Preparation method of stable isotope labeled methotrexate internal standard reagent
A stable isotope, methotrexate technology, applied in the directions of isotope introduction of heterocyclic compounds, isotope introduction of organic compounds, organic chemical methods, etc., can solve the problems of high price and rare raw materials, and achieve low cost, short synthesis steps, and reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
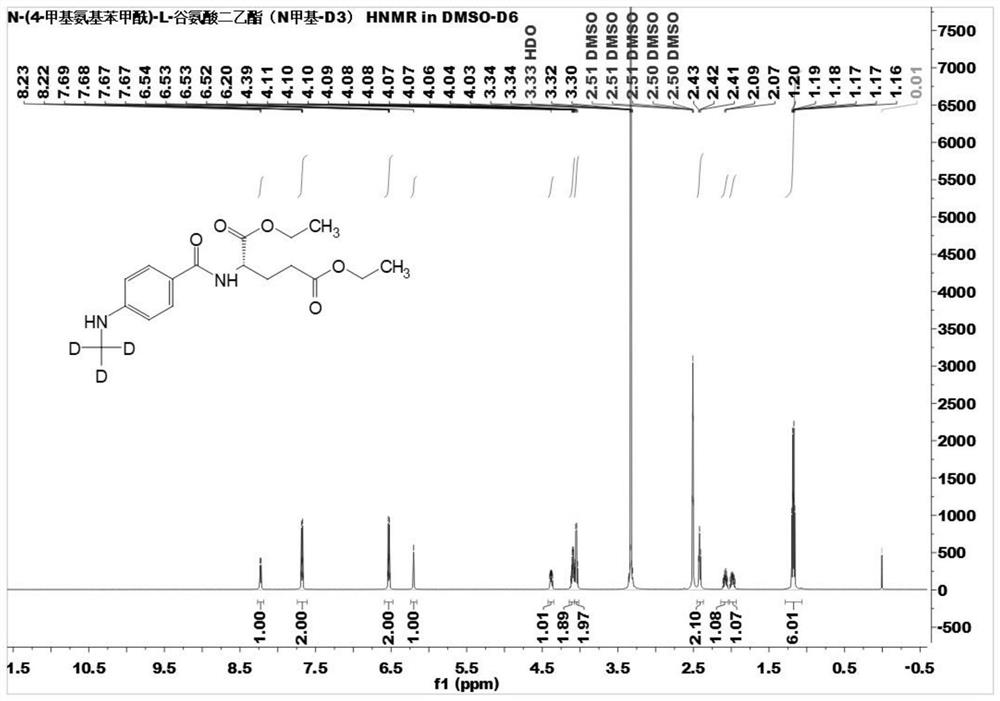
[0058] Example 1: Diethyl N-(4-methylaminobenzoyl)-L-glutamate (N-methyl-D 3 )preparation
[0059]
[0060] In a 100mL three-necked flask, add deuterated methylbenzenesulfonate (1.42g, 7.5mmol), N-(4-aminobenzoyl)-L-diethyl glutamate (2.74g, 8.25mmol) , Tetrabutylammonium bromide (12.46g, 37.5mmol), 10mL of 20% aqueous potassium carbonate solution, N, N-dimethylformamide (30mL); reacted at 70°C for 20 hours; TLC traced the reaction of the reaction materials to complete Afterwards, the reaction solution was cooled to room temperature, 200 mL of water was added, extracted with ethyl acetate (3×100 mL), the organic phases were combined, washed successively with saturated sodium bicarbonate solution and saturated sodium chloride solution, and dried by adding anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain stable isotope labeled N-(4-methylaminobenzoyl)-L-glutamic acid diethyl ester...
Embodiment 2
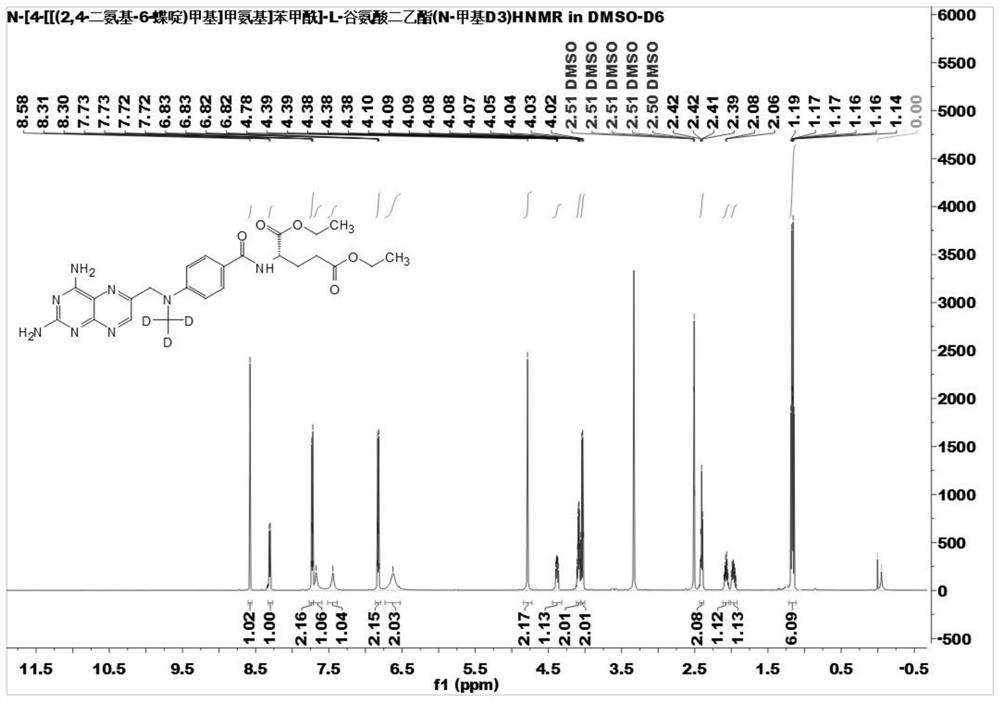
[0061] Example 2: Diethyl N-[4-[[(2,4-diamino-6-pteridine)methyl]methylamino]benzoyl]-L-glutamate (N-methyl-D 3 ) preparation
[0062]
[0063] In a 100mL three-neck flask, add stable isotope labeled N-(4-methylaminobenzoyl)-L-glutamic acid diethyl ester (N-methyl-D 3 ) (0.63g, 1.84mmol), 6-(bromomethyl)-2,4-pteridine diamine hydrobromide (0.69g, 1.84mmol), N, N-dimethylacetamide (20mL), Diisopropylethylamine (0.71g, 5.52mmol); reacted at 50°C for 16 hours; TLC tracked the reaction raw materials after the reaction was complete, distilled off the solvent under reduced pressure, added 20mL of water, and washed with ethyl acetate (3 × 30mL ) extraction, combined organic phases, washed with saturated sodium bicarbonate solution successively, washed with saturated sodium chloride solution, added anhydrous sodium sulfate to dry, evaporated under reduced pressure to remove the solvent, and the residue obtained N-[4-[[ (2,4-Diamino-6-pteridine)methyl]methylamino]benzoyl]-L-glutam...
Embodiment 3
[0064] Embodiment 3: methotrexate (N-methyl-D 3 ) preparation
[0065]
[0066] In a 50mL three-necked flask, add N-[4-[[(2,4-diamino-6-pteridine)methyl]methylamino]benzoyl]-L-glutamic acid diethyl ester (N-form Base-D 3 ) (0.17g, 0.33mmol), methanol (5mL), sodium hydroxide (0.04g, 1mmol); and reacted at 30°C for 5 hours; TLC traced that the reaction raw materials were completely reacted, the solvent was distilled off under reduced pressure, and 10mL of water was added, Regulate the pH value to 7 with hydrochloric acid of 1mol / L, stand and refrigerate and then crystallize; filter and collect the precipitated yellow crystals, obtain stable isotope labeled methotrexate (N-methyl-D 3 ) (0.12g, yield 80%). 1 H NMR (DMSO-D 6 , 600MHz): δ12.33(brs, 2H), 8.58(s, 1H), 8.19(d, J=6Hz, 1H), 7.74(d, J=6Hz, 1H), 7.67(brs, 1H), 7.45 (brs, 1H), 6.83(d, J=6Hz, 2H), 6.63(brs, 2H), 4.78(s, 2H), 4.37~4.33(m, 1H), 2.32(t, J=6Hz, 2H) , 2.08~2.02(m, 1H), 1.95~1.88(m, 1H), see attached Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com