Preparation method and application of bicyclol
A bicyclic alcohol and bicyclic alkyd technology, applied in the field of medicine, can solve the problems of cumbersome process, high standards, production accidents, etc., and achieve the effect of stable process yield and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] In the first aspect, the embodiments of the present invention provide a method for preparing bicyclic alcohol, comprising the following steps:
[0022] Step S10, adding bicyclic alkyd and base to the reaction solvent;
[0023] Step S20, adding methyl p-toluenesulfonate solution for reaction to obtain bicyclic alcohol.
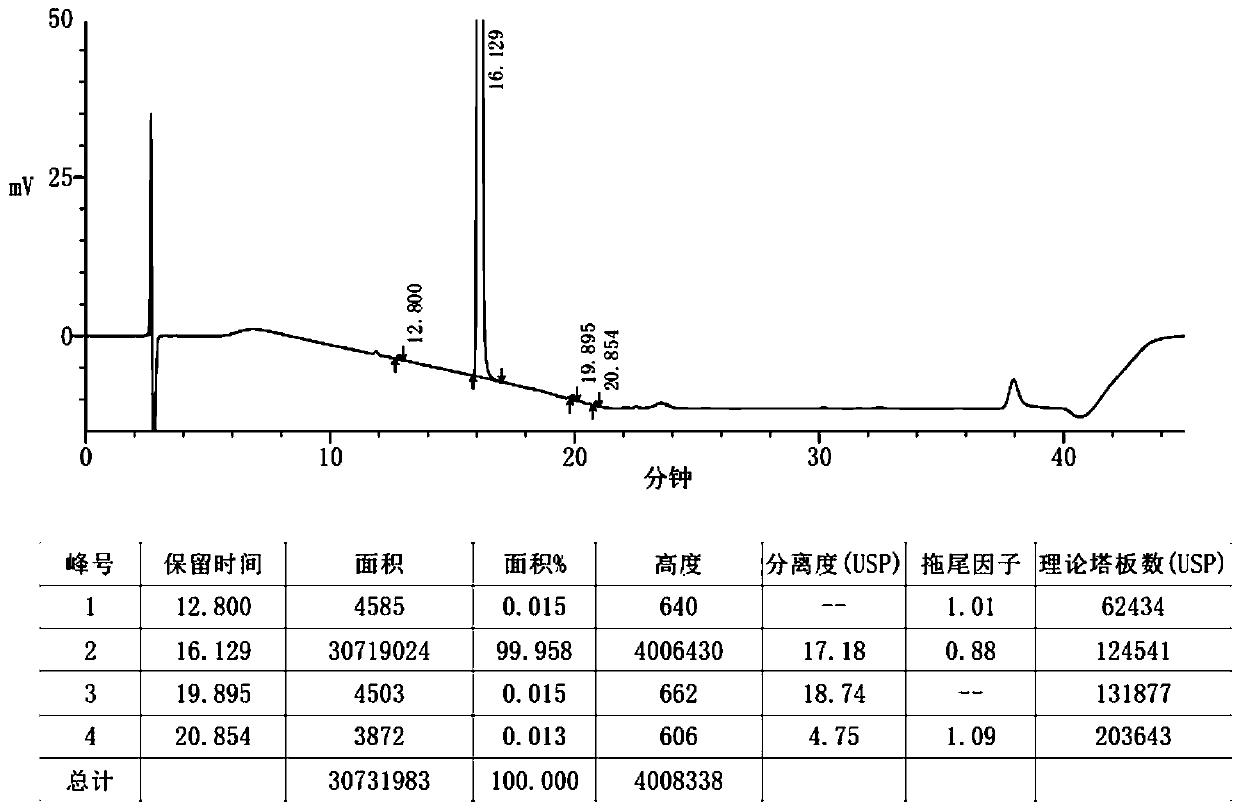
[0024] In the preparation method of the bicyclic alcohol provided in this example, a new methylating reagent, methyl p-toluenesulfonate, is used to avoid the use of methyl p-toluenesulfonate, which is prone to poisoning and explosion, is not suitable for commercial purchase, and is very likely to produce dangerous formazan during use. base reagents, thereby overcoming the disadvantages of harsh post-treatment and low yields caused by the use of methylation reagents that are prone to poisonous and explosive production. Through simple process operations, mild experimental conditions, and high yields The raw material medicine meeting the quality requiremen...
Embodiment 1
[0049] Step S1, at 0°C~5°C, add 10.0g (26.6mmol) of bicyclic alkyd and 0.96g (39.9mmol, 1.5eq) of sodium hydride into 80ml of acetonitrile, and stir for about 20min.
[0050] In step S2, 20ml of acetonitrile solution dissolved with 5.9g (31.9mmol, 1.2eq) of methyl p-toluenesulfonate was added dropwise, the addition time was about 30min, and the reaction was stirred at 0°C~5°C for about 6h.
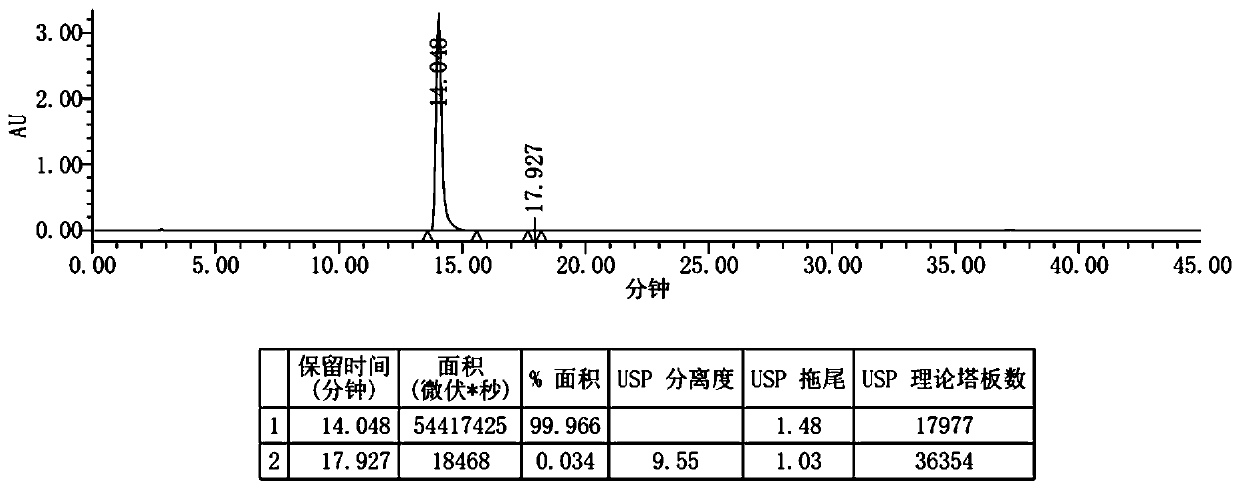
[0051] Step S3, TLC monitors the completion of the reaction, filters, collects the filtrate, adds 300ml of water dropwise under stirring at 0°C~5°C, keeps stirring at room temperature for 2 hours after the dropwise addition, filters, and dries the filter cake to obtain 7.9g of crude bicycloalcohol, bicycloalcohol The crude product yield was 76.2%.
[0052] Step S4, reflux the crude product of bicyclic alcohol with 80ml of toluene to dissolve it, add 0.5g of activated carbon for decolorization for 30min, heat filter, stir the filtrate at room temperature for 1h, precipitate a solid, filter,...
Embodiment 2
[0055] Step S1, at 45°C~50°C, add 10.0g (26.6mmol) of bicyclic alkyd and 0.64g (26.6mmol, 1.0eq) of sodium hydride into 15ml of dichloromethane, and stir for about 20min.
[0056] Step S2, 5ml of dichloromethane solution dissolved with 5.0g (26.6mmol, 1.0eq) of methyl p-toluenesulfonate was added dropwise, the dropwise addition time was about 30min, and the reaction was stirred at 45°C~50°C for about 3h.
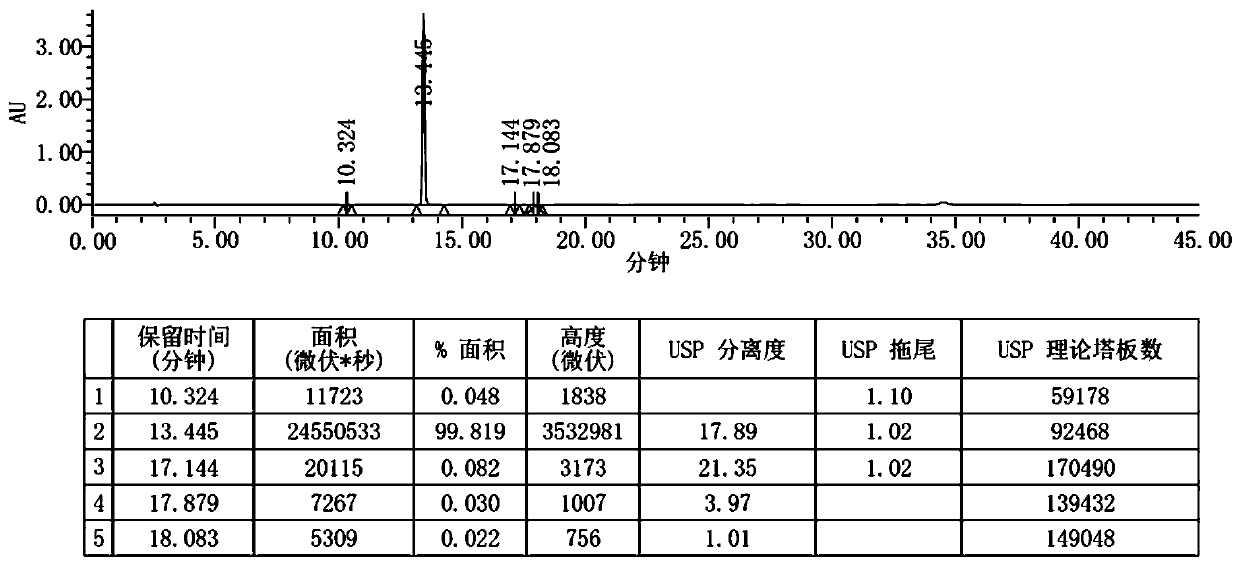
[0057] Step S3, TLC monitors the completion of the reaction, filters, collects the filtrate, adds 100ml of water dropwise under stirring at 45°C~50°C, keeps stirring at room temperature for 2 hours after the dropwise addition, filters, and dries the filter cake to obtain 7.8g of crude bicycloalcohol, bicycloalcohol The crude product yield was 75.2%.
[0058] Step S4, reflux the crude product of bicyclic alcohol with 80ml of toluene to dissolve it, add 0.5g of activated carbon for decolorization for 30min, heat filter, stir the filtrate at room temperature for 1h, precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com