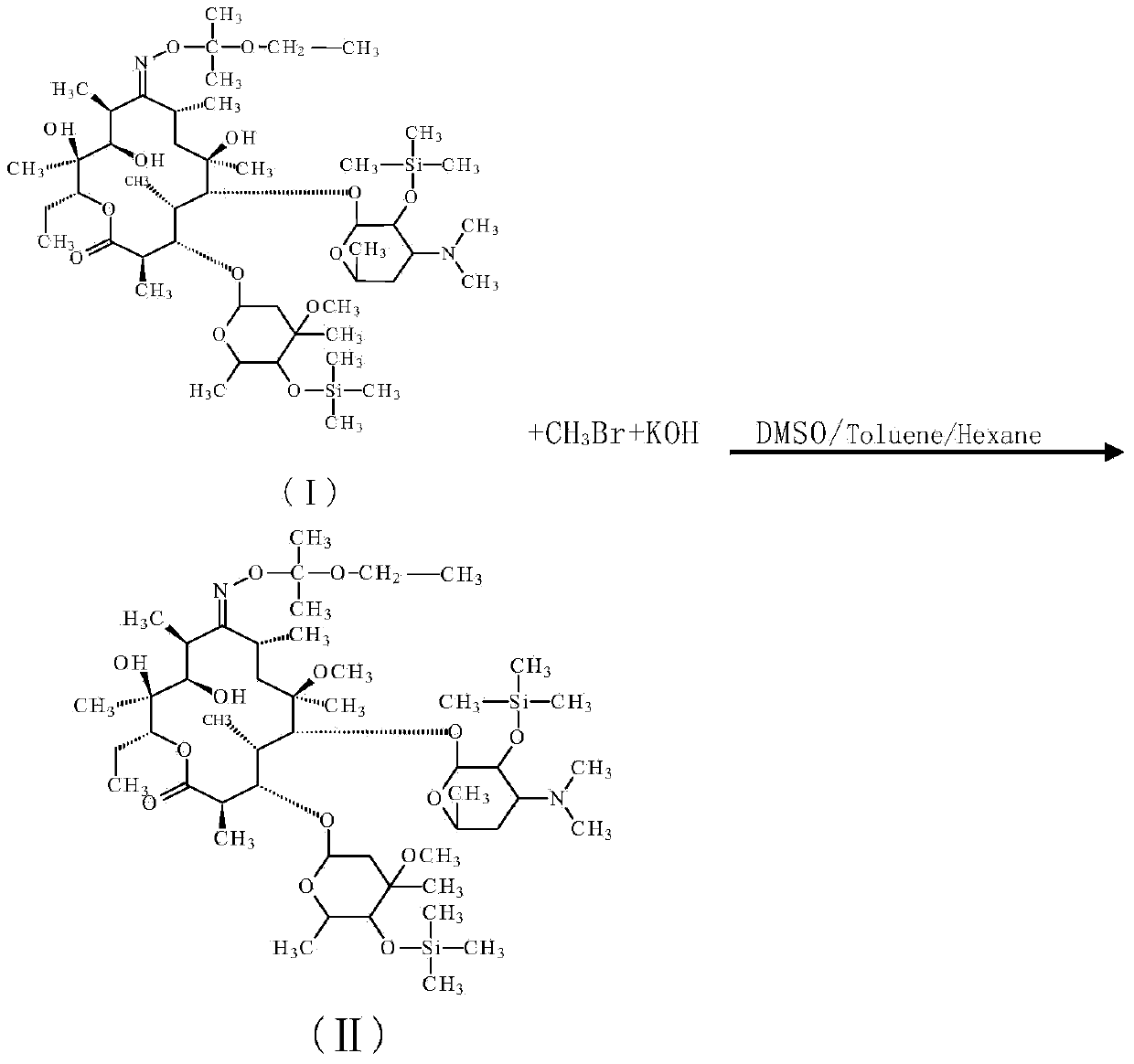
Preparation method for Clarithromycin intermediate
A technology for clarithromycin and intermediates, applied in the field of preparation of clarithromycin intermediates, can solve the problems of difficult to realize industrialized production, high recovery cost, low yield and the like, and achieves low pollution, sufficient reaction and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 1000ml reaction flask equipped with a stirrer and a thermometer, add 20g compound I (content 96%, 0.02mol), toluene 150ml (130g), stir to dissolve, add dimethyl sulfoxide 150ml (165g), control the temperature 15~ 20°C, add 0.03mol of methylation reagent, 1.4g of potassium hydroxide (content ≥ 96%, 0.024mol), control the temperature at 15-20°C for 15min, add 150ml of water, stop the reaction, HPLC detection, the purity of compound II is 73.6%, the reaction raw material is 17.5%, and the by-product is 4.0%.
Embodiment 2
[0030] The preparation method is the same as in Example 1, the difference is that the amount of Compound I, toluene and dimethyl sulfoxide is adjusted, the reaction time is adjusted, and the conversion rate under different reaction conditions is tested, as shown in Table 1.
[0031] Table 1: Effects of different solvent ratios and reaction times on conversion.
[0032] Proportion (g:ml:ml) 20 minutes to react 30 minutes to react 40 minutes to react Compound 1: Toluene: DMSO = 1:3:3 55% 65% 58% Compound 1: Toluene: DMSO = 1:5:5 48% 64% 63% Compound 1: Toluene: DMSO = 1:7:7 47% 65% 70%
[0033] Compound 1: Toluene: DMSO = 1:10:10 43% 70% 72%
[0034] It can be seen from Table 1 that as the amount of solvent increases, the reaction rate slows down and the conversion rate increases, and the amount of solvent being more than 10 times that of compound 1 is of little significance.
[0035] The second group of embodiments: t...
Embodiment 3
[0037] In a 500ml reaction flask equipped with a stirrer and a thermometer, add 20g of compound I (content 96%, 0.02mol), 50g of toluene, 10g of n-hexane, stir to dissolve, add 100g of dimethyl sulfoxide, and control the temperature at 15-20°C , add 0.03 mol of methylating reagent, 1.4 g of potassium hydroxide (content ≥ 96%, 0.024 mol), control the temperature at 15-20 ° C for 50 min, add 50 ml of water, terminate the reaction, HPLC detection, compound II purity is 76.4%, The reaction raw material is 15.1%, and the by-product is 3.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com