixed liquid from methyl p-toluenesulfonate and 2-ethyl-4,5-dihydrooxazoline, and shaking and heating to obtain powder; b) adding the powder to hydrogen chloride, heating for reaction, drying by evaporation, adding a saturated sodium hydroxide solution, filtering, washing and drying to obtain a white solid; c) mixing the white solid with methanol, adding sodium hydroxide and carbon disulfide at low temperature, precipitating the solution after reaction, filtering and washing the precipitate, and grinding the precipitate to obtain fine powder; d) mixing sodium hypophosphite,melamine, chlorinated phosphate, tetrachlorobisphenol A and magnesium hydroxide into a mixture, adding the mixture to decalin, then adding a coupling agent, and stirring to obtain a base solution; e)adding a pentaerythritol stearate and polyethylene wax composition into the base solution, adding the fine powder to obtain dispersion liquid, and shaking the dispersion liquid to obtain the composite flame retardant pretreatment liquid.
Owner:德清舒华泡沫座椅有限公司
Preparation of 3,5-di(2-cyano-isopropyl)-toluene
The invention relates to a novel synthesis process of pharmaceutical intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene, belonging to the chemical synthesis technology field. The invention uses 5-methyl-1, 3-dimethyl phthalate as the material, synthesizes anastrozole intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene by esterification, reduction, brominating, cyaniding and methylation reaction. The reduction method using borohydrides is performed in aether solvent, the method can completely avoid using high-toxicity solvent such as CCl4 or the like; in the methylation reaction, expensive iodomethane is replaced by p-toluenesulfonic acid methyl; the purity of product 3, 5-bi(2-cyanoindole-isopropyl)-toluene (2) is high.
Owner:苏州第壹制药有限公司
A kind of preparation method of clarithromycin intermediate
ActiveCN103992346BImprove conversion rateFully methylatedGroup 4/14 element organic compoundsPotassium hydroxideClarithromycin
The invention discloses a preparation method for a Clarithromycin intermediate. The method is characterized in that (2',4"-O-bistrimethylsilyl)-erythromycin A-9[O-(1-ethoxy-1-methylethyl)]oxime is used as a raw material, toluene, hexane and dimethyl sulfoxide are used as solvents, bromomethane, iodomethane and methyl p-toluenesulfonate are used as methylation reagents, potassium hydroxide is used as a catalyst, and methylation is carried out so as to prepare the Clarithromycin intermediate. According to the invention, through selection of a solvent system, cooperative control of the amounts of the reaction reagent and the catalyst and synergism of temperature and time, the preparation method for the Clarithromycin intermediate has the advantages of simple process, stable operation, a high conversion rate, low cost, a small amount of waste gas, waste water and industrial residue and suitability for industrial production.
Owner:ZHEJIANG BETTER PHARMA
Preparation method of stable isotope labeled methotrexate internal standard reagent
ActiveCN114456170AReduce usageHigh chemical purityIsotope introduction to heterocyclic compoundsComponent separationStable Isotope LabelingIsotopic labeling
The invention relates to a preparation method of a stable isotope labeled methotrexate internal standard reagent for monitoring the blood concentration of a clinical treatment drug, and belongs to the field of research and development of standard substances for monitoring the blood concentration of the clinical treatment drug. The preparation method comprises the following steps: by taking stable isotope labeled methyl p-toluenesulfonate as a raw material, carrying out addition, condensation and hydrolysis, and finally separating and purifying to obtain the stable isotope labeled methotrexate. The process route has the advantages that raw materials required for synthesis are simple and easy to obtain, reaction conditions are mild, a highly toxic methylation reagent is not used, and a final product is high in total yield and easy to separate and purify. The chemical purity of the final product stable isotope labeled methotrexate can reach 99% or above, and the isotope abundance can reach 99% or above. The stable isotope labeled methotrexate internal standard reagent prepared by the preparation method can meet the technical requirements of the stable isotope labeled internal standard reagent required by a liquid chromatography-tandem mass spectrometry method for monitoring the blood concentration of clinical treatment drugs.
Owner:谱同生物医药科技常州有限公司
A kind of polyurethane adhesive and preparation method thereof
ActiveCN104031240BGood yellowing resistanceImprove anti-agingPolyureas/polyurethane adhesivesEpoxyPolyurethane adhesive
The invention provides a polyurethane adhesive and a preparation method thereof. The polyurethane adhesive comprises diphenylmethane diisocyanate, styrene grafted polyether polyol, epoxide resin, methyl p-toluenesulfonate, polypropylene glycol, polyoxypropylene pentaerythritol ester, an amine catalyst, isophorone diamine, zeolite, castor oil, N-ethyl-N-zinc phenyl dithiocarbamate, resorcinol, triphenylphosphine, a silane coupling agent and an emulsifying agent. The preparation method comprises the following steps of: firstly mixing the diphenylmethane diisocyanate, the amine catalyst, the epoxide resin, the methyl p-toluenesulfonate, the polypropylene glycol, the polyoxypropylene pentaerythritol ester and the zeolite, and then adding the resorcinol and the triphenylphosphine; stirring, and then adding a mixture of the styrene grafted polyether polyol, the isophorone diamine, the castor oil, the N-ethyl-N-zinc phenyl dithiocarbamate, the silane coupling agent and the emulsifying agent to obtain the polyurethane adhesive. The polyurethane adhesive provided by the invention has the advantages of excellent yellowing resistance, ageing resistance, heat resistant property, hydrolysis resistant property and bonding strength.
Owner:上海汉司实业有限公司
A kind of synthetic method of anti-influenza drug
The invention discloses a method for synthesizing a new type of anti-influenza drug baloxavir distil, which uses thiosalicylic acid compound 1 and 1-(halogenated methyl)-2,3-difluorobenzene compound 2 as starting materials. Compound 3 was obtained by direct docking, and then PPA was used to close the ring to obtain 7,8-difluorodibenzo[b,e]thiazin-11(6H)-one compound 4, and then the key compound 4 was obtained under the catalysis of a chiral enzyme. Chiral Thiazepam Intermediate Compound 5. Then, compound 5 is directly condensed with the key chiral fragment compound 6 through Mitsubobu reaction to obtain compound 7. Finally, the alkyl protection is removed and condensed with ((methoxycarbonyl)oxy)4-toluenesulfonate methyl ester to obtain the final product compound 9. Loxavir dipivoxil. This synthesis route reduces the difficulty of scale-up of route process operations, reduces the generation of by-products, improves product purity, and reduces route costs. The specific route is:
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
A high-yield, high-purity dast source powder synthesis process
The invention discloses a high-yield high-purity DAST source powder synthetic process. The synthetic process comprises the following two steps: 1, reacting 4-methylpyridine with methyl p-toluenesulfonate in the presence of absolute ethyl alcohol serving as a solvent to obtain an absolute ethyl alcohol solution of 4-methyl-N-methylpyridine tosilate; and 2, reacting 4-methyl-N-methylpyridine tosilate with p-dimethylaminobenzaldehyde in the presence of absolute ethyl alcohol serving as a solvent and under the catalytic action of di-n-butylamine or piperidine, so as to obtain high-yield (85-95%) high-quality (90-95%) DAST source powder. According to the synthetic process, absolute ethyl alcohol is used as a reaction solvent, harm of poisonous and harmful solvents such as methylbenzene and methyl alcohol to the body of an operator can be avoided and the pollution of waste liquid to the environment can be avoided. The research success of the high-yield high-purity DAST source powder synthetic process is beneficial to culture of high-quality large-sized DAST crystal, thereby laying a good material and theoretical basis for research on the DAST crystal and related products.
Owner:CHINA ELECTRONICS TECH GRP NO 46 RES INST
Preparation method of quick self-drying cleaning liquid for oil lens
InactiveCN108641824AImprove adsorption capacityReduce adhesionInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsGraphiteDiethyl ether
The invention discloses a preparation method of a quick self-drying cleaning liquid for an oil lens. The preparation method comprises the following operation steps: (1) adding nano graphite into the methyl trimethoxy silane with a weight of 4-6 times of the weight of the nano graphite, heating the mixture to 88-94 DEG C, keeping the temperature for 6-8 hours, heating the mixture to 125-130 DEG C until the liquid is completely volatilized to obtain modified nano graphite; and (2) uniformly mixing methyl p-toluenesulfonate, bis-(sodium sulfopropyl)-disulfide, myrcene, the modified nano graphiteand diethyl ether, and carrying out ultrasonic treatment for 15-20 minutes to obtain the quick self-drying cleaning liquid for the oil lens. The preparation method of the quick self-drying cleaning liquid for the oil lens is simple in operation and low in cost, is green and environmentally friendly, and cannot damage the oil lens, the cleaning liquid does not remain on the surface of the lens, andthe drying speed is high.
Owner:朱清
Method for improving flame retardant property of polyurethane foam
InactiveCN109096531AImprove flame retardant performanceProduce environmental protectionPhosphateSolvent
The invention relates to the field of modification of foamed plastics, in particular to a method for improving the flame retardant property of polyurethane foam. The method comprises the following steps: firstly, washing the polyurethane foam; secondly, preparing a pretreatment liquid: (a) preparing methyl p-toluenesulfonate and 2-ethyl-4,5-dihydrozoline into a mixed liquid, oscillating and carrying out heating reaction to obtain powder; (b) adding the powder into hydrogen chloride for reacting, evaporating to dryness and dropwise adding sodium hydroxide, filtrating, washing and drying to obtain a white solid; (c) mixing the white solid with methanol, adding sodium hydroxide and carbon disulfide, reacting for separating out precipitate, filtering, washing and grinding to obtain fine powder; (d) mixing sodium hypophosphite, melamine, chlorinated phosphate, tetrachlorobisphenol A and magnesium hydroxide into a mixture, and adding a solvent and a coupling agent to obtain a base liquid; (e) adding a composition into the base liquid, adding the fine powder to obtain dispersion liquid and oscillating to obtain a pretreatment liquid; thirdly, impregnating the polyurethane foam with the pretreatment liquid; fourthly, impregnating the polyurethane foam with copper-containing industrial waste liquid.
Owner:德清舒华泡沫座椅有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com